Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Ali 5
Caricato da
Losi Graham0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
62 visualizzazioni7 pagineenergy balance lectures
Titolo originale
ALI 5
Copyright
© © All Rights Reserved
Formati disponibili
PPTX, PDF, TXT o leggi online da Scribd
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoenergy balance lectures
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PPTX, PDF, TXT o leggi online su Scribd
0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
62 visualizzazioni7 pagineAli 5
Caricato da
Losi Grahamenergy balance lectures
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PPTX, PDF, TXT o leggi online su Scribd
Sei sulla pagina 1di 7
Enthalpy Change in Non-Reactive Processes
• Change in enthalpy can occur as a result of:
• (i) temperature change;
• (ii) change of phase;
• (iii) mixing or solution; and
• (iv) reaction.
• Change in Temperature
• Sensible heat: Heat transferred to raise or lower the
temperature of a material.
• Sensible heat change: change in the enthalpy of a
system due to variation in temperature.
• Sensible heat change is determined using a property
of matter called the heat capacity at constant
pressure (CP); J gmol-1K-1, cal g-1 °C-1, Btu lb-1 °C
• The term specific heat refers to heat capacity
expressed on a per-unit-mass basis.
• Tables B.3-B.6 in Appendix B list Cp values for
several organic and inorganic compounds.
• Additional Cp data and information about
estimating heat capacities can be found in
references such as Chemical Engineers'
Handbook, Handbook of Chemistry and
Physics and International Critical Tables.
• When Cp is constant, the change in enthalpy
of a substance due to change in temperature
at constant pressure is:
• M is either mass or moles of the substance
depending on the dimensions of Cp, T 1 is the
initial temperature and T 2 is the final
temperature.
• The corresponding change in specific enthalpy
is:
• Example 5.1 Sensible heat change with
constant Cp
• What is the enthalpy of 150 g formic acid at
70°C and 1 atm relative to 25°C and 1 atm?
• Solution:
• From Table B.5, Cp for formic acid in the
temperature range of interest is 0.524 cal g- 1
oC- 1. Substituting into Eq (5.12)"
• ∆H = (150 g) (0.524 cal g-~ ~ (70 - 25)~
• ∆H = 3537.0 cal
• or
• ∆H = 3.54 kcal.
• Relative to H=0 at 25°C the enthalpy of formic
acid at 70°C is 3.54 kcal.
• Heat capacities for most substances vary with
temperature.
• This means that when we calculate enthalpy
change due to change in temperature, the value
of CP itself varies over the range of ∆T.
• Heat capacities are often tabulated as
polynomial functions of temperature, such as:
• Coefficients a, b, c and d for a number of
substances are given in Table B.3 in Appendix B.
• We can assume that heat capacity is constant & results for
sensible heat change which approximate the true value.
• Because the temperature range of interest in bioprocessing
is relatively small, assuming constant heat capacity for some
materials does not introduce large errors.
• Cp data may not be available at all temperatures; heat
capacities like most of those listed in Tables B.5 and B.6 are
applicable only at a specified temperature or temperature
range.
• As an example, in Table B.5 the heat capacity for liquid
acetone between 24.2°C & 49.4 °C is 0.538 cal g-1 °C-1 even
though this value will vary within the temperature range.
• A useful rule of thumb for organic liquids near room
temperature is that Cp increases by 0.001-0.002 cal g-1 oC-1.
• One method for calculating sensible heat change
when CP varies with temperature involves use of
the mean heat capacity, Cpm.
• Table B.4 in Appendix B lists mean heat capacities
for several common gases.
• These values are based on changes in enthalpy
relative to a single reference temperature, Tref=
0°C.
• To determine the change in enthalpy for a change
in temperature from T1 to T2, read the values o f
Cpm at T1 and T2 and calculate:
Potrebbero piacerti anche
- 1 2 3 Literature Review 4 Experiment Objective 5 Methodology 6 Results 7 Discussions 8 Conclusion & Recommendations 9 References 10 AppendicesDocumento16 pagine1 2 3 Literature Review 4 Experiment Objective 5 Methodology 6 Results 7 Discussions 8 Conclusion & Recommendations 9 References 10 Appendicesmonkeystar12100% (3)
- CombustionDocumento46 pagineCombustionIrene Kaye AceroNessuna valutazione finora
- Lecture2 GranulopoiesisDocumento9 pagineLecture2 GranulopoiesisAfifa Prima GittaNessuna valutazione finora
- Inside:: Issue 4 - February 2004 Bi-Monthly Warhammer E-ZineDocumento40 pagineInside:: Issue 4 - February 2004 Bi-Monthly Warhammer E-ZineJoe BloggsNessuna valutazione finora
- Toeic: Check Your English Vocabulary ForDocumento41 pagineToeic: Check Your English Vocabulary ForEva Ibáñez RamosNessuna valutazione finora
- Practice Makes Perfect in Chemistry: The Physical Behavior of MatterDa EverandPractice Makes Perfect in Chemistry: The Physical Behavior of MatterValutazione: 5 su 5 stelle5/5 (1)
- Chapter 4 (Heat Effects)Documento67 pagineChapter 4 (Heat Effects)yohannes lemiNessuna valutazione finora
- Design of Penstock: Reference Code:IS 11639 (Part 2)Documento4 pagineDesign of Penstock: Reference Code:IS 11639 (Part 2)sunchitk100% (3)
- Manual For Experiment With Bomb CalorimeterDocumento12 pagineManual For Experiment With Bomb CalorimeterArunSutharNessuna valutazione finora
- Evolution Army 3 R DadDocumento341 pagineEvolution Army 3 R DadStanisław DisęNessuna valutazione finora
- SF RKJ 5 N TD67 K8 ZWCZIHfk Mgdoj 0 Ec TTYM2 Su LRG HDocumento45 pagineSF RKJ 5 N TD67 K8 ZWCZIHfk Mgdoj 0 Ec TTYM2 Su LRG HdiscordewrNessuna valutazione finora
- 12 7 2018 8 10 51 Am721@444Documento11 pagine12 7 2018 8 10 51 Am721@444xaytopsNessuna valutazione finora
- Thermo Heat EffectsDocumento61 pagineThermo Heat EffectsMayFifthNessuna valutazione finora
- Thermal PropertiesDocumento35 pagineThermal PropertiesMD. Humayun KobirNessuna valutazione finora
- Applied Thermodynamics: BSEE 2019-2023 3 Semester Pakistan Institute of Engineering and Applied Sciences, IslamabadDocumento46 pagineApplied Thermodynamics: BSEE 2019-2023 3 Semester Pakistan Institute of Engineering and Applied Sciences, IslamabadAliNessuna valutazione finora
- Week 6 Lecture 2Documento22 pagineWeek 6 Lecture 2muhammadtalhabilal6666Nessuna valutazione finora
- CH 2Documento41 pagineCH 2etayhailuNessuna valutazione finora
- Panas ReaksiDocumento62 paginePanas ReaksielangNessuna valutazione finora
- Panas Reaksi 3aDocumento62 paginePanas Reaksi 3aDahniarIkaNessuna valutazione finora
- Heat EffectDocumento22 pagineHeat EffectTMedhin MisganawNessuna valutazione finora
- Che 320 Part ADocumento41 pagineChe 320 Part AFreddie UzokweNessuna valutazione finora
- CTDCHA2 - Learning Unit 3 2019Documento39 pagineCTDCHA2 - Learning Unit 3 2019Brandon GreenwoodNessuna valutazione finora
- Chapter 8Documento19 pagineChapter 8احمد غالبNessuna valutazione finora
- Chapter 17 ThermochemistryDocumento72 pagineChapter 17 ThermochemistryUma FadziliaNessuna valutazione finora
- Thermodynamics Chp4 NotesDocumento24 pagineThermodynamics Chp4 NotesFJ100% (1)
- Chapter 3 - Heat EffectDocumento24 pagineChapter 3 - Heat EffectNur Liyana zamzuryNessuna valutazione finora
- Thermodynamics-I: Fall 2019Documento3 pagineThermodynamics-I: Fall 2019ali hamzaNessuna valutazione finora
- Lab 1 CalorimeterDocumento4 pagineLab 1 CalorimeterAndrian NasirNessuna valutazione finora
- 5.2 - Calculation of Enthalpy ChangesDocumento5 pagine5.2 - Calculation of Enthalpy ChangesNguyenHoangMinhDucNessuna valutazione finora
- Chapter 4Documento64 pagineChapter 4Biru EsheteNessuna valutazione finora
- Steady State Nonisothermal Reactor DesignDocumento59 pagineSteady State Nonisothermal Reactor DesignLin Xian XingNessuna valutazione finora
- First Law of ThermodynamicsDocumento67 pagineFirst Law of ThermodynamicsRhodelyn TolentinoNessuna valutazione finora
- EMM2503 Chapter FourDocumento19 pagineEMM2503 Chapter FourFarhan AdhliNessuna valutazione finora
- Ramadan Youssef Sakr Moustafa - Lecture 3-1st-2nd Laws On CombustionDocumento46 pagineRamadan Youssef Sakr Moustafa - Lecture 3-1st-2nd Laws On CombustionAbhinash KumarNessuna valutazione finora
- Assignment 2: (Subject: Ch.E. 401 Chemical Reactor Design) Related To CLO1Documento4 pagineAssignment 2: (Subject: Ch.E. 401 Chemical Reactor Design) Related To CLO1imtiazNessuna valutazione finora
- Fundamental Process VariablesDocumento36 pagineFundamental Process VariablesIfiok UsoroNessuna valutazione finora
- CCTD101B Notes 5 - Internal Energy and EnthalpyDocumento5 pagineCCTD101B Notes 5 - Internal Energy and EnthalpyKibwe TrimNessuna valutazione finora
- Thermodynamics-I: Fall 2016Documento4 pagineThermodynamics-I: Fall 2016ghaniNessuna valutazione finora
- Heat of CombustionDocumento9 pagineHeat of CombustionlollihopNessuna valutazione finora
- 04 Flame TemperatureDocumento38 pagine04 Flame TemperatureGnanasambandan SwaminathanNessuna valutazione finora
- Week 7 Lecture 1Documento20 pagineWeek 7 Lecture 1muhammadtalhabilal6666Nessuna valutazione finora
- ChemicalDocumento34 pagineChemicalVia Ysabel ManacopNessuna valutazione finora
- The Calorimetric Method of Determining The IntegralDocumento3 pagineThe Calorimetric Method of Determining The IntegralLoveFreequencyNessuna valutazione finora
- Thermal PropertiesDocumento21 pagineThermal Propertiesebrahim ftiesNessuna valutazione finora
- Topic 5 Energetics-ThermochemistryDocumento45 pagineTopic 5 Energetics-ThermochemistryLucia PesentiNessuna valutazione finora
- Adiabatic Flame TemperatureDocumento5 pagineAdiabatic Flame TemperatureJagdeep SekhonNessuna valutazione finora
- Chapter 4 Heat EffectsDocumento6 pagineChapter 4 Heat Effectsariana religiosoNessuna valutazione finora
- Thermodynamics IDocumento34 pagineThermodynamics IJannineNessuna valutazione finora
- Thermochemistry: Ron RobertsonDocumento21 pagineThermochemistry: Ron RobertsonRobin CelisNessuna valutazione finora
- 2.5 (A) Enthalpy: Chapter 2. The First LawDocumento71 pagine2.5 (A) Enthalpy: Chapter 2. The First Lawnabila OktavianiNessuna valutazione finora
- Lecture 11Documento14 pagineLecture 11sanyamjain51150Nessuna valutazione finora
- Thermodynamic Processes: (3) Isobaric Processes: Those Processes Which Take Place at Constant Pressure Are CalledDocumento16 pagineThermodynamic Processes: (3) Isobaric Processes: Those Processes Which Take Place at Constant Pressure Are CalledKarwan AliNessuna valutazione finora
- Chapter 1Documento27 pagineChapter 1Handoko PhandaNessuna valutazione finora
- Litium Bromide Water Absorption RefrigerationDocumento12 pagineLitium Bromide Water Absorption RefrigerationAnees FahimNessuna valutazione finora
- Example Kimia Fizik PDFDocumento32 pagineExample Kimia Fizik PDFMiaHusnaNessuna valutazione finora
- Mean Heat CapacityDocumento2 pagineMean Heat CapacityAlbert Ameka0% (1)
- Thermodynamics I: Energy Analysis of Closed SystemsDocumento19 pagineThermodynamics I: Energy Analysis of Closed SystemsManmit SinghNessuna valutazione finora
- Edexcel IAL Chemistry Unit2Documento33 pagineEdexcel IAL Chemistry Unit2Aliya K100% (1)
- Experiment 2 - CalorimetryDocumento4 pagineExperiment 2 - CalorimetryJHON MARK YANONGNessuna valutazione finora
- Module 5 - ThermochemistryDocumento11 pagineModule 5 - ThermochemistryKarel Grace ColotNessuna valutazione finora
- Adiabatic Flame TemperatureDocumento5 pagineAdiabatic Flame TemperatureRaghav SharmaNessuna valutazione finora
- 04 Deaktivasi Katalisis HeterogenDocumento44 pagine04 Deaktivasi Katalisis HeterogenNajwaAinayaZawaidNessuna valutazione finora
- Chemistry Unit 4 Part 3 ReallyacademicsDocumento35 pagineChemistry Unit 4 Part 3 ReallyacademicsWill AndyNessuna valutazione finora
- Class VI&VII: Heat EffectsDocumento31 pagineClass VI&VII: Heat EffectsChanakun KaewkhamsaenNessuna valutazione finora
- 50114a Isolemfi 50114a MonoDocumento2 pagine50114a Isolemfi 50114a MonoUsama AwadNessuna valutazione finora
- Government College of Nursing Jodhpur: Practice Teaching On-Probability Sampling TechniqueDocumento11 pagineGovernment College of Nursing Jodhpur: Practice Teaching On-Probability Sampling TechniquepriyankaNessuna valutazione finora
- Man As God Created Him, ThemDocumento3 pagineMan As God Created Him, ThemBOEN YATORNessuna valutazione finora
- Cable To Metal Surface, Cathodic - CAHAAW3Documento2 pagineCable To Metal Surface, Cathodic - CAHAAW3lhanx2Nessuna valutazione finora
- Pg2022 ResultDocumento86 paginePg2022 ResultkapilNessuna valutazione finora
- 40 People vs. Rafanan, Jr.Documento10 pagine40 People vs. Rafanan, Jr.Simeon TutaanNessuna valutazione finora
- Pityriasis VersicolorDocumento10 paginePityriasis Versicolorketty putriNessuna valutazione finora
- PDF Chapter 5 The Expenditure Cycle Part I Summary - CompressDocumento5 paginePDF Chapter 5 The Expenditure Cycle Part I Summary - CompressCassiopeia Cashmere GodheidNessuna valutazione finora
- Gas Dynamics and Jet Propulsion 2marksDocumento15 pagineGas Dynamics and Jet Propulsion 2marksAbdul rahumanNessuna valutazione finora
- Patrick Meyer Reliability Understanding Statistics 2010Documento160 paginePatrick Meyer Reliability Understanding Statistics 2010jcgueinj100% (1)
- 1 in 8.5 60KG PSC Sleepers TurnoutDocumento9 pagine1 in 8.5 60KG PSC Sleepers Turnoutrailway maintenanceNessuna valutazione finora
- LSCM Course OutlineDocumento13 pagineLSCM Course OutlineDeep SachetiNessuna valutazione finora
- Prevention of Waterborne DiseasesDocumento2 paginePrevention of Waterborne DiseasesRixin JamtshoNessuna valutazione finora
- QSP 04bDocumento35 pagineQSP 04bakrastogi94843Nessuna valutazione finora
- Unsuccessful MT-SM DeliveryDocumento2 pagineUnsuccessful MT-SM DeliveryPitam MaitiNessuna valutazione finora
- Cloud Comp PPT 1Documento12 pagineCloud Comp PPT 1Kanishk MehtaNessuna valutazione finora
- How To Block HTTP DDoS Attack With Cisco ASA FirewallDocumento4 pagineHow To Block HTTP DDoS Attack With Cisco ASA Firewallabdel taibNessuna valutazione finora
- India TeenagersDocumento3 pagineIndia TeenagersPaul Babu ThundathilNessuna valutazione finora
- Bolt Grade Markings and Strength ChartDocumento2 pagineBolt Grade Markings and Strength ChartGregory GaschteffNessuna valutazione finora
- Sept Dec 2018 Darjeeling CoDocumento6 pagineSept Dec 2018 Darjeeling Conajihah zakariaNessuna valutazione finora
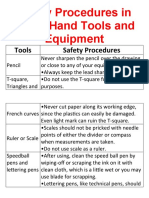
- Safety Procedures in Using Hand Tools and EquipmentDocumento12 pagineSafety Procedures in Using Hand Tools and EquipmentJan IcejimenezNessuna valutazione finora
- ყვავილები ელჯერნონისთვისDocumento348 pagineყვავილები ელჯერნონისთვისNia NorakidzeNessuna valutazione finora
- Lesson 6 - Vibration ControlDocumento62 pagineLesson 6 - Vibration ControlIzzat IkramNessuna valutazione finora
- Lecture 14 Direct Digital ManufacturingDocumento27 pagineLecture 14 Direct Digital Manufacturingshanur begulaji0% (1)
- SASS Prelims 2017 4E5N ADocumento9 pagineSASS Prelims 2017 4E5N ADamien SeowNessuna valutazione finora