Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Sinul
Caricato da
Cucos Natalia0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
102 visualizzazioni38 pagineThis document provides an overview of breast anatomy relevant to plastic surgery. It describes the borders and components of the breast, including parenchyma, Cooper's ligaments, the nipple-areolar complex, vascular supply, lymphatics, innervation, and associated musculature. Gynecomastia, the enlargement of male breast tissue, is also briefly discussed.
Descrizione originale:
breast
Titolo originale
sinul
Copyright
© © All Rights Reserved
Formati disponibili
PPTX, PDF, TXT o leggi online da Scribd
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoThis document provides an overview of breast anatomy relevant to plastic surgery. It describes the borders and components of the breast, including parenchyma, Cooper's ligaments, the nipple-areolar complex, vascular supply, lymphatics, innervation, and associated musculature. Gynecomastia, the enlargement of male breast tissue, is also briefly discussed.
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PPTX, PDF, TXT o leggi online su Scribd
0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
102 visualizzazioni38 pagineSinul
Caricato da
Cucos NataliaThis document provides an overview of breast anatomy relevant to plastic surgery. It describes the borders and components of the breast, including parenchyma, Cooper's ligaments, the nipple-areolar complex, vascular supply, lymphatics, innervation, and associated musculature. Gynecomastia, the enlargement of male breast tissue, is also briefly discussed.
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PPTX, PDF, TXT o leggi online su Scribd
Sei sulla pagina 1di 38
Breast ( anatomy, reduction,
tuberous breast deformity,
gynecomastia)
Anatomy for plastic surgery of the breast
Parenchymal borders:
• Superior border: clavicle
• Medial border: sternum
• Inferior border: inframammary
fold
• Lateral border: anterior border
of the latissimus dorsi
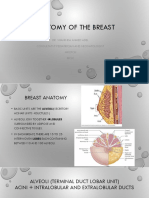
Parenchyma
• The functioning parenchyma produces milk in the post-partum
period. Adipose tissue comprises a significant amount of the
breast volume, representing 50–70% of the breast volume.
• With age and the hormonal changes of menopause, the
glandular tissue of the breast involutes, increasing the adipose
to parenchymal tissue ratio.
• The Cooper’s ligaments provide
numerous interconnections between
the deep and superficial fascial layers.
These ligaments pass through ad
invest in the breast parenchyma
securing to the pectoralis fascia. With
attenuation of these support structures,
breast ptosis will develop.
Nipple areola complex
• The nipple areola complex is the primary
landmark of the breast.
• The nipple itself may project as much as
≥1 cm, with a diameter of approximately
4–7 mm.
• The areola consists of pigmented skin
surrounding the nipple proper and is on
average approximately 4.2–4.5 cm in
diameter.
• The areola consists of keratinized,
stratified epithelium and contains not only
the lactiferous sinus openings, but also
sebaceous glands and the Montgomery
glands.
• Deep to the nipple and areolar there are
smooth muscle fibers which are arranged
circumferentially and radially. These
fibers are attached to the thick
connective tissue of the areola and are
responsible for nipple erection.
Vascularity
• The breast has a rich vascular supply
from multiple arterial sources.
• The primary arterial supply includes
three main sources: the internal
mammary perforators, lateral thoracic
artery and the anterolateral intercostal
perforators.
• Additional arterial supply includes the
thoracoacromial artery and its perforators
and the vessels of the serratus anterior.
• The internal mammary perforators enter
the superior medial portion of the breast
via the second through sixth intercostal
spaces. The second and third perforators
are the predominant of these perforating
vessels. Because of their larger caliber
the second or third perforators are the
preferred recipient vessels for free tissue
reconstruction using the internal
mammary perforators.
• Supplying the superolateral aspect
of the breast is the lateral thoracic
or external mammary artery. This
vessel is a primary branch of the
axillary artery and enters the
breast after passing around the
lateral border of the pectoralis
major muscle at the inferior aspect
of the axilla. It distributes its
branches in the upper outer
quadrant of the breast.
• The lateral intercostal vessels
represent an additional important
blood supply of the breast. The
lateral breast receives anterior
intercostal arteries from the third
through sixth interspaces. These
vessels perforate the serratus
anterior just lateral to the pectoral
border. Lateral intercostal vessels
enter the breast at the anterior
margin of the latissimus dorsi to
supply the lateral breast and
overlying skin.
• Medial intercostal perforators are
responsible for direct supply of the
inferior central portion of the breast
inferior to the nipple areolar
complex.
• Venous drainage of the breast is via
two systems. The subdermal venous
plexus above the superficial fascia is
quite variable and represents the
superficial system. The veins arise
from the periareolar venous plexus
within the parenchyma, the
superficial systems anastomose with
the deep system. The deep system
parallels the arterial supply with the
veins paired to their respective
arteries. Venous perforators following
the internal mammary perforators
drain via the internal mammary vein
to the innominate vein. The lateral
thoracic veins drain via the azygos
vein into the superior vena cava.
• Vascular anatomy is also of importance with regard to the recipient site for microvascular
anastomosis when free tissue transfer is used for breast reconstruction.
• The thoracodorsal vessels have been used, particularly when the reconstruction is immediately
postmastectomy. The thoracodorsal artery is often small (<2 mm) and may have insufficient flow.
The axillary vessels can be technically difficult for the assistant, since they must operate across
the chest. In addition, the axillary system may limit flap movement and shaping the breast.
• The use of the internal mammary vessels as a recipient site facilitates shaping the medial portion
of the breast. However, the technique requires partial rib resection and eliminates the opportunity
for a potential coronary artery bypass graft.
• The internal mammary vessel may be preferred in delayed cases, especially in patients who
have had adjuvant radiation, as dissection of axillary vessels can be very difficult.
Lymphatics
• The predominance of lymph
drainage of the breast is via the
interlobular lymphatic vessels to the
subareolar plexus. Lymph is
directed toward the axillary lymph
nodes.
• This drainage is parallel to the
venous drainage of the breast.
Lateral lymphatics course around
the edge of the pectoralis major
toward the pectoral lymph nodes.
Additional lymphatics course
through the pectoral muscles to the
apical lymph nodes. From the
axillary lymph nodes, lymph drains
into the subclavian and
supraclavicular lymph nodes.
Innervation
• Sensory innervation has three
major nerve distributions which
include the anterior lateral
intercostals, the medial intercostals,
and the cervical plexus.
• The anterior rami of the lateral
cutaneous nerves of the
intercostals provide sensation to
the lateral portion of the breast
extending to and including the
nipple areolar complex. The breast
demonstrates a dermatomal pattern
derived from the anterolateral and
anteromedial branches of the
intercostal nerves (T3–T5).
• Branches of the cervical plexus
provide the superior medial sensory
innervation.
• Intercostal segmental nerves
contribute the remainder of the
breast sensation and can be
considered the primary sensory
nerves. The third through sixth
anterolateral intercostal nerves pass
through the interdigitations of the
serratus muscles to enter the lateral
aspect of the breast.
• Along the medial border of the
breast, the second through sixth
anteromedial intercostal nerves
enter the breast parenchyma
alongside the internal mammary
perforating vessels. These sensory
nerves provide innervation to the
medial breast and nipple areolar
complex.1
Musculature
• The muscles directly
associated with the breast
include the pectoralis major,
serratus anterior, external
oblique and the superior
portion of the rectus
abdominis
Pectoralis major
• Origin- medial clavicle and lateral
sternum
• Insertion- on the humerus
• Blood suplly: toracoacromial artery;
intercostal perforators from the internal
mammary artery
• Inervation: medial and lateral anterior
thoracic nerves
• Action: flex; adduct and rotate the arm
medially.
• The pectoralis major is extremely
important in both aesthetic and
reconstructive breast surgery, since
it provides muscle coverage for the
breast implant
Serratus anterior
• Origin is the outer surface of the upper borders of
the first through eighth ribs
• Insertion is on the deep surface of the scapula
• Vascular supply is derived equally from the
lateral thoracic artery and branches from the
thoracodorsal artery
• The long thoracic nerve serves to innervate the
serratus anterior, which acts to rotate the
scapula, raising the point of the shoulder and
drawingthe scapula forward toward the body.
• Because the serratus anterior underlies the
lateral aspect of the breast, in aesthetic surgery,
blunt elevation of the pectoralis major laterally
inadvertently elevates a small portion of the
serratus muscle. To completely cover the implant
with muscle in reconstructive surgery, often the
serratus anterior must be elevated sharply to
obtain a sufficient muscle layer to provide
coverage.
Rectus abdominis
• Origin at the crest of the pubis
and interpubic ligament to its
insertion at the xiphoid process
and cartilages of the fifth through
seventh ribs.
• It acts to compress the abdomen
and flex the spine
• When placing an implant for
breast reconstruction, in
attempting to achieve complete
coverage with muscle, the rectus
fascia must often be elevated to
place the implant sufficiently
caudal.
External oblique
• Its origin is from the lower eight ribs, and its insertion
is along the anterior half of the iliac crest and the
aponeurosis of the linea alba from the xiphoid to the
pubis
• It acts to compress the abdomen, flex and laterally
rotate the spine, and depress the ribs.
• Elevated along with the rectusabdominis fascia to
provide inferior coverage of the breast implant during
reconstructive surgery
• In aesthetic surgery, placement of the implant
inferiorly is usually not below these fascial
attachments. If the implant is placed behind the
fascia, the implant often “rides too high” and may
result in a “double bubble” effect, wherein the breast
parenchyma slides over and off the implant
Gynecomastia
Gynecomastia
• Gynecomastia is enlargement of the male breast
and is caused by an increase in ductal tissue,.
stroma, and/or fat. Most frequently, the changes
occur at the time of hormonal change: infancy,
adolescence, and old age.
• The most common cause of gynecomastia is
unknown (idiopathic).
• In all three age groups (neonatal, adolescent,
and older men), gynecomastia appears to be
related to either an increase in estrogens, a
decrease in androgens, or a deficit in androgen
receptors.
• The incidence of gynecomastia rises again in
older men (age > 65 years).
COMMON CAUSES OF GYNECOMASTIA
DIAGNOSIS
PATHOLOGY
• A careful history and physical examination
• Three types of gynecomastia is the most important part of any workup
have been described: florid, for gynecomastia.
fibrous, and intermediate. The
6
• The history notes the time of onset of the
gynecomastia, symptoms associated with
florid type is characterized by an the gynecomastia, drug use.
increase in ductal tissue and • Physical examination includes assessment
vascularity. of the breast gland and includes the nature
of the tissue, isolated masses, and
• The fibrous type has more tenderness. The thyroid is evaluated for
stromal fibrosis with few ducts. enlargement. The testes are examined for
asymmetry, masses, enlargement, or
• The intermediate type is a atrophy.
mixture of the two. • Laboratory evaluation is based on the
findings of the history and physical
examination
CLASSIFICATION
Simon, Hoffman, and Kahn divided gynecomastia into four grades:
1
grade 1: small enlargement, no skin excess
grade 2 a: moderate enlargement, no skin excess
b: moderate enlargement with extra skin
grade 3: marked enlargement with extra skm
Letterman and Schuster' created a classification system based on the
type of correction:
1: intra-areolar incision with no excess skin
2: intra-areolar incision with mild redundancy corrected with
excision of skin
through a superior periareolar scar
3: excision of chest skin with or without shifting the nipple.
Rohrich et al.,in a paper discussing the utility of ultrasound-assisted liposuction
in the treatment of gynecomastia, developed the following classification
grade I: minimal hypertrophy (<250 g of breast tissue) without ptosis
grade II: moderate hypertrophy (250 to 500 g of breast tissue) without ptosis
grade III: severe hypertrophy (>500 g breast tissue) with grade I ptosis
grade IV: severe hypertrophy with grade II or III ptosis
TREATMENT OF GYNECOMASTIA
• The goal of surgery is:
- to remove the excess breast tissue and skin,
- ensure adequate positioning of the nipple-areola complex,
- ensure symmetry between the breasts and chest wall,
- to avoid significant scarring
• Most fibrous or solid Simon stage 1 or 2a lesions are treated
with surgical excision or more recently, in selected cases, with
ultrasonic liposuction:with sharp tip cannulas, power-assisted
liposuction, or ultrasound-assisted liposuction.
• If surgical excision is chosen, a periareolar incision is
performed.
• The skin incision is placed at the junction of the areola and skin.
• After the incision is made, a cuf of tissue 1 to 1.5 an in
thickness is preserved directly deep to the nipple/areola
complex. This maneuver prevents postoperative nipple/areola
depression or adherence of the nipple/areola to the chest wall.
• When liposuction is unsuccessful at
removing all of the tissue required to
achieve a good result, the pull-through
technique is added.
• In this technique, either the lateral or
periareolar incision is opened slightly
(about 1.5 em) and the residual tissue is
grasped. The tissue is pulled out through
the wound and removed with scissors or
electrocautery. The pull-through
resection is performed until the desired
contour is achieved.
• All patients are treated with compression
garments for at least 1 month
COMPLICATIONS
• Complications include inadequate resection, overresection, excess
skin, complex scars, hematoma, seroma, partial nipple necrosis, suture
line dehiscence, pain, loss of nipple sensation, and infection.
• Potential risks of ultrasonic liposuction include thermal burns and skin
necrosis, because one of the byproducts of ultrasonic energy is heat.
• This is avoided by using cool towels over the skin and avoiding
superficial planes near the skin surface.
TUBEROUS BREAST
DEFORMITY
• Tuberous breast deformity describes a spectrum of aberrant breast
morphology first reported by Rees and Aston
• There are several features of the tuberous breast that are important to
identify before management. These include a constricted base,
contraction of the skin envelope, relative micromastia, enlarged
diameter of the nipple-areola complex and herniation of breast
parenchyma through the nipple-areola complex.
• Although the exact etiology has not
been elucidated, it is generally
accepted that this disorder has an
embryologic origin.Most reports have
speculated that the superficial
investing fascia of the breast is
abnormal and constricted at the base
of the breast. This constriction at the
base and deficiency at the areola is
responsible for the reduced base
diameter and areolar herniation
Classification
Von Heimburg:
Type 1: hypoplasia of the lower
medial quadrant
Type II: hypoplasia of the lower
medial and lateral quadrants
with sufficient skin in the
subareolar area
Type III: hypoplasia of the lower
medial and lateral quadrants
with a deficiency of the
subareolar skin
Type IV: severe breast constriction
with minimal breast base
Grolleau Classification
Type 1: lower medial quadrant deficiency
• Type II: lower medial and lateral quadrant deficiency
Type Ill: deficiency of all four quadrants
Treatment
• The goals of surgery are to restore volume to the hypoplastic breast(s),
expand the lower pole by releasing the tethering fibrous attachments or
bands between the breast parenchyma and deep fascial and pectoralis
muscle and also between the breast parenchyma and skin, and where
necessary reduce the areola size and recess the herniated breast tissue.
• The Mandrekas technique is
illustrated. (Above, left) A
periareolar approach is
advocated. (Above, center)
The dissection proceeds in
the subcutaneous plane to
the pectoral fascia. (Above,
right) The dissection
continues to the desired
inframammary fold. (Below,
left) The inferior pole of the
breast is exteriorized, and the
constrictive band is divided
vertically. (Below, right)
Finally, the areola is reduced,
and the breast is recontoured
Potrebbero piacerti anche
- SinulDocumento86 pagineSinulCucos NataliaNessuna valutazione finora
- Anatomy of The Pectoral RegionDocumento42 pagineAnatomy of The Pectoral Regionomotayojane21Nessuna valutazione finora
- Anatomy Screeningcervix by DR. VACHASPATIDocumento52 pagineAnatomy Screeningcervix by DR. VACHASPATIOshydh PojnNessuna valutazione finora
- Anatomy of The UterusDocumento21 pagineAnatomy of The UterusSalman KhanNessuna valutazione finora
- P. Cavity: (Female Reproductive Organs)Documento38 pagineP. Cavity: (Female Reproductive Organs)SAKARIYE MAXAMEDNessuna valutazione finora
- Anatomical Considerations During G-Laparoscopic SurgeryDocumento28 pagineAnatomical Considerations During G-Laparoscopic SurgeryMahia RahmanNessuna valutazione finora
- Abdominal Wall and HerniaDocumento35 pagineAbdominal Wall and HerniaMohammad BanisalmanNessuna valutazione finora
- Uterus Fallopian Tube and OvaryDocumento35 pagineUterus Fallopian Tube and Ovaryvijaya pranaviNessuna valutazione finora
- Lec.1.Anatomy of PregnancyDocumento53 pagineLec.1.Anatomy of PregnancyManal AsadNessuna valutazione finora
- Anatomy of GIT For PCII Students..Documento106 pagineAnatomy of GIT For PCII Students..AMANUEL HABTEWOLDNessuna valutazione finora
- Female Genital OrgansDocumento40 pagineFemale Genital OrgansShimmering MoonNessuna valutazione finora
- Anatomy of Breast: DR Ram Manohar Lohia Institute of Medical Sciences, LucknowDocumento103 pagineAnatomy of Breast: DR Ram Manohar Lohia Institute of Medical Sciences, LucknowMohammad Ahmad AyasrahNessuna valutazione finora
- Anatomy of Fallopian Tube & OvaryDocumento89 pagineAnatomy of Fallopian Tube & OvaryAsma AijazNessuna valutazione finora
- Surgical-ANATOMY OF Anterior Abdominal WallDocumento83 pagineSurgical-ANATOMY OF Anterior Abdominal WallSyed Irfan ArifNessuna valutazione finora
- Anatomy of The Breast1Documento13 pagineAnatomy of The Breast1Shahinda Ahmed AdelNessuna valutazione finora
- AnatDocumento45 pagineAnatRinxas VerinxtNessuna valutazione finora
- Breast Anatomy With Clinical CorrelationDocumento27 pagineBreast Anatomy With Clinical CorrelationDionix Cruz100% (2)
- Anatomy and Physiology of The VaginaDocumento74 pagineAnatomy and Physiology of The VaginaRacquel BurrowesNessuna valutazione finora
- Breast AnatomyDocumento15 pagineBreast Anatomyvarun100% (1)
- Anterolateral Abdominal Wall and Abdominal IncisionsDocumento42 pagineAnterolateral Abdominal Wall and Abdominal IncisionsSamar AhmadNessuna valutazione finora
- Abdomen and Abdominal WallDocumento40 pagineAbdomen and Abdominal WallAlex ChagalaNessuna valutazione finora
- Embryology and Functional Anatomy of The BreastDocumento20 pagineEmbryology and Functional Anatomy of The BreastseidkeNessuna valutazione finora
- Abdominal Region Part 2Documento58 pagineAbdominal Region Part 2Swati LataNessuna valutazione finora
- Breast - Mammary GlandDocumento16 pagineBreast - Mammary GlandEniola DaramolaNessuna valutazione finora
- Uterus and Ovary UltrasoundDocumento144 pagineUterus and Ovary Ultrasoundisicheipraise3Nessuna valutazione finora
- Anatomy of Female Reproductive SystemDocumento68 pagineAnatomy of Female Reproductive SystemdodoNessuna valutazione finora
- 4rd Lecture - AbdomenDocumento26 pagine4rd Lecture - AbdomenZainab Jamal SiddiquiNessuna valutazione finora
- Anatomy and Embryology of Bladder: Dr. Deepesh Kalra Institute of Urology Madras Medical College, ChennaiDocumento33 pagineAnatomy and Embryology of Bladder: Dr. Deepesh Kalra Institute of Urology Madras Medical College, ChennaiFatima Zahra Rahim ArchiNessuna valutazione finora
- 4) Mammary Gland 220413 Upnm2Documento22 pagine4) Mammary Gland 220413 Upnm2AaronMaroonFive100% (1)
- Maternal Anatomy WilliamsDocumento60 pagineMaternal Anatomy WilliamsZari Novela100% (2)
- Female Genital Organs.Documento18 pagineFemale Genital Organs.Shimmering MoonNessuna valutazione finora
- Topography Femoral RegionDocumento40 pagineTopography Femoral RegionmonaNessuna valutazione finora
- Perineal TearsDocumento49 paginePerineal TearsvisakhaNessuna valutazione finora
- Vagina Anat, Episiotomy and MGMT of Cervical TearDocumento50 pagineVagina Anat, Episiotomy and MGMT of Cervical TearCapricious BibekNessuna valutazione finora
- Intro Lab Anatomi Rps (2024)Documento117 pagineIntro Lab Anatomi Rps (2024)Jason Maxwell mcguireNessuna valutazione finora
- Abdominal WallDocumento56 pagineAbdominal WallAHMAD KHANNessuna valutazione finora
- PT Physiology of PuerperiumDocumento14 paginePT Physiology of PuerperiumPragati BholeNessuna valutazione finora
- Revisit of Male & Female Genital Tracts Semester VIIDocumento50 pagineRevisit of Male & Female Genital Tracts Semester VIIDr. AyshaNessuna valutazione finora
- 07 - Uterus, Uterine Tubes, OvariesDocumento52 pagine07 - Uterus, Uterine Tubes, Ovariesck4realNessuna valutazione finora
- AnatomicallyDocumento3 pagineAnatomicallyAjayDeep NallabothulaNessuna valutazione finora
- CBL 2Documento20 pagineCBL 2Hammad AkramNessuna valutazione finora
- Anatomy of Uterus and Vagina and PudendumDocumento38 pagineAnatomy of Uterus and Vagina and Pudendumgugus aminaNessuna valutazione finora
- Female PerineumDocumento27 pagineFemale Perineumkaartikey dubeNessuna valutazione finora
- Anterior Abdominal Wall& Inguinal Canal 2023Documento26 pagineAnterior Abdominal Wall& Inguinal Canal 2023GanapathyGaneshNessuna valutazione finora
- Ca BreastDocumento64 pagineCa Breastadina.batajuNessuna valutazione finora
- Perineum and UG TriangleDocumento27 paginePerineum and UG TriangleAbiola NerdNessuna valutazione finora
- Maternal AnatomyDocumento96 pagineMaternal AnatomyIbnu RahmanNessuna valutazione finora
- UntitledDocumento7 pagineUntitledDania Ibraheem100% (1)
- Student Name: ALI HASSAN RAZA STUDENT I D: 2018304027Documento6 pagineStudent Name: ALI HASSAN RAZA STUDENT I D: 2018304027Sayed AsifNessuna valutazione finora
- Perineum AnatomyDocumento23 paginePerineum Anatomyrohitrohillapalwal999Nessuna valutazione finora
- Anatomy of Pregnancy NewDocumento42 pagineAnatomy of Pregnancy NewZaroon Abdullah KhanNessuna valutazione finora
- Relations of ThoraxDocumento28 pagineRelations of ThoraxAnigha PrasadNessuna valutazione finora
- Anatomy and PhysiologyDocumento3 pagineAnatomy and PhysiologyJade AltarejosNessuna valutazione finora
- Inguinal Canal: DR - Lubna NazliDocumento28 pagineInguinal Canal: DR - Lubna NazliAhmed AminNessuna valutazione finora
- UterusDocumento34 pagineUterushammad992Nessuna valutazione finora
- Urinary Bladder, Rectum and Anal CanalDocumento34 pagineUrinary Bladder, Rectum and Anal CanalIbe ClementNessuna valutazione finora
- Uterus 1Documento13 pagineUterus 1shrutik91Nessuna valutazione finora
- Female Reproductive SystemDocumento40 pagineFemale Reproductive SystemSAYMABANUNessuna valutazione finora
- Female Reproductive System (Yuni)Documento36 pagineFemale Reproductive System (Yuni)Ayi Abdul BasithNessuna valutazione finora
- (Mebooksfree Net) Cor&pro&pla&sur&nel&buc&2nd PDFDocumento527 pagine(Mebooksfree Net) Cor&pro&pla&sur&nel&buc&2nd PDFCucos Natalia100% (1)
- Hialuronidaza PDFDocumento8 pagineHialuronidaza PDFCucos NataliaNessuna valutazione finora
- Essentials of Plastic Surgery, 2nd EditionDocumento1.351 pagineEssentials of Plastic Surgery, 2nd EditionIcleanu Alexandru84% (19)
- 16 ISS Baker Haddon 1974Documento10 pagine16 ISS Baker Haddon 1974Parnil SinghNessuna valutazione finora
- Menstrual Disorders Associated With Thyroid Dysfunction: Ramya M. R., Parvathavarthini, Darshan Savery, R. SankareswariDocumento5 pagineMenstrual Disorders Associated With Thyroid Dysfunction: Ramya M. R., Parvathavarthini, Darshan Savery, R. SankareswariCucos NataliaNessuna valutazione finora
- Amputation Stumps H. E.: Harding Langdale-Kelham, London, EnglandDocumento3 pagineAmputation Stumps H. E.: Harding Langdale-Kelham, London, EnglandCucos NataliaNessuna valutazione finora
- 04 Rasulic Acta 1Documento6 pagine04 Rasulic Acta 1Cucos NataliaNessuna valutazione finora
- Essentials of Plastic Surgery, 2nd EditionDocumento1.351 pagineEssentials of Plastic Surgery, 2nd EditionIcleanu Alexandru84% (19)
- NeliganDocumento1.404 pagineNeliganCucos Natalia100% (2)
- Neuroma of HandDocumento5 pagineNeuroma of HandCucos NataliaNessuna valutazione finora
- Surgical Treatment of Strabismus in AdultsDocumento1 paginaSurgical Treatment of Strabismus in AdultsCucos NataliaNessuna valutazione finora
- Alogrefele de NerviDocumento11 pagineAlogrefele de NerviCucos NataliaNessuna valutazione finora
- Leziunea de Peroneu ComunDocumento16 pagineLeziunea de Peroneu ComunCucos NataliaNessuna valutazione finora
- Brachial PlexusDocumento42 pagineBrachial PlexusPrince DuNessuna valutazione finora
- Apk Retention Exam RevieweeDocumento9 pagineApk Retention Exam RevieweeKennie RamirezNessuna valutazione finora
- Cervical RadiculopathyDocumento7 pagineCervical Radiculopathyrhymescsf100% (1)
- 2014-10-17 Trail Guide To The Body CH 2Documento4 pagine2014-10-17 Trail Guide To The Body CH 2Ben WisherNessuna valutazione finora
- The Way of Thinking in Brachial Plexus InjuryDocumento48 pagineThe Way of Thinking in Brachial Plexus InjuryYoonHeeNyNessuna valutazione finora
- Physical Therapy Protocols For Conditions of Shoulder RegionDocumento111 paginePhysical Therapy Protocols For Conditions of Shoulder RegionPieng Napa100% (1)
- L3 AP 101 Mock Questions Exam Guide V101.2Documento24 pagineL3 AP 101 Mock Questions Exam Guide V101.2Sergiu TitcuNessuna valutazione finora
- Massage Therapy For Bruxism, TMJ SyndromeDocumento39 pagineMassage Therapy For Bruxism, TMJ SyndromeAdam Kesher100% (1)
- MRMDocumento39 pagineMRMZafirnur Amrin JasmanNessuna valutazione finora
- Brachial Plexus PowerpointDocumento32 pagineBrachial Plexus PowerpointZulkarnain Syamsuri100% (1)
- Anatomy 1 - MSS (Upper Limb)Documento127 pagineAnatomy 1 - MSS (Upper Limb)Abdullah As'adNessuna valutazione finora
- Acumed® Clavicle PlatingDocumento68 pagineAcumed® Clavicle Platingthomsoon01Nessuna valutazione finora
- Breast Anatomy and Mammography PositioningDocumento38 pagineBreast Anatomy and Mammography Positioningjitendra guptaNessuna valutazione finora
- Assessing The Breasts and Axillae: DelegationDocumento4 pagineAssessing The Breasts and Axillae: DelegationVioletteNessuna valutazione finora
- Anatomy 1.4 Upper Limbs - Muscle and FasciaDocumento10 pagineAnatomy 1.4 Upper Limbs - Muscle and Fascialovelots1234Nessuna valutazione finora
- Neuro Handbook (SUNY)Documento100 pagineNeuro Handbook (SUNY)Serge KhelemsKy100% (3)
- Mnemonics - AnatomyDocumento3 pagineMnemonics - Anatomymastac741Nessuna valutazione finora
- Anatomy Mnemonics UsmleDocumento65 pagineAnatomy Mnemonics UsmleHoney HoneyNessuna valutazione finora
- The Shoulder GirdleDocumento26 pagineThe Shoulder GirdleVeena VishwanathNessuna valutazione finora
- Master Degree in Plastic Surgery ThesisDocumento117 pagineMaster Degree in Plastic Surgery ThesisMohamed Ahmed El-RoubyNessuna valutazione finora
- Anatomy - Pectoral Region and Axilla PDFDocumento4 pagineAnatomy - Pectoral Region and Axilla PDFAngel KimNessuna valutazione finora
- Lambou Delto PectoralDocumento9 pagineLambou Delto PectoralalexNessuna valutazione finora
- Brachial PlexusDocumento62 pagineBrachial PlexusTutankhamoun Akhenatoun100% (2)
- Univ of Michigan - Gross Anatomy - Muscles TablesDocumento41 pagineUniv of Michigan - Gross Anatomy - Muscles TablesDarren Lim100% (1)
- BreastDocumento27 pagineBreastRasha HelmyNessuna valutazione finora
- History ExaminationDocumento18 pagineHistory ExaminationMohammed FaragNessuna valutazione finora
- 01 Muscles of The Chest WallDocumento10 pagine01 Muscles of The Chest WallAlvaro Mantilla GarcíaNessuna valutazione finora
- Touch For Health Level IDocumento2 pagineTouch For Health Level ISilvia De AlejandroNessuna valutazione finora
- Serdev Suture Techniques Breast Lift - Prof Nikolay P Serdev MD PHDDocumento4 pagineSerdev Suture Techniques Breast Lift - Prof Nikolay P Serdev MD PHDjobetobaNessuna valutazione finora
- Muscles of The Back Region - Listed Alphabetically Muscle Origin Insertion Action Innervation Artery NotesDocumento32 pagineMuscles of The Back Region - Listed Alphabetically Muscle Origin Insertion Action Innervation Artery NotesMaria Celina Lomboy SerapioNessuna valutazione finora