Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Phase Diagram
Caricato da
zainal arifin0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
51 visualizzazioni36 paginePhase diagrams provide information about the equilibrium phases that exist at different temperatures and compositions for multi-component materials. They show how microstructure and properties depend on thermal treatments.
Binary phase diagrams map the relationships between temperature, composition, and phases for alloy systems containing two components. They show single-phase and two-phase regions and can be used to determine phase fractions at different conditions. Isomorphous systems have complete solubility and a simple diagram while eutectic systems have a eutectic point where three phases meet in equilibrium.
Microstructure development depends on whether equilibrium or non-equilibrium cooling is used, with faster cooling limiting diffusion and producing nonequilibrium phases and compositions. Phase diagrams are thus important
Descrizione originale:
Titolo originale
4. Phase diagram.pptx
Copyright
© © All Rights Reserved
Formati disponibili
PPTX, PDF, TXT o leggi online da Scribd
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoPhase diagrams provide information about the equilibrium phases that exist at different temperatures and compositions for multi-component materials. They show how microstructure and properties depend on thermal treatments.
Binary phase diagrams map the relationships between temperature, composition, and phases for alloy systems containing two components. They show single-phase and two-phase regions and can be used to determine phase fractions at different conditions. Isomorphous systems have complete solubility and a simple diagram while eutectic systems have a eutectic point where three phases meet in equilibrium.
Microstructure development depends on whether equilibrium or non-equilibrium cooling is used, with faster cooling limiting diffusion and producing nonequilibrium phases and compositions. Phase diagrams are thus important
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PPTX, PDF, TXT o leggi online su Scribd
0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
51 visualizzazioni36 paginePhase Diagram
Caricato da
zainal arifinPhase diagrams provide information about the equilibrium phases that exist at different temperatures and compositions for multi-component materials. They show how microstructure and properties depend on thermal treatments.
Binary phase diagrams map the relationships between temperature, composition, and phases for alloy systems containing two components. They show single-phase and two-phase regions and can be used to determine phase fractions at different conditions. Isomorphous systems have complete solubility and a simple diagram while eutectic systems have a eutectic point where three phases meet in equilibrium.
Microstructure development depends on whether equilibrium or non-equilibrium cooling is used, with faster cooling limiting diffusion and producing nonequilibrium phases and compositions. Phase diagrams are thus important
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PPTX, PDF, TXT o leggi online su Scribd
Sei sulla pagina 1di 36
Phase Diagrams
Mohammad Rais Alfiansyah Taufiq S.T., M.Sc.
Why Study this?
• Properties of materials determined by microstructure
• Change microstructure with thermal treatment
• Most phase diagrams are stable / equilibrium state / microstructure
• Some nonequilibrium structure more desirable due to better
properties
• Strong correlation between microstructure and mechanical
properties
Definition and Basics
• Components pure metals or compounds form alloy
• Copper-Zinc brass Cu and Zn
• Solute a liquid that dissolves a solid, liquid or gaseous solute
• Solvent a substance dissolved in another substance
• System specific body of materials under consideration (ladle of
molten steel)
• Or series of possible alloys consisting of the same components
(iron-carbon system)
• Phase a homogeneous portion of a system that has uniform
physical and chemical characteristics.
What is phase?
Solubility Limit
• Most alloys systems, specific
temperature there is a
maximum concentration of solute
atoms in solvent
• Called as Solubility Limit
• Additional solute/compound
become another solid solution
having distinctly different
composition
Phase and Microstructure
• Pure metals are a phase
• Every solid, liquid and gaseous solution
• Example Sugar-water syrup, solid sugar
• There is a boundary separating the phase
• Systems s composed of two or more phases are termed “mixtures” or
“heterogeneous systems.”
• Mixtures show distinguish properties compare to single phase
• Mechanical behaviour depend on microstructure
• Number of phases, their proportions, their distribution and arrangement
The Gibbs Phase Rule
• proposed by the nineteenth-century physicist J. Willard Gibbs
• P is the number of phases present
• F is termed the number of degrees of freedom (e.g., temperature,
pressure, composition)
• C represents the number of components in the system
• N s the number of noncompositional variables (e.g., temperature
and pressure).
Phase Equilibrium
• Definition
• Equilibrium in terms of a thermodynamic quantity called the free energy
• Free energy function of internal energy, randomness of atoms or molecules
• Equilibrium if free energy minimum in specific (temp, pressure, comp)
• Stable, no change indefinitely, change of (temp, pressure, comp) higher free
energy
• In this context phase eq is condition where two phases or more exist in
a time
Metastable
• In solid systems, state of eq. never completely achieved due to
extremely slow rate of eq.
• Called as metastable or non-equilibrium
• Commonly more practical than equilibrium one
• Heat treatment in steel and aluminium
More in phase transformation
Phase Diagram
phase diagram / equilibrium diagram
the information about the control of the phase structure
Controllable parameters (temperature, pressure, and composition)
Phase diagrams constructed by these parameters
One Component / Unary Phase
Diagrams
• Phase diagram of one-component
system, in which composition is
held constant
• unary phase diagram
• pressure–temperature (or P–T)
diagram
• Example of H2O
Binary Phase Diagrams
• Temperature and Composition as variable parameters, pressure
held constant ~~ 1 atm
• Consist of 2 components
• Maps that represent the relationships between temperature and the
compositions and quantities of phases at equilibrium effecting
microstructure of alloys
Binary Isomorphous Systems
• Common example, Cu-Ni alloy
• Temperature ordinate, composition abscissa
• FCC crystal structure of Cu-Ni below 1080oC
• Cu-Ni soluble to each other, nearly identical atomic radii and
electronegativities, and similar valences
• isomorphous because of this complete liquid and solid solubility of
the two components.
• Liquid, solid solution, mixture (α+L)
• α,β,γ etc. represent solid solution of combined metals (alloys)
Determine phase weight fraction
• For α and L are straight forward, can be noted from abscissa
• But for α+L phase
Volume fraction
• For multiphase alloys, it is often more convenient to specify relative
phase amount in terms of volume fraction rather than mass fraction
Development of microstructure in
isomorphous alloys
• Equilibrium Cooling
1. Reducing temperature slowly considering equilibrium phase
2. Readjustment and redistribution
3. Diffusion
4. Long time
• Non-equilibrium Cooling
1. Fast cooling
2. Limit time for readjustment
3. Microstructure is different
Equilibrium Cooling
• Start at a, 1300C L phase 35 wt% Ni-
65 wt% Cu
• At b, 1260C α start to grow, L (35 Ni)
and α (46 Ni)
• At c, L (32 Ni) and α (43 Ni)
• At d, almost all L alter to α, α (35 Ni)
and L (24 Ni)
• After d, 35 wt% Ni- 65 wt% Cu
• After all L alter to α, there is no new
phase
Non-equilibrium Cooling
• Start at a’, 1300C L phase 35 wt% Ni-65
wt% Cu
• At b’, 1260C α start to grow, 46 wt% Ni-54
wt% Cu α(46Ni)]
• At c’, L (29 Ni) and α (40 Ni)
• Diffusion rate slowing as temperature
dropping, grain of α at c consist of 2 solid
layer α(46Ni) & α(40 Ni)
• Phase α with different Ni concentration
• Concentration represent with its average
Non-equilibrium Cooling
• Rise of phase L fraction drop of α fraction in α + L phase
• Solidus line shifting to higher Ni concentration
• At c’, solidus line show average Ni conc (α(42 Ni))
• At 1220C in non-eq cooling, solidification still on going
• Solidification will stop at e’, α(31 Ni) averaging α(35 Ni)
• The slower of cooling process, the smaller of displacement.
• Forming low-melting element at core (cored structure), can be prevented
with heat treatment homogenization atom diffusion
Mechanical properties of
isomorphous alloys
Binary eutectic systems
• Cu-Ag Alloy binary eutectic system
• three single-phase regions are found on the diagram: a, b, and liquid
• The α phase rich copper solution, silver solute, FCC structure
• The β phase rich silver solution, copper solute, FCC
• Pure copper α, pure silver β
• the solubility in each of these solid phases is limited, Below BEG line
• Eutectic system :
• three phase may be
eq at eutectic
isotherm point
• Single phase
separated from each
other by two-phase
• Another example :
lead and tin low
temperature solder
• CE (71.9 wt% Ag) and
TE 779C/1434F
quiz
• At 500°C (930°F), what is the maximum solubility
• (a) of Cu in Ag?
• (b) Of Ag in Cu?
Sn-Pb
problem
• For a 40 wt% Sn–60 wt%
Pb alloy at 150?C (300?F),
(a) What phase(s) is (are)
present?
(b) What is (are) the
composition(s) of the
phase(s)?
(c) mass fraction and
(d) volume fraction. At 150C
take the densities of Pb and
Sn to be 11.23 and 7.24
g/cm3, respectively
• Composition of α
corresponds to tie line
α/(α+β) 10 wt% Sn–90 wt%
Pb
• Composition of β
corresponds to tie line
β/(α+β) 98 wt% Sn–2 wt%
Pb
• determine the density of each phase
• Volume fraction of β = 1 - volume fraction α
= 1 – 0,57 = 0,43
Development of microstructure in
eutectic alloys
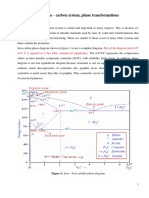
The iron–iron carbide (Fe–Fe3C)
phase diagram
Ferrite α BCC
Austenite γ FCC
Ternary Phase Diagrams
These diagrams are based on those found in Phase Diagrams for Ceramacists (Levin et al., 1964).
Potrebbero piacerti anche
- Maxwell-Boltzmann DistributionDocumento8 pagineMaxwell-Boltzmann DistributionlamyantingNessuna valutazione finora
- Chapter2 Bonding and PropertiesDocumento71 pagineChapter2 Bonding and PropertiesShahd AlhamaydaNessuna valutazione finora
- Gases: Larry Brown Tom HolmeDocumento52 pagineGases: Larry Brown Tom Holmemuhammad ali shakeelNessuna valutazione finora
- Physical Chemistry Chapter 8 LaidlerDocumento46 paginePhysical Chemistry Chapter 8 LaidlerCody Ewell0% (1)
- Naturally Occurring Uranium Consists of Two Isotopes, Uranium-235 and Uranium-238. Both Isotopes Are Radioactive. They Emit Alpha RadiationDocumento27 pagineNaturally Occurring Uranium Consists of Two Isotopes, Uranium-235 and Uranium-238. Both Isotopes Are Radioactive. They Emit Alpha RadiationRoszelan MajidNessuna valutazione finora
- Objectives: The Nature of MatterDocumento31 pagineObjectives: The Nature of MatterGene DacayoNessuna valutazione finora
- Chapter 11 CorrosionDocumento38 pagineChapter 11 CorrosionNaveenesh RajNessuna valutazione finora
- Corrosion Kinetics BasheerDocumento9 pagineCorrosion Kinetics Basheerchenabeel0% (1)
- MolecularSymmetry Raman IrDocumento37 pagineMolecularSymmetry Raman IrSare GomezNessuna valutazione finora
- wk7 (3) - Fe-C SystemDocumento12 paginewk7 (3) - Fe-C Systemsaeed khaledNessuna valutazione finora
- Ceramic Material and Their Magnetic Properties and Its ApplicationsDocumento16 pagineCeramic Material and Their Magnetic Properties and Its Applicationsarindham chakrabortyNessuna valutazione finora
- The Ideal Gas Law - Chemistry LibreTextsDocumento8 pagineThe Ideal Gas Law - Chemistry LibreTextsJovenil BacatanNessuna valutazione finora
- Binary Alloy SystemDocumento17 pagineBinary Alloy SystemAhmas SyaakirNessuna valutazione finora
- Resistance Vs Temperature Experiment Lab ReportDocumento7 pagineResistance Vs Temperature Experiment Lab ReportEmily Gatlin67% (3)
- Issues To Address... : Chapter 5-1Documento58 pagineIssues To Address... : Chapter 5-1Nasser SANessuna valutazione finora
- 22 - Fracture Toughness & Toughening MechDocumento30 pagine22 - Fracture Toughness & Toughening MechMd. Rafiqul IslamNessuna valutazione finora
- 2-BMCG2323 Manufaturing MaterialsDocumento91 pagine2-BMCG2323 Manufaturing Materialshemarubini96Nessuna valutazione finora
- c1 Mechanical PropertiesDocumento46 paginec1 Mechanical PropertiesHusnal TaufiqNessuna valutazione finora
- Material Science and Metallurgy: Unit I Structure of MaterialsDocumento127 pagineMaterial Science and Metallurgy: Unit I Structure of MaterialsViraj Babar100% (1)
- Chapter1 - Symmetry and Point GroupDocumento111 pagineChapter1 - Symmetry and Point GroupChia Wee Keat100% (1)
- Group 2 Properties of MaterialsDocumento24 pagineGroup 2 Properties of MaterialsjohnpaulshobayanNessuna valutazione finora
- Imperfections in Solid Materials - Ch4Documento40 pagineImperfections in Solid Materials - Ch4aa454Nessuna valutazione finora
- Iron Carbon Phase DiagramDocumento4 pagineIron Carbon Phase DiagramMizanur RahmanNessuna valutazione finora
- Structure of Crystalline Solids - Callister BookDocumento56 pagineStructure of Crystalline Solids - Callister BookKhushiNessuna valutazione finora
- CopperDocumento18 pagineCopperaimanhazimNessuna valutazione finora
- lectut-MTN-513-pdf-Structure of Crystalline CeramicsDocumento53 paginelectut-MTN-513-pdf-Structure of Crystalline CeramicsAkash AgarwalNessuna valutazione finora
- Structure of Ceramics: Part 3: MME 467 Ceramics For Advanced ApplicationsDocumento15 pagineStructure of Ceramics: Part 3: MME 467 Ceramics For Advanced ApplicationsMd. Rafiqul IslamNessuna valutazione finora
- Capacitance Lab ExerciseDocumento5 pagineCapacitance Lab ExerciseCrmz CastilloNessuna valutazione finora
- Chapter 2Documento59 pagineChapter 2Temesgen Zeleke100% (1)
- MetalDocumento57 pagineMetalPrashant PuriNessuna valutazione finora
- Chapter-6 Mechanical Properties of MetalsDocumento35 pagineChapter-6 Mechanical Properties of Metalssamuel mekuriawNessuna valutazione finora
- TEM Lecture CrystallineDocumento30 pagineTEM Lecture CrystallineSyed Abdul AhadNessuna valutazione finora
- Chapter 4 Mechanical Properties of MetalsDocumento19 pagineChapter 4 Mechanical Properties of MetalsKeith Tanaka MagakaNessuna valutazione finora
- Material Science and Engineering: Engr. Joseph Benedict N. PrimDocumento107 pagineMaterial Science and Engineering: Engr. Joseph Benedict N. PrimJOSEPH BENEDICT PRIMNessuna valutazione finora
- Corrosion Measurement UNIT-5: CHE-545-172 DR Ime B.ObotDocumento48 pagineCorrosion Measurement UNIT-5: CHE-545-172 DR Ime B.ObotArielNessuna valutazione finora
- Coefficient of Friction Lab Write UpDocumento5 pagineCoefficient of Friction Lab Write UpPOH HSIN TANNessuna valutazione finora
- 09 - Electron MicrosDocumento63 pagine09 - Electron Microsnayonaega100% (2)
- 8.2 Final Fracture ToughnessDocumento19 pagine8.2 Final Fracture ToughnessHenry Theodore DaquinagNessuna valutazione finora
- 1 - Lecture Notes For 1st Cycle Test 07022016Documento17 pagine1 - Lecture Notes For 1st Cycle Test 07022016SaiPraneethNessuna valutazione finora
- Unit 4 MSE Heat Treatment of MetalsDocumento85 pagineUnit 4 MSE Heat Treatment of MetalsRushikesh KaleNessuna valutazione finora
- Electrochem Practice TestDocumento41 pagineElectrochem Practice TestaaaaaNessuna valutazione finora
- Nucleophilic Substitution Reactions (SN1, SN2) AND Elimination Reactions (E1, E2)Documento11 pagineNucleophilic Substitution Reactions (SN1, SN2) AND Elimination Reactions (E1, E2)Makhdoom JahaniaNessuna valutazione finora
- MM PDF Ia1Documento109 pagineMM PDF Ia1M.41Mohd AnasNessuna valutazione finora
- Lec5 PDFDocumento37 pagineLec5 PDFharikiranNessuna valutazione finora
- Formal Report Exp6Documento7 pagineFormal Report Exp6Rachel CajilesNessuna valutazione finora
- CHNS oDocumento7 pagineCHNS oAnonymous xcJJIqETwNessuna valutazione finora
- Types of SolidsDocumento39 pagineTypes of SolidsTHARIK ANWAR100% (2)
- Bonding and Properties 2018 PDFDocumento31 pagineBonding and Properties 2018 PDFJackNessuna valutazione finora
- Course Content: No. Title Slide NoDocumento54 pagineCourse Content: No. Title Slide NoDilip YadavNessuna valutazione finora
- Phase DiagramsDocumento55 paginePhase DiagramsNeelu TiruvayipatiNessuna valutazione finora
- Ceramics Tech #ElectroceramicsDocumento51 pagineCeramics Tech #Electroceramicsnawa100% (2)
- Martensitic Stainless SteelsDocumento8 pagineMartensitic Stainless SteelsAdilmar E. NatãnyNessuna valutazione finora
- Uv Visible SpectrosDocumento14 pagineUv Visible SpectrosDevanshi JadaunNessuna valutazione finora
- Indexing Diffraction PatternsDocumento35 pagineIndexing Diffraction PatternsYasirFaheem100% (1)
- Structure and BondingDocumento12 pagineStructure and BondingNisha JodhanNessuna valutazione finora
- Surface Hardening of SteelDocumento50 pagineSurface Hardening of SteelTeptep GonzalesNessuna valutazione finora
- All Functional Materials For Material EngineeringDocumento27 pagineAll Functional Materials For Material Engineeringeddula ganeshNessuna valutazione finora
- Microstructure of A Lead-Tin AlloyDocumento55 pagineMicrostructure of A Lead-Tin AlloyThaya GanapathyNessuna valutazione finora
- Definitions: Components and PhasesDocumento42 pagineDefinitions: Components and Phasesabhilash reddyNessuna valutazione finora
- Electron Beam Melted Ti-6Al-4V: Microstructure, Texture and Mechanical Behavior of The As-Built and Heat-Treated MaterialDocumento15 pagineElectron Beam Melted Ti-6Al-4V: Microstructure, Texture and Mechanical Behavior of The As-Built and Heat-Treated MaterialDiep BerteauNessuna valutazione finora
- A Review of Particulate Reinforced Aluminum Mat - 2020 - Transactions of NonferrDocumento34 pagineA Review of Particulate Reinforced Aluminum Mat - 2020 - Transactions of Nonferrmohd asrofi muslimNessuna valutazione finora
- Lecture 1-4 - IMSE - Introduction + MetallographyDocumento32 pagineLecture 1-4 - IMSE - Introduction + Metallographyvicky dasNessuna valutazione finora
- Cast Iron - A Predictable Material 25 Years of ModDocumento14 pagineCast Iron - A Predictable Material 25 Years of ModScribdtsNessuna valutazione finora
- Brochure PDFDocumento3 pagineBrochure PDFAldo HernándezNessuna valutazione finora
- Effect of Heat Treatment On Corrosion Behaviour of Welded AA6061 Aluminium Alloy in SeawaterDocumento9 pagineEffect of Heat Treatment On Corrosion Behaviour of Welded AA6061 Aluminium Alloy in SeawaterMohamed RamadanNessuna valutazione finora
- Failure Analysis Furnace Radiant TubesDocumento13 pagineFailure Analysis Furnace Radiant Tubesjohan garciaNessuna valutazione finora
- 1 Sample PreparationDocumento490 pagine1 Sample PreparationVyakulShahNessuna valutazione finora
- Discussion MaterialDocumento3 pagineDiscussion Materialmuhd afhamNessuna valutazione finora
- Aerospace Material Specification: AMS5629™ Rev. JDocumento10 pagineAerospace Material Specification: AMS5629™ Rev. JDavid SalgueroNessuna valutazione finora
- Abstract Book - ICFME-2022Documento105 pagineAbstract Book - ICFME-2022S R E E N I V A S ANessuna valutazione finora
- Technical Report of PipingDocumento370 pagineTechnical Report of PipingYudo Heru Pribadi100% (1)
- Catalyst TubeDocumento8 pagineCatalyst TubePradeep SinghNessuna valutazione finora
- Mechanical and Electrochemical Characterization of Ti-12Mo-5Zr Alloy For Biomedical ApplicationDocumento4 pagineMechanical and Electrochemical Characterization of Ti-12Mo-5Zr Alloy For Biomedical ApplicationhijekeNessuna valutazione finora
- Proceeding ICOME 2015Documento184 pagineProceeding ICOME 2015Sally ChytNessuna valutazione finora
- Plant Layout Quiz 3 AnswersDocumento2 paginePlant Layout Quiz 3 AnswersSurya ANessuna valutazione finora
- Ceramicos PDFDocumento288 pagineCeramicos PDFElvis PalaciosNessuna valutazione finora
- J Matdes 2016 09 029Documento8 pagineJ Matdes 2016 09 029GUSTAVO LOPEZ MENDOZANessuna valutazione finora
- CF7 DPR Manuscript ProceedingsDocumento6 pagineCF7 DPR Manuscript ProceedingsD P RAO PALAPARTINessuna valutazione finora
- CHP 1-3 PDFDocumento94 pagineCHP 1-3 PDFvsudarsanNessuna valutazione finora
- IC-CMTP2 Abstract BookDocumento216 pagineIC-CMTP2 Abstract Bookzeroo85Nessuna valutazione finora
- Introduction To CeramicsDocumento39 pagineIntroduction To CeramicsRasika MalodeNessuna valutazione finora
- Seminar Presentation PPT On Reactive Powder Concrete Civil EngineeringDocumento39 pagineSeminar Presentation PPT On Reactive Powder Concrete Civil Engineeringshreekanth bapatNessuna valutazione finora
- The Characterization of Cast Fe-Cr-C Alloy: ArticleDocumento5 pagineThe Characterization of Cast Fe-Cr-C Alloy: Articlerohith kumarNessuna valutazione finora
- Characterization of Cobalt Chromium HAPDocumento10 pagineCharacterization of Cobalt Chromium HAPmasinski fakultetNessuna valutazione finora
- Welding TMCP SteelsDocumento7 pagineWelding TMCP SteelsElias Kapa100% (1)
- Manufacturing and Application of Stainless Steels: Andrea Di SchinoDocumento262 pagineManufacturing and Application of Stainless Steels: Andrea Di SchinoJohanes DarmawanNessuna valutazione finora
- Aluminium Alloy 6061-T651 WeldedDocumento9 pagineAluminium Alloy 6061-T651 WeldedBagandi ManurungNessuna valutazione finora
- Fatigue Testing of The StentDocumento13 pagineFatigue Testing of The StentPooja BhatiNessuna valutazione finora
- Unit-V Investment Casting of SuperalloysDocumento36 pagineUnit-V Investment Casting of SuperalloysJ JhansibaiNessuna valutazione finora