Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Useful Constants - Standard Reduction Potentials
Caricato da
Jana Padua0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
1 visualizzazioni1 paginaTitolo originale
Useful constants - Standard Reduction Potentials
Copyright
© © All Rights Reserved
Formati disponibili
PDF, TXT o leggi online da Scribd
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PDF, TXT o leggi online su Scribd
0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
1 visualizzazioni1 paginaUseful Constants - Standard Reduction Potentials
Caricato da
Jana PaduaCopyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PDF, TXT o leggi online su Scribd
Sei sulla pagina 1di 1
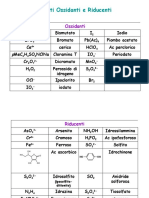
Standard Reduction Potentials at 25oC Eo (V)
F2(g) + 2e– → 2F–(aq) +2.87
Ce4+(aq) + e– → Ce3+(aq) +1.61
Au+(aq) + e– → Au(s) +1.68
MnO4–(aq) + 8H+(aq) + 5e– → Mn2+(aq) + 4H2O +1.51
Cl2(g) + 2e– → 2Cl–(aq) +1.36
Cr2O72– + 14H+(aq) + 6e– → 2Cr3+(aq) + 7H2O +1.33
O2(g) + 4H+ + 4e− ⇌ 2H2O(l) +1.23
IO3–(aq) + 6H+(aq) + 5e– → I-(aq) + 3H2O +1.20
Ag+(aq) + e– → Ag(s) +0.80
Hg22+(aq) + 2e– → 2Hg(s) +0.79
Fe3+(aq) + e– → Fe2+(aq) +0.77
I2(s) + 2e– → 2I–(aq) +0.54
Cu+(aq) + e– → Cu(s) +0.52
VO2+(aq) + 2H+(aq) + e– → V3+(aq) + H2O +0.36
Cu2+(aq) + 2e– → Cu(s) +0.34
Cu2+(aq) + e– → Cu+(aq) +0.15
Sn4+(aq) + 2e– → Sn2+(aq) +0.15
2 H+(aq) + 2e– → H2(g) 0.00
Pb2+(aq) + 2e– → Pb(s) -0.13
Sn2+(aq) + 2e– → Sn(s) -0.14
Co2+(aq) + 2e– → Co(s) -0.28
Tl+(aq) + e– → Tl(s) -0.34
Cd2+(aq) + 2e– → Cd(s) -0.40
Cr3+(aq) + e– → Cr2+(aq) -0.41
Fe2+(aq) + 2e– → Fe(s) -0.44
Cr3+(aq) + 3e– → Cr(s) -0.74
Zn2+(aq) + 2e– → Zn(s) -0.76
K+(aq) + e– → K(s) -2.93
Li+(aq) + e– → Li(s) -3.05
Potrebbero piacerti anche
- Lezione 10 - ChimicaDocumento14 pagineLezione 10 - ChimicaLuigi BellancaNessuna valutazione finora
- 2439-20100622-Soluzioni Compito 18 06 10Documento4 pagine2439-20100622-Soluzioni Compito 18 06 10Michele PassucciNessuna valutazione finora
- Potenziali Elettrochimici Comuni (Common Electrochemical Potentials)Documento2 paginePotenziali Elettrochimici Comuni (Common Electrochemical Potentials)m_i_Nessuna valutazione finora
- Table of Standard Reduction PotentialsDocumento4 pagineTable of Standard Reduction PotentialsStefan NathNessuna valutazione finora
- Tabella PotenzialiDocumento3 pagineTabella PotenzialiChina33Nessuna valutazione finora
- Tabelle Potenziali REDOX Ordine Potenziale DecrescenteDocumento11 pagineTabelle Potenziali REDOX Ordine Potenziale DecrescenteAlessandro PiovanoNessuna valutazione finora
- Standard Reduction Potentials in Aqueous Solution at 25oc MidtermDocumento2 pagineStandard Reduction Potentials in Aqueous Solution at 25oc MidtermChintana AeritNessuna valutazione finora
- 3 Module 2 Electrochimie H2011Documento17 pagine3 Module 2 Electrochimie H2011Mourad Rabah100% (1)
- Tabelle EsameDocumento1 paginaTabelle Esamedonato.cannito03Nessuna valutazione finora
- Respostas NOX PDFDocumento1 paginaRespostas NOX PDFLinn SantosNessuna valutazione finora
- Esercizi RedoxDocumento2 pagineEsercizi RedoxevaNessuna valutazione finora
- Tabela_de_Potenciais-Padro_apndice_E-13Documento1 paginaTabela_de_Potenciais-Padro_apndice_E-13Davi AlexandreNessuna valutazione finora
- Apêndice E Livro Brown (Potencial de Redução)Documento1 paginaApêndice E Livro Brown (Potencial de Redução)alvesNessuna valutazione finora
- Soluzioni Capitolo17 BradyBluDocumento5 pagineSoluzioni Capitolo17 BradyBluDavideNessuna valutazione finora
- 3b-Reazioni in Soluzione Parte 2Documento27 pagine3b-Reazioni in Soluzione Parte 2Jose Daniel100% (1)
- Esercizi - I ParteDocumento27 pagineEsercizi - I Parteapi-3706692100% (5)
- Reactii Chimia AnorganicaDocumento18 pagineReactii Chimia AnorganicaDyvonD1Nessuna valutazione finora
- Bilanciamento Forma MolecolareDocumento2 pagineBilanciamento Forma Molecolareelia dettiNessuna valutazione finora
- BILANCIAMENTODocumento2 pagineBILANCIAMENTOelia dettiNessuna valutazione finora
- Esercizi NomenclaturaDocumento5 pagineEsercizi NomenclaturaPublio AurelioNessuna valutazione finora
- Potenziali RedoxDocumento15 paginePotenziali RedoxAndreaChiarappaNessuna valutazione finora
- PileDocumento6 paginePilealbsNessuna valutazione finora
- STECHIOMETRIADocumento1 paginaSTECHIOMETRIAgenius981392Nessuna valutazione finora
- Q RedoxDocumento26 pagineQ RedoxAndrea BonfissutoNessuna valutazione finora
- CGI5Documento10 pagineCGI5Nava GambardellaNessuna valutazione finora
- Balancing Redox Reactions Worksheet - KeyDocumento3 pagineBalancing Redox Reactions Worksheet - KeySamFredricksonNessuna valutazione finora
- Soluzioni 2B INFDocumento1 paginaSoluzioni 2B INFLorenzo ConteNessuna valutazione finora
- Numero Di Ossidazione e Nomenclatura PDFDocumento6 pagineNumero Di Ossidazione e Nomenclatura PDFDanial AhmadNessuna valutazione finora
- Reactii ChimiceDocumento29 pagineReactii Chimicemarkiza07100% (1)
- Ajuste Reac Quimicas ValDocumento3 pagineAjuste Reac Quimicas ValAlbert Céspedes EsteveNessuna valutazione finora
- Bagatti Cap09 Entalpia PDFDocumento2 pagineBagatti Cap09 Entalpia PDFIlaria CalòNessuna valutazione finora
- Tabella Potenziali StandardDocumento11 pagineTabella Potenziali StandardNicola PieriNessuna valutazione finora
- 30c7 3198 File PDFDocumento12 pagine30c7 3198 File PDFVirginiaNessuna valutazione finora
- REAZIONI REDOX Extra SoluzioniDocumento2 pagineREAZIONI REDOX Extra SoluzioniBon MafNessuna valutazione finora
- Valitutti Soluzioni Esercizi 82074 c21Documento5 pagineValitutti Soluzioni Esercizi 82074 c21Lorenzo PaganNessuna valutazione finora
- Reazioni Acido BaseDocumento8 pagineReazioni Acido BaseEmiliano NaticchioniNessuna valutazione finora
- Nomenclatura e ReazioniDocumento11 pagineNomenclatura e Reazionifrancesca_b93Nessuna valutazione finora
- Lezione 16Documento33 pagineLezione 16Anonymous eGAqqjNessuna valutazione finora
- RedoxDocumento2 pagineRedoxemilianoNessuna valutazione finora
- 3 Nomenclatura 2021Documento14 pagine3 Nomenclatura 2021serenaNessuna valutazione finora
- Analisi Med IIDocumento59 pagineAnalisi Med IIrodrigue yanNessuna valutazione finora
- Stechioxfis 02Documento7 pagineStechioxfis 02andrew wallenNessuna valutazione finora
- Esercitazione 2_Fondamenti Di Chimica_2023Documento2 pagineEsercitazione 2_Fondamenti Di Chimica_2023fdales03Nessuna valutazione finora
- Chimica Inorganica 8Documento26 pagineChimica Inorganica 8doinita7Nessuna valutazione finora
- Equilibri SolubDocumento5 pagineEquilibri SolubVincenzo BiondiNessuna valutazione finora
- 04 Esercizi RedoxDocumento5 pagine04 Esercizi RedoxHadar DavidoffNessuna valutazione finora
- Esercizi RedoxDocumento5 pagineEsercizi RedoxMatilde BorselliNessuna valutazione finora
- Bilanciamento EserciziDocumento2 pagineBilanciamento EserciziRoberto FiorilloNessuna valutazione finora
- Chimica - BilanciamentiDocumento2 pagineChimica - Bilanciamentiniko08niko08Nessuna valutazione finora
- Valitutti Esploriamo Soluzioni 14Documento6 pagineValitutti Esploriamo Soluzioni 14Roberto FiorilloNessuna valutazione finora
- Lezione 11Documento25 pagineLezione 11Gean Paulo PerticaNessuna valutazione finora
- Lab Química Informe 5Documento2 pagineLab Química Informe 5Tania Sofia Torres RomeroNessuna valutazione finora
- Ciclopentano - Propeno-2Documento2 pagineCiclopentano - Propeno-2Lenin Rubén ChávezNessuna valutazione finora
- 1Documento4 pagine1tung352012Nessuna valutazione finora
- Redox 2012Documento17 pagineRedox 2012Robert RothNessuna valutazione finora
- Eje. CombustiónDocumento2 pagineEje. CombustiónDaniela DucheNessuna valutazione finora
- Esercizi - I Parte-Tad01Documento5 pagineEsercizi - I Parte-Tad01Matteo BlackRebel IllariNessuna valutazione finora