Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
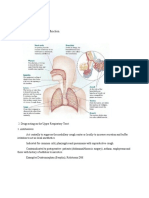
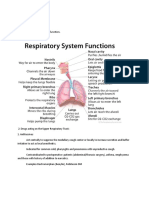
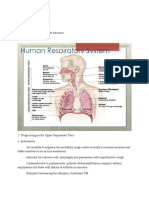
Intranasal Steroids in Pediatrics
Caricato da
Kishore Chandki0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
90 visualizzazioni39 pagineNasal Sprays: Steroids in children: Presentation for Pediatricians
Copyright
© Attribution Non-Commercial (BY-NC)
Formati disponibili
PPT, PDF, TXT o leggi online da Scribd
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoNasal Sprays: Steroids in children: Presentation for Pediatricians
Copyright:
Attribution Non-Commercial (BY-NC)
Formati disponibili
Scarica in formato PPT, PDF, TXT o leggi online su Scribd
0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
90 visualizzazioni39 pagineIntranasal Steroids in Pediatrics
Caricato da
Kishore ChandkiNasal Sprays: Steroids in children: Presentation for Pediatricians
Copyright:
Attribution Non-Commercial (BY-NC)
Formati disponibili
Scarica in formato PPT, PDF, TXT o leggi online su Scribd
Sei sulla pagina 1di 39
Histamine: pruritus, sneezing, rhinorrhea
AcetyIchoIin : stimulates glandular secretion
omparison of Various Approaches
to the Treatment of AIIergic Rhinitis
Sneezing Discharge Itch ongestion Side effects
Antihistamines
traditionaI (A) +++ +++ +++ + +++
Nonsedating
(NSA) +++ ++ +++ + - to +
AzeIastine +++ ++ +++ + - to +
Decongestants - + - +++ ++
NSA +
decongestants +++ +++ +++ +++ ++
Leukotriene antag.* + to ++ + to ++ + to ++ ++ - to +
romoIyn ++ + + + -
NasaI S (NS) +++ +++ +++ +++ +
NSA + NS ++++ ++++ ++++ ++++ +
Immunotherapy +++ +++ +++ +++ + to ++
= Presumed; no data on individual symptoms Nayak AS, et al. Ann Allergy Asthma Immunol.
2002;88:592-600. ++++ = Strongly positive effect; + = Minimal effect
Drug Iass Itch /
Sneeze
Discharge BIock Impaired
smeII
NasaI
Preparations
Antihistamines
** Azelastine,
Olopatadine
AntichoIinergics
** ** ** pratropium
Decongestants
** ** lometazoli
ne
ast eII
StabiIizers
** $odium
cromoglcate
orticosteroids
omparison of Various Approaches
to the Treatment of AIIergic Rhinitis
&nmedicated
Decongestant Antihistamine Steroids
SaIine 0.65% NaI SaIine 0.65% NaI
&se preserative &se preserative
free one free one
(BenzaIkonium I) (BenzaIkonium I)
if stinging if stinging
sensation sensation
OxymetazoIine OxymetazoIine
0.01% 0.01%
0.025% 0.025%
0.05% 0.05%
0.1% 0.1%
aution in HTN, aution in HTN,
seizures, thyroid seizures, thyroid
Ds, cardiac Ds Ds, cardiac Ds
For short For short- -term use term use
onIy onIy
1. 1.AzeIastine AzeIastine
2. 2.OIopatadine OIopatadine
As effective as oraI As effective as oraI
antihistamines, antihistamines,
doNOT use doNOT use
together together
SE: drowsiness, SE: drowsiness,
bitter taste in the bitter taste in the
mouth and mouth and
headache headache
W W FIuticasone FIuticasone
W W ometasone ometasone
W W Budesonide Budesonide
W W BecIomethason BecIomethason
W W FIunisoIide FIunisoIide
W W TriamcinoIone TriamcinoIone
Do not offer Do not offer
immediate reIief of immediate reIief of
sinus congestion sinus congestion
ast eII Stab
romoIyn romoIyn
sodium sodium
OnIy effective if OnIy effective if
used on a used on a
reguIar, reguIar,
consistent consistent
basis up to four basis up to four
times a day times a day
Effects not Effects not
apparent for 2 apparent for 2- -4 4
wks wks
ay be ay be
combined with combined with
others others
AntichoIinergic
Ipratropium Ipratropium
bromide bromide
In 1960, the first nasaI steroid spray, In 1960, the first nasaI steroid spray, Decadron Turbinaire, Turbinaire,
was introduced in the &nited States. AIthough very was introduced in the &nited States. AIthough very
effective, too much of the drug was absorbed into the effective, too much of the drug was absorbed into the
bIoodstream, which resuIted in side effects and Iimited its bIoodstream, which resuIted in side effects and Iimited its
use use
A different medication, A different medication, BecIomethasone, was initiaIIy , was initiaIIy
marketed in the 1970s & has been weII toIerated marketed in the 1970s & has been weII toIerated
1
st
Gen: becIomethasone, fIunisoIide, triamcinoIone : becIomethasone, fIunisoIide, triamcinoIone
2
nd
Gen: budesonide, fIuticasone, mometasone (faster : budesonide, fIuticasone, mometasone (faster
acting & more potent than the other nasaI steroids, with no acting & more potent than the other nasaI steroids, with no
difference in side effects) difference in side effects)
TraditionaIIy been reserved for patients with severe TraditionaIIy been reserved for patients with severe
aIIergic symptoms not controIIed by antihistamines aIone aIIergic symptoms not controIIed by antihistamines aIone
RecentIy, the use of these agents as a RecentIy, the use of these agents as a first-Iine therapy
has become more common, especiaIIy after the reIease of has become more common, especiaIIy after the reIease of
newer formuIations requiring onIy once or twice daiIy newer formuIations requiring onIy once or twice daiIy
dosing dosing
In a recent consensus paper, the American oIIege of In a recent consensus paper, the American oIIege of
AIIergy, Asthma, and ImmunoIogy Iisted intranasaI steroids AIIergy, Asthma, and ImmunoIogy Iisted intranasaI steroids
as the as the most effective therapy in controIIing the symptoms
of aIIergic rhinitis of aIIergic rhinitis
1 Allergy Clin Immunol 2002 Mar; 109:426 1 Allergy Clin Immunol 2002 Mar; 109:426- -32 32
ork through a variety of mechanisms ork through a variety of mechanisms
It is beIieved that the benefits of these agents are IargeIy It is beIieved that the benefits of these agents are IargeIy
due to the inhibition of proinfIammatory secretions, such as due to the inhibition of proinfIammatory secretions, such as
interIeukins, tumor necrosis factor interIeukins, tumor necrosis factor- -aIpha, histamine, aIpha, histamine,
tryptase, and Ieukotrienes, as weII as a reduction in the tryptase, and Ieukotrienes, as weII as a reduction in the
numbers or apoptosis of infIammatory ceIIs in the nasaI numbers or apoptosis of infIammatory ceIIs in the nasaI
epitheIium epitheIium
The finaI resuIt of these actions is a reduction in T The finaI resuIt of these actions is a reduction in T
Iymphocytes, eosinophiIs, basophiIs, monocytes, and mast Iymphocytes, eosinophiIs, basophiIs, monocytes, and mast
ceIIs within the upper airway, producing a decrease in nasaI ceIIs within the upper airway, producing a decrease in nasaI
congestion, rhinorrhea, sneezing, and itching congestion, rhinorrhea, sneezing, and itching
reduction of
symptoms and exacerbations
reduction of
mucosaI infIammation
reduction of
Iate phase reactions
priming
nasaI hyperresponsiveness
1
reduction of
mucosaI mast ceIIs
reduction of
acute aIIergic reactions
2
W suppression of
gIanduIar activity
and vascuIar Ieakage
W induction of
vasoconstriction
3
Potency represents the reIative binding affinity of the Potency represents the reIative binding affinity of the
drug for gIucocorticoid receptors, as determined by the drug for gIucocorticoid receptors, as determined by the
reciprocaI of the reIative amount of drug needed to repIace reciprocaI of the reIative amount of drug needed to repIace
50% of bound dexamethasone (the positive controI) 50% of bound dexamethasone (the positive controI)
Anti Anti- -infIammatory activity aIso assessed by the degree of infIammatory activity aIso assessed by the degree of
cytokine inhibition. In vitro studies of histamine reIease cytokine inhibition. In vitro studies of histamine reIease
show fIuticasone > mometasone > budesonide > show fIuticasone > mometasone > budesonide >
becIomethasone = triamcinoIone becIomethasone = triamcinoIone
ometasone & fIuticasone have aIso shown the greatest ometasone & fIuticasone have aIso shown the greatest
inhibition of interIeukins (IL inhibition of interIeukins (IL- -4 and IL 4 and IL- -5) in T ceII sampIes 5) in T ceII sampIes
taken from heaIthy donors taken from heaIthy donors
Budesonide, becIomethasone, & triamcinoIone aIso Budesonide, becIomethasone, & triamcinoIone aIso
inhibit IL inhibit IL- -4 and IL 4 and IL- -5, but require higher concentrations of 5, but require higher concentrations of
drug to do so drug to do so
Efficacy aIso dependent on the degree of IipophiIicity Efficacy aIso dependent on the degree of IipophiIicity
The more highIy IipophiIic the agent, the higher and faster The more highIy IipophiIic the agent, the higher and faster
the rate of uptake by the nasaI mucosa. This resuIts in an the rate of uptake by the nasaI mucosa. This resuIts in an
increased penetration of gIucocorticoid receptors and a increased penetration of gIucocorticoid receptors and a
proIonged effect proIonged effect
The intranasaI steroids can be ranked from highest degree The intranasaI steroids can be ranked from highest degree
of IipophiIicity to Iowest as foIIows (therefore poor systemic of IipophiIicity to Iowest as foIIows (therefore poor systemic
absorption): mometasone > fIuticasone > becIomethasone > absorption): mometasone > fIuticasone > becIomethasone >
budesonide > triamcinoIone > fIunisoIide budesonide > triamcinoIone > fIunisoIide
ost intranasaI steroid products work within the first ost intranasaI steroid products work within the first
severaI days of use, with some producing symptomatic severaI days of use, with some producing symptomatic
reIief in as few as 12 hours after the first dose reIief in as few as 12 hours after the first dose
There is typicaIIy a 3 to 7 day period before fuII benefit is There is typicaIIy a 3 to 7 day period before fuII benefit is
seen seen
The use of the intranasaI route for The use of the intranasaI route for
drug administration significantIy drug administration significantIy
Iessens the risk for systemic adverse Iessens the risk for systemic adverse
effects effects
FIuticasone & mometasone have a FIuticasone & mometasone have a
higher degree of IipophiIicity, higher degree of IipophiIicity,
therefore producing a faster rate of therefore producing a faster rate of
absorption, Ionger retention time in absorption, Ionger retention time in
the nasaI tissues, and minimaI the nasaI tissues, and minimaI
absorption in the gastrointestinaI absorption in the gastrointestinaI
tract. The systemic bioavaiIabiIity of tract. The systemic bioavaiIabiIity of
these two drugs is minimaI, which these two drugs is minimaI, which
reduces systemic adverse effects reduces systemic adverse effects
0
5
10
15
20
25
Mometa Flutic Budes Beclo Flunisol
Bioavail
AII currentIy avaiIabIe intranasaI steroids are effective in AII currentIy avaiIabIe intranasaI steroids are effective in
controIIing the symptoms of aIIergic rhinitis controIIing the symptoms of aIIergic rhinitis
In two recent meta In two recent meta- -anaIyses, intranasaI steroids were anaIyses, intranasaI steroids were
found to be equaI to or better than oraI antihistamines in found to be equaI to or better than oraI antihistamines in
reducing symptoms of congestion, rhinorrhea, sneezing, reducing symptoms of congestion, rhinorrhea, sneezing,
and ocuIar itching and ocuIar itching
Despite their differences in potency, pharmacokinetics, Despite their differences in potency, pharmacokinetics,
and pharmacodynamics, studies comparing intranasaI and pharmacodynamics, studies comparing intranasaI
steroids have faiIed to demonstrate any cIinicaIIy significant steroids have faiIed to demonstrate any cIinicaIIy significant
differences among the intranasaI steroids currentIy in use differences among the intranasaI steroids currentIy in use
GeneraIIy weII toIerated GeneraIIy weII toIerated
ost frequent AE (5 ost frequent AE (5- -10%): nasaI irritation, stuffiness, dry 10%): nasaI irritation, stuffiness, dry
nose & mouth, minor nasaI bIeeding, sneezing, throat nose & mouth, minor nasaI bIeeding, sneezing, throat
discomfort, nausea, discomfort, nausea, Headache, & dizziness. ore frequent , & dizziness. ore frequent
with the oIder agents, but severaI of those products have with the oIder agents, but severaI of those products have
been reformuIated as aqueous (AQ) preparations to reduce been reformuIated as aqueous (AQ) preparations to reduce
adverse effects adverse effects
AIthough rare, chiIdren on Iong AIthough rare, chiIdren on Iong- -term therapy shouId be term therapy shouId be
monitored for irritation of monitored for irritation of nasaI septum uIceration or uIceration or
perforation. LocaIized andida infections of the nose and perforation. LocaIized andida infections of the nose and
pharynx can occur, but are infrequent. Hypersensitivity pharynx can occur, but are infrequent. Hypersensitivity
reactions, with faciaI edema, rash, pruritus, and anaphyIaxis reactions, with faciaI edema, rash, pruritus, and anaphyIaxis
or anaphyIactoid reactions have occurred with these or anaphyIactoid reactions have occurred with these
agents, but appear to be rare agents, but appear to be rare
Because of the Iimited systemic avaiIabiIity with the Because of the Iimited systemic avaiIabiIity with the
newer agents, the risk of newer agents, the risk of adrenaI suppression is minimaI
Excessive doses of the oIder agents, such as Excessive doses of the oIder agents, such as
becIomethasone, administered over a proIonged period becIomethasone, administered over a proIonged period
couId potentiaIIy Iead to suppression of hypothaIamic couId potentiaIIy Iead to suppression of hypothaIamic- -
pituitary pituitary- -adrenaI (HPA) axis function or signs of adrenaI (HPA) axis function or signs of
hypercorticism, incIuding cushingoid features, arthraIgia, hypercorticism, incIuding cushingoid features, arthraIgia,
and myaIgia and myaIgia
Reviews of cIinicaI studies expIored the effects of Reviews of cIinicaI studies expIored the effects of
intranasaI steroids on the basaI index function (by intranasaI steroids on the basaI index function (by
measuring cortisoI IeveIs) & generaIIy showed no measuring cortisoI IeveIs) & generaIIy showed no
significant effects of becIomethasone dipropionate 200 significant effects of becIomethasone dipropionate 200- -800 800
g/day, triamcinoIone acetonide 220 g/day, fIuticasone g/day, triamcinoIone acetonide 220 g/day, fIuticasone
propionate 200 g/day, & mometasone furoate 200 g/day. propionate 200 g/day, & mometasone furoate 200 g/day.
Data suggest that the drugs have IittIe or no effect on the Data suggest that the drugs have IittIe or no effect on the
HPA axis when administered at recommended dosages HPA axis when administered at recommended dosages
It has been suggested that It has been suggested that mometasone or fluticasone
may be preferred in chiIdren requiring chronic therapy. Both may be preferred in chiIdren requiring chronic therapy. Both
have been shown to have minimaI effect on the HPA axis in have been shown to have minimaI effect on the HPA axis in
chiIdren during cIinicaI triaIs, even at high doses or after chiIdren during cIinicaI triaIs, even at high doses or after
proIonged periods of reguIar use proIonged periods of reguIar use
One of the greatest concerns for most famiIies is the One of the greatest concerns for most famiIies is the
effect of Iong effect of Iong- -term steroid use on growth term steroid use on growth
In 1998, the Food and Drug Administration mandated that In 1998, the Food and Drug Administration mandated that
aII inhaIed and intranasaI steroid products carry a warning aII inhaIed and intranasaI steroid products carry a warning
regarding the risk of growth suppression. This warning was regarding the risk of growth suppression. This warning was
based, in part, on data from a year based, in part, on data from a year- -Iong triaI of intranasaI Iong triaI of intranasaI
becIomethasone becIomethasone
A smaII, but statisticaIIy significant reduction in growth A smaII, but statisticaIIy significant reduction in growth
veIocity was reported in a 12 veIocity was reported in a 12- -month study of young chiIdren month study of young chiIdren
(aged 6 (aged 6- -9 yrs) treated for perenniaI aIIergic rhinitis with 9 yrs) treated for perenniaI aIIergic rhinitis with
becIomethasone dipropionate 336 g/day. The treated group becIomethasone dipropionate 336 g/day. The treated group
had sIower growth rates of 0.013 cm/day or 5 cm/year had sIower growth rates of 0.013 cm/day or 5 cm/year
compared with a group receiving pIacebo, 0.017 cm/day or compared with a group receiving pIacebo, 0.017 cm/day or
5.9 cm/year 5.9 cm/year
Data evaIuating the effects of nasaI inhaIed steroids on Data evaIuating the effects of nasaI inhaIed steroids on
chiIdren are Iimited chiIdren are Iimited
Questions that future investigations couId address Questions that future investigations couId address
incIude which agents significantIy affect growth, whether incIude which agents significantIy affect growth, whether
growth returns to normaI after the drug is stopped, and growth returns to normaI after the drug is stopped, and
whether there is an additive effect when concomitant oraI &
inhaIed steroids are administered for treatment of asthma steroids are administered for treatment of asthma
hiIe the recent studies are encouraging, it is important hiIe the recent studies are encouraging, it is important
to remember the Iack of Iong to remember the Iack of Iong- -term studies documenting term studies documenting
safety. &ntiI those data are avaiIabIe, it appears safety. &ntiI those data are avaiIabIe, it appears prudent to
seIect those agents with minimaI systemic avaiIabiIity to to
reduce the risk of growth impairment and assess growth at reduce the risk of growth impairment and assess growth at
reguIar intervaIs during treatment reguIar intervaIs during treatment
AIIergic rhinitis is a very common chronic iIIness affecting 10 to 40% of AIIergic rhinitis is a very common chronic iIIness affecting 10 to 40% of
chiIdren worIdwide. SeasonaI aIIergic rhinitis (hay fever) is most common chiIdren worIdwide. SeasonaI aIIergic rhinitis (hay fever) is most common
around springtime. Symptoms are mostIy sneezing, a runny nose and watery around springtime. Symptoms are mostIy sneezing, a runny nose and watery
eyes. e Iooked for triaIs that compared antihistamines (either oraI or topicaI) eyes. e Iooked for triaIs that compared antihistamines (either oraI or topicaI)
in addition to a topicaI nasaI steroid with a topicaI nasaI steroid aIone in in addition to a topicaI nasaI steroid with a topicaI nasaI steroid aIone in
chiIdren who had aIIergic rhinitis. e wanted to know whether adding chiIdren who had aIIergic rhinitis. e wanted to know whether adding
antihistamines (oraI or topicaI) in the therapy of chiIdren with aIIergic rhinitis antihistamines (oraI or topicaI) in the therapy of chiIdren with aIIergic rhinitis
who aIready use topicaI nasaI steroids wouId have additionaI benefits for them. who aIready use topicaI nasaI steroids wouId have additionaI benefits for them.
e found one triaI that had been carried out in chiIdren comparing oraI e found one triaI that had been carried out in chiIdren comparing oraI
antihistamines in addition to topicaI nasaI steroids with topicaI nasaI steroids antihistamines in addition to topicaI nasaI steroids with topicaI nasaI steroids
aIone but it did not provide sufficient data to draw any concIusions. ost of the aIone but it did not provide sufficient data to draw any concIusions. ost of the
triaIs focused onIy on aduIts or incIuded a smaII number of chiIdren. triaIs focused onIy on aduIts or incIuded a smaII number of chiIdren.
&nfortunateIy, the triaIs which incIuded chiIdren aIong with aduIts did not report &nfortunateIy, the triaIs which incIuded chiIdren aIong with aduIts did not report
whether there were any differences in the effect of treatment or adverse effects whether there were any differences in the effect of treatment or adverse effects
in chiIdren in comparison with aduIts. e are therefore unabIe to draw a in chiIdren in comparison with aduIts. e are therefore unabIe to draw a
concIusion as to whether or not this combination therapy has beneficiaI effect concIusion as to whether or not this combination therapy has beneficiaI effect
in chiIdren with aIIergic rhinitis or whether the benefits are acceptabIe in terms in chiIdren with aIIergic rhinitis or whether the benefits are acceptabIe in terms
of the adverse effects. of the adverse effects.
AdenoidaI hypertrophy is generaIIy considered a common condition AdenoidaI hypertrophy is generaIIy considered a common condition
of chiIdhood and represents one of the most frequent indications for of chiIdhood and represents one of the most frequent indications for
surgery in chiIdren. In Iess severe cases, non surgery in chiIdren. In Iess severe cases, non- -surgicaI interventions surgicaI interventions
may be considered, however few medicaI aIternatives are currentIy may be considered, however few medicaI aIternatives are currentIy
avaiIabIe. This review was conducted to assess the effectiveness of avaiIabIe. This review was conducted to assess the effectiveness of
intranasaI corticosteroids for improving nasaI airway obstruction in intranasaI corticosteroids for improving nasaI airway obstruction in
chiIdren aged 0 to 12 years with moderate to severe adenoidaI chiIdren aged 0 to 12 years with moderate to severe adenoidaI
hypertrophy hypertrophy
Evidence derived from five of the six randomised controIIed triaIs Evidence derived from five of the six randomised controIIed triaIs
incIuded in this review suggests that intranasaI steroids may incIuded in this review suggests that intranasaI steroids may
significantIy improve symptoms of nasaI obstruction in chiIdren with significantIy improve symptoms of nasaI obstruction in chiIdren with
adenoidaI hypertrophy and that this improvement may be associated adenoidaI hypertrophy and that this improvement may be associated
with the reduction of adenoid size. One study did not find a significant with the reduction of adenoid size. One study did not find a significant
improvement in nasaI obstruction symptoms. Further Iarge and high improvement in nasaI obstruction symptoms. Further Iarge and high- -
quaIity randomised controIIed triaIs are warranted. quaIity randomised controIIed triaIs are warranted.
TopicaI nasaI corticosteroids
are mainstay of treatment for
ethmoidaI poIyposis
NasaI drops are preferabIe for
nasaI poIyposis and probabIy
aIso for chronic rhinosinusitis.
These are used in the 'head
upside down position' in order
to reach the osteomeataI
compIex at which the sinuses
drain.
Management of Intermittent AR
Avoid Allergens
Mild ntermittent AR Moderate-Severe ntermittent AR
Nasal H
1
blocker / $pra
Oral H
1
blocker
Decongestants
LTRA
Nasal H
1
blocker / Spray
Oral H
1
blocker
Decongestants/LTRA/Chromone
FLUTICASONE - 2 sprays/nostril OD
LTRA Leukotriene Receptor Antagonists
AIIergic Rhinitis & its Impact on Asthma (ARIA) GuideIines
AIIergic Rhinitis & its Impact on Asthma (ARIA) GuideIines
anagement oI !ersistent AR
Avoid Allergens
Nasal H
1
blocker
Oral H
1
blocker / LTRA
Decongestants / Chromone
Intranasal CS / OTA$ON/
/FLUTICASONE
Review patients aIter 2-4 weeks
Step up iI no improvement Continue: 1 month iI improvement
Agent TN Age Bioa
vaiIa
biIity
Doses
BecIomethasone
Avoid in
chiIdren!
6 17
6-12 years: 1 spray (42 mcg) in each nostril
twice daily Adults: 1-2 sprays in each nostril
twice daily
FIunisoIide 6 20-50
6-14 years: 1 spray (25 mcg) in each nostril
three times daily Adults: 1 spray in each
nostril three times daily or 2 sprays in each
nostril twice daily
TriamcinoIone
ortispray
(Fourrts)
2 22
2-12 years: 1 spray (55 mcg) in each nostril
once daily Adults: 2 sprays in each nostril
once daily
Budesonide 6 11
6 years & Adults: 2 sprays (32 mcg/spray)
in each nostril twice daily or 4 sprays in each
nostril once daily
FIuticasone
FIohaIe,
FIixonase
>4 <2
4-12 years: 1 spray (50 mcg) in each nostril
once daily Adults: 2 sprays in each nostril
once daily
ometasone
Nasonex,
omate
>3 <0.1
3-12 years: 1 spray (50 mcg) in each nostril
once daily Adults: 2 sprays in each nostril
once daily
Agent TN Age Bioa
vaiIa
biIity
Doses
Ipratropium >5 >5
%wo strengths (0.03% and 0.06%): only
effective for runny noses& can literally "turn
off the faucet." two sprays 3 to 4 times per
day in each nostril
romoIyn
Sodium
O%
1-2 sprays in each nostril 4 times per day
more effective in younger people with high
gE
OIopatadine
# + Mast Cell
Stabilizer)
OIamyst
(Fourrts)
>12 >12
?>6 6
1 dose = 600 3g, 2 sprays twice a day each
nostril
AzeIastine,
H1RA
>12 >12
SAR: 1 or 2 sprays per nostril twice daily.
%he 0.15% formulation may also be
administered as 2 sprays per nostril once
daily. PAR: 0.15% formulation- 2 sprays per
nostril twice daily.
icIesonide,
Steroid
>12 >12
Take antihistamines for sneezing, runny nose, itchy nose Take antihistamines for sneezing, runny nose, itchy nose
and throat and throat
Take decongestants for nasaI congestion onIy Take decongestants for nasaI congestion onIy
AntichoIinergic medicine such as Ipratropium Bromide AntichoIinergic medicine such as Ipratropium Bromide
may heIp with intractabIe runny noses may heIp with intractabIe runny noses
NasaI steroids are safe and effective on a runny, itchy, NasaI steroids are safe and effective on a runny, itchy,
and particuIarIy stuffy nose and particuIarIy stuffy nose
ombination of antihistamine, decongestant, and steroid ombination of antihistamine, decongestant, and steroid
inhaIers are a good choice for moderate or severe hay inhaIers are a good choice for moderate or severe hay
fever fever
TopicaI nasaI decongestant shouId be Iimited to use for TopicaI nasaI decongestant shouId be Iimited to use for
3 to 5 days maximum 3 to 5 days maximum
ost potent anti ost potent anti- -infIammatory agents for AR infIammatory agents for AR
IntranasaI: acts IocaIIy IntranasaI: acts IocaIIy
GoaI: To controI symptoms with Iowest possibIe dose GoaI: To controI symptoms with Iowest possibIe dose
>90% achieve symptomatic reIief >90% achieve symptomatic reIief
ost effective when started severaI days before exposure ost effective when started severaI days before exposure
and used on reguIar basis and used on reguIar basis
Therapeutic efficacy within 1 Therapeutic efficacy within 1- -3 days, but max efficacy 3 days, but max efficacy
may take up to 3 weeks may take up to 3 weeks
ompIiance is criticaI ompIiance is criticaI
Effective in treatment of aII nasaI symptoms incIuding Effective in treatment of aII nasaI symptoms incIuding
obstruction obstruction
First Iine pharmacotherapy for persistent aIIergic rhinitis First Iine pharmacotherapy for persistent aIIergic rhinitis
At present, no data on the recommended duration At present, no data on the recommended duration
It is usuaI practice for patients to have a triaI of one of the It is usuaI practice for patients to have a triaI of one of the
nasaI steroid sprays at the first consuItation nasaI steroid sprays at the first consuItation
The patients can be reviewed at a month towards the The patients can be reviewed at a month towards the
compIetion of the bottIe of spray compIetion of the bottIe of spray
Patients with partiaI recovery from AR shouId be Patients with partiaI recovery from AR shouId be
continued for another month continued for another month
Patients who are totaIIy symptom free couId have their Patients who are totaIIy symptom free couId have their
treatment stopped. The Iatter patients shouId be advised treatment stopped. The Iatter patients shouId be advised
that the symptoms may recur and thus repeat courses of that the symptoms may recur and thus repeat courses of
treatment can be given treatment can be given
For the patients who do not receive any benefit despite For the patients who do not receive any benefit despite
triaI of two different sprays shouId have a nasaI evaIuation triaI of two different sprays shouId have a nasaI evaIuation
& an aIIergy work & an aIIergy work- -up done up done
Singapore Med 1 2002 Vol 43(8) : 412 Singapore Med 1 2002 Vol 43(8) : 412- -414 414
ith seasonaI aIIergies, daiIy use of these sprays shouId ith seasonaI aIIergies, daiIy use of these sprays shouId
begin 1 to 2 weeks before the aIIergy season and continue begin 1 to 2 weeks before the aIIergy season and continue
throughout the season throughout the season
In year round or perenniaI aIIergic rhinitis, particuIarIy if In year round or perenniaI aIIergic rhinitis, particuIarIy if
unresponsive to treatments, daiIy use of intranasaI steroids unresponsive to treatments, daiIy use of intranasaI steroids
has been found very effective in controIIing symptoms, has been found very effective in controIIing symptoms,
particuIarIy nasaI congestion particuIarIy nasaI congestion
NasaI steroids may aIso heIp improve the sense of smeII, NasaI steroids may aIso heIp improve the sense of smeII,
which is frequentIy diminished in aIIergic rhinitis which is frequentIy diminished in aIIergic rhinitis
IntranasaI steroids are usefuI in chiIdren with IntranasaI steroids are usefuI in chiIdren with
aIIergic aIIergic- - seasonaI & perenniaI rhinitis seasonaI & perenniaI rhinitis
Effective in treating both earIy Effective in treating both earIy- - & Iate & Iate- -phase phase
responses responses
Good controI of aII nasaI symptoms with usuaIIy Good controI of aII nasaI symptoms with usuaIIy
once daiIy administration once daiIy administration
AII agents are safe & effective. The usage in chiIdren AII agents are safe & effective. The usage in chiIdren
starts from age three (ometasone) and two starts from age three (ometasone) and two
(TriamcinoIone) (TriamcinoIone)
Thank you for staying awake!
or, you may now wake up &
ask questions!
Potrebbero piacerti anche
- Nsaids / Orthodontic Courses by Indian Dental AcademyDocumento22 pagineNsaids / Orthodontic Courses by Indian Dental Academyindian dental academyNessuna valutazione finora
- Chemistry in Everyday LifeDocumento71 pagineChemistry in Everyday LifePratima SinghNessuna valutazione finora
- ACUTE POISONING TREATMENTDocumento28 pagineACUTE POISONING TREATMENTMirna auliaNessuna valutazione finora
- Respiratory DrugsDocumento19 pagineRespiratory DrugsBryan MedranoNessuna valutazione finora
- Parmacology Respirasi: DR - Saiful BatubaraDocumento24 pagineParmacology Respirasi: DR - Saiful BatubaraEryanda DinataNessuna valutazione finora
- Antihistamine BlockersDocumento12 pagineAntihistamine Blockersyra capiliNessuna valutazione finora
- Chapter 16 - Preoperative MedicationDocumento4 pagineChapter 16 - Preoperative MedicationFanny Ari SandyNessuna valutazione finora
- Allergic RhinitisDocumento55 pagineAllergic RhinitisZZNessuna valutazione finora
- BeclomethasoneDocumento2 pagineBeclomethasoneDiane Bonita HerreraNessuna valutazione finora
- B233 Exam 5test Mapfall2010Documento5 pagineB233 Exam 5test Mapfall2010Katie L HollingsworthNessuna valutazione finora
- Allergic RhinitisDocumento53 pagineAllergic RhinitisYousfNessuna valutazione finora
- Journal of International Medical Research 1986 Busson 53 62Documento10 pagineJournal of International Medical Research 1986 Busson 53 62Eureka HimitsuNessuna valutazione finora
- Introduction to AntibioticsDocumento2 pagineIntroduction to AntibioticsMysael Acosta FloresNessuna valutazione finora
- Respiratory Drugs GuideDocumento56 pagineRespiratory Drugs GuideIra G. Delos Santos100% (1)
- Anticholinergic: Anticholinergics: Generic and Brand NamesDocumento6 pagineAnticholinergic: Anticholinergics: Generic and Brand NamesSaffery Gly LayuganNessuna valutazione finora
- Ïy Ïy Ïy ÏyDocumento4 pagineÏy Ïy Ïy ÏyAriel P. VasquezNessuna valutazione finora
- AnaphylaxisDocumento39 pagineAnaphylaxisLydia Novalista100% (1)
- Pharm Exam Ii NotesDocumento24 paginePharm Exam Ii Noteskatiana louisNessuna valutazione finora
- Unit-15 Chemistry in Everyday Life 2023Documento16 pagineUnit-15 Chemistry in Everyday Life 2023jagannathanNessuna valutazione finora
- Pharmacology Chapter 42 p-1Documento14 paginePharmacology Chapter 42 p-1sho bartNessuna valutazione finora
- AcetylcysteineDocumento2 pagineAcetylcysteineGwyn RosalesNessuna valutazione finora
- Drug Study DrugDocumento5 pagineDrug Study Drugjl frusaNessuna valutazione finora
- Gastrointestinal System .Documento6 pagineGastrointestinal System .Jayward BucayuNessuna valutazione finora
- Anaphylaxis: By: O. Ahmadi, MD. Professor Assistant of Esfahan Medical School, Emergency Department of Al-Zahra HospitalDocumento39 pagineAnaphylaxis: By: O. Ahmadi, MD. Professor Assistant of Esfahan Medical School, Emergency Department of Al-Zahra HospitalBudi SetyanugrahaNessuna valutazione finora
- Modern Medicine Analgesics: AspirinDocumento5 pagineModern Medicine Analgesics: Aspirinmuhamad_ariff_3Nessuna valutazione finora
- Drugs On RespiratoryDocumento17 pagineDrugs On RespiratoryIrwan M. IskoberNessuna valutazione finora
- Pharmacology Respiratory DrugsDocumento51 paginePharmacology Respiratory DrugsAngel DamoNessuna valutazione finora
- Camille I BSM 3 Phama Midterm ActivityDocumento8 pagineCamille I BSM 3 Phama Midterm ActivityJanine VegaNessuna valutazione finora
- Drug Administration 2011Documento98 pagineDrug Administration 2011bagir_dm10Nessuna valutazione finora
- Respiratory Drugs GuideDocumento56 pagineRespiratory Drugs GuideShang MacarayonNessuna valutazione finora
- Case Study No.4 The Telltale Heart: Group 2 Nuñez, Refuerzo, Abalos, Almonte, AlmueteDocumento11 pagineCase Study No.4 The Telltale Heart: Group 2 Nuñez, Refuerzo, Abalos, Almonte, AlmueteRejeanne MonroyNessuna valutazione finora
- Drugs For AsthmaDocumento1 paginaDrugs For Asthmakamil malikNessuna valutazione finora
- Periodontal Treatment in Medically CompromisedDocumento14 paginePeriodontal Treatment in Medically CompromisedAbdallah Essam Al-ZireeniNessuna valutazione finora
- Janine Vega BSM 3 Phama Midterm ActivityDocumento8 pagineJanine Vega BSM 3 Phama Midterm ActivityJanine VegaNessuna valutazione finora
- Anti-Tubercular Drugs: CLASSIFICATION: On Basis of Clinical ApplicationDocumento21 pagineAnti-Tubercular Drugs: CLASSIFICATION: On Basis of Clinical ApplicationSimran SinghNessuna valutazione finora
- ArthritisDocumento69 pagineArthritisKavya sriNessuna valutazione finora
- Jelie Rose BSM 3 Phama Midterm ActivityDocumento8 pagineJelie Rose BSM 3 Phama Midterm ActivityJanine VegaNessuna valutazione finora
- Gastrointestinal DrugsDocumento49 pagineGastrointestinal DrugsMae Antonette OrlinaNessuna valutazione finora
- Persistent Asthma - Prof DR SidhartaniDocumento18 paginePersistent Asthma - Prof DR SidhartaniRuki HartawanNessuna valutazione finora
- Pharmacology 2 14.05.2021Documento3 paginePharmacology 2 14.05.2021VIJAY BHILWADENessuna valutazione finora
- Respiratory SystemDocumento28 pagineRespiratory SystemRaghda NimerNessuna valutazione finora
- Medicine (Pharmaceutical Chemistry)Documento33 pagineMedicine (Pharmaceutical Chemistry)Crizaldo MempinNessuna valutazione finora
- БронхиальнаяDocumento51 pagineБронхиальнаяDaniel FunkNessuna valutazione finora
- ER Intubation GuideDocumento79 pagineER Intubation Guidezulham effendyNessuna valutazione finora
- Chemistry Project MedicineDocumento25 pagineChemistry Project MedicineMridul SomaniNessuna valutazione finora
- Versus: By: Kathleen Desouza & Rennette GarciaDocumento14 pagineVersus: By: Kathleen Desouza & Rennette GarciakatNessuna valutazione finora
- Antiinflammatory Drugs: Toya AriawanDocumento27 pagineAntiinflammatory Drugs: Toya Ariawanlast100% (1)
- Ranitidine and Omeprazole Nursing CareDocumento3 pagineRanitidine and Omeprazole Nursing CareJose Jr A PerunaNessuna valutazione finora
- Drugsbank: Drugs Used in PediatricsDocumento6 pagineDrugsbank: Drugs Used in PediatricsNur Ekayani SyamNessuna valutazione finora
- Premedication and Other Prophylactic MeasuresDocumento39 paginePremedication and Other Prophylactic Measuresanugerah_buang3842Nessuna valutazione finora
- NCM 112 Study Guide MidtermDocumento42 pagineNCM 112 Study Guide MidtermMark Nathaniel ValerioNessuna valutazione finora
- Invitro Dissolution and Assay of "Ibuprofen"Tablet.Documento59 pagineInvitro Dissolution and Assay of "Ibuprofen"Tablet.Md.Moniruzzaman100% (3)
- Group 2 Activity 8 Instrumentation 4 Phchem LabDocumento3 pagineGroup 2 Activity 8 Instrumentation 4 Phchem LabJeyma DacumosNessuna valutazione finora
- Role of Drugs in Orthodontics / Orthodontic Courses by Indian Dental AcademyDocumento239 pagineRole of Drugs in Orthodontics / Orthodontic Courses by Indian Dental Academyindian dental academyNessuna valutazione finora
- Interaksi Obat 1Documento54 pagineInteraksi Obat 1Rada DoloksaribuNessuna valutazione finora
- NAUSEA AND VOMITING GUIDEDocumento8 pagineNAUSEA AND VOMITING GUIDESri Ayu NingsihNessuna valutazione finora
- What You Should Know: D.1 Pharmaceutical Products and Drug Action Drug DevelopmentDocumento53 pagineWhat You Should Know: D.1 Pharmaceutical Products and Drug Action Drug DevelopmentRania Samir Shaaban MohamedNessuna valutazione finora
- Basic Pharmacology And Drug Calculations [Practice Questions And Answers]Da EverandBasic Pharmacology And Drug Calculations [Practice Questions And Answers]Valutazione: 4 su 5 stelle4/5 (1)
- RabiesDocumento35 pagineRabiesKishore ChandkiNessuna valutazione finora
- Azithromycin in PediatricsDocumento40 pagineAzithromycin in PediatricsKishore ChandkiNessuna valutazione finora
- AntihistaminesDocumento83 pagineAntihistaminesKishore ChandkiNessuna valutazione finora
- Albendazole in PediatricsDocumento38 pagineAlbendazole in PediatricsKishore ChandkiNessuna valutazione finora
- Immunization Review GPDocumento46 pagineImmunization Review GPKishore ChandkiNessuna valutazione finora
- Head Circumference of Indian ChildrenDocumento1 paginaHead Circumference of Indian ChildrenKishore ChandkiNessuna valutazione finora
- Pneumococcal VaccinesDocumento38 paginePneumococcal VaccinesKishore Chandki100% (1)
- Inhalers in Pediatric AsthmaDocumento53 pagineInhalers in Pediatric AsthmaKishore Chandki100% (2)
- Enteral Feeding in LBW NeonatesDocumento93 pagineEnteral Feeding in LBW NeonatesKishore ChandkiNessuna valutazione finora
- Length of Indian ChildrenDocumento2 pagineLength of Indian ChildrenKishore ChandkiNessuna valutazione finora
- Some Algorithms in PediatricsDocumento15 pagineSome Algorithms in PediatricsKishore ChandkiNessuna valutazione finora
- Interesting Case of EncephalopathyDocumento18 pagineInteresting Case of EncephalopathyKishore ChandkiNessuna valutazione finora
- TOFDocumento6 pagineTOFKishore ChandkiNessuna valutazione finora
- Newer Insulins in PediatricsDocumento24 pagineNewer Insulins in PediatricsKishore ChandkiNessuna valutazione finora
- Acute Diarrhea in ChildrenDocumento53 pagineAcute Diarrhea in ChildrenKishore ChandkiNessuna valutazione finora
- Enteral Feeding LBWDocumento93 pagineEnteral Feeding LBWKishore ChandkiNessuna valutazione finora
- Chicken Pox StudentsDocumento19 pagineChicken Pox StudentsKishore ChandkiNessuna valutazione finora
- Ger GPDocumento72 pagineGer GPKishore ChandkiNessuna valutazione finora
- Oral IronDocumento72 pagineOral IronKishore ChandkiNessuna valutazione finora
- Autologous Transfusion Strategies to Reduce Allogeneic Blood UseDocumento31 pagineAutologous Transfusion Strategies to Reduce Allogeneic Blood Usethalida24100% (1)
- Safety Data Sheet: Product Name: MOBILGEAR 600 XP 320Documento11 pagineSafety Data Sheet: Product Name: MOBILGEAR 600 XP 320RupeshBabu SankariyaNessuna valutazione finora
- Records of Patient Medical CheckupsDocumento237 pagineRecords of Patient Medical CheckupsPanca WirawanNessuna valutazione finora
- Annexure 'CD - 01' FORMAT FOR COURSE CURRICULUMDocumento4 pagineAnnexure 'CD - 01' FORMAT FOR COURSE CURRICULUMYash TiwariNessuna valutazione finora
- Exercise and Physical Activity For Older - VanBeveren 2012 PDFDocumento22 pagineExercise and Physical Activity For Older - VanBeveren 2012 PDFJuani CantellanosNessuna valutazione finora
- National Health Trends and Traditional Medicine ActsDocumento32 pagineNational Health Trends and Traditional Medicine ActsArleneNessuna valutazione finora
- Rheumatoid Arthritis: Pathophysiology PathophysiologyDocumento34 pagineRheumatoid Arthritis: Pathophysiology PathophysiologyOmair RiazNessuna valutazione finora
- Gval ResumeDocumento1 paginaGval Resumeapi-403123903Nessuna valutazione finora
- Aloe Vera-A Wonder Plant Its History, Cultivation and Medicinal Uses PDFDocumento4 pagineAloe Vera-A Wonder Plant Its History, Cultivation and Medicinal Uses PDFveronyk28Nessuna valutazione finora
- Machine Learning Predicts 5-Chloro-1 - (2 - Phenylethyl) - 1h-Indole-2,3-Dione As A Drug Target For Fructose Bisphosphate Aldolase in Plasmodium FalciparumDocumento7 pagineMachine Learning Predicts 5-Chloro-1 - (2 - Phenylethyl) - 1h-Indole-2,3-Dione As A Drug Target For Fructose Bisphosphate Aldolase in Plasmodium FalciparumInternational Journal of Innovative Science and Research TechnologyNessuna valutazione finora
- Obama Mind ControlDocumento68 pagineObama Mind Controlkdnkgljfg67% (3)
- Bu SuryaniDocumento68 pagineBu SuryaniMaulana SaputraNessuna valutazione finora
- The 38 Bach RemediesDocumento20 pagineThe 38 Bach RemediesSriram Bharat100% (1)
- ColostomyDocumento5 pagineColostomyZhyraine Iraj D. Caluza100% (1)
- Obat SpesialisDocumento16 pagineObat SpesialisLAILATUL AFIYAHNessuna valutazione finora
- TrichDocumento30 pagineTrichwadige4668Nessuna valutazione finora
- Elderly Care IndiaDocumento3 pagineElderly Care IndiakasurvarNessuna valutazione finora
- CPV/CCV Ag (3 Lines) : VcheckDocumento2 pagineCPV/CCV Ag (3 Lines) : VcheckFoamfab WattikaNessuna valutazione finora
- 1 - General Indications and Contraindications - 2019 - Lumbar Interbody FusionsDocumento12 pagine1 - General Indications and Contraindications - 2019 - Lumbar Interbody FusionsSergiu MalinNessuna valutazione finora
- 16-Week Marathon Training Program with Weekly SessionsDocumento4 pagine16-Week Marathon Training Program with Weekly SessionsMaria Del Carmen García FermínNessuna valutazione finora
- Supine Cervical Traction After Anterior Cervical Diskectomy and FusionDocumento4 pagineSupine Cervical Traction After Anterior Cervical Diskectomy and FusionOscar NgNessuna valutazione finora
- Standard Case Report Checklist and Template For AuthorsDocumento5 pagineStandard Case Report Checklist and Template For AuthorsArief MunandharNessuna valutazione finora
- Aspirin: The widely used pain reliever and fever reducerDocumento4 pagineAspirin: The widely used pain reliever and fever reducerEithel EithelNessuna valutazione finora
- Fetal SkullDocumento34 pagineFetal SkullNeelofur Ibran Ali85% (20)
- 05 - SPSF3 04 B2 PDFDocumento20 pagine05 - SPSF3 04 B2 PDFCikgu RoshailaNessuna valutazione finora
- 6th Grade (Level F) Spelling ListsDocumento36 pagine6th Grade (Level F) Spelling ListsArmaan100% (1)
- Compartment SyndromeDocumento29 pagineCompartment SyndromeFazmial UjirNessuna valutazione finora
- 45 PDFDocumento8 pagine45 PDFChika FebrianiNessuna valutazione finora
- EMS Drug DilutionDocumento21 pagineEMS Drug Dilutionthompson godfreyNessuna valutazione finora
- The Art of Healthy Eating KidsDocumento122 pagineThe Art of Healthy Eating KidsSenka SkenderovicNessuna valutazione finora























































![Basic Pharmacology And Drug Calculations [Practice Questions And Answers]](https://imgv2-2-f.scribdassets.com/img/word_document/475660044/149x198/2c7fc45015/1691161640?v=1)

















































