Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
3875S2 02 Soreth
Caricato da
Carloos Semper0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
11 visualizzazioni20 paginejj
Titolo originale
3875S2_02_Soreth
Copyright
© © All Rights Reserved
Formati disponibili
PPT, PDF, TXT o leggi online da Scribd
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentojj
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PPT, PDF, TXT o leggi online su Scribd
0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
11 visualizzazioni20 pagine3875S2 02 Soreth
Caricato da
Carloos Semperjj
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PPT, PDF, TXT o leggi online su Scribd
Sei sulla pagina 1di 20
Development of Antibiotics for
Otitis Media:
Past, Present, and Future
Janice Soreth, M.D.
Director
Division of Anti-Infective Drug Products
Outline of the Past and Present
1977 Guidance
1992 Points-to-Consider Document
1992 IDSA/FDA Guidelines
1998 FDA Draft Guidance
Back to the Future?
tympanocentesis for all?
single v. multiple?
clinical-only studies?
placebo-controlled trials?
trial versus trials
Bottom line: What do we need, to know the
drug works and is safe for children with
otitis media?
Otitis Media:
Incidence & Epidemiology
25,000,000 visits for otitis media yearly in
the U.S.
accounts for 1 out of 3 pediatric office visits
by 1 year of age:
62% > 1 episode AOM; 17 % > 3
by 3 years of age:
80% > 1 episode AOM; 46 % > 3
categories: occasional AOM to otitis prone
1977 FDA Guidance on AOM
number of trials: not addressed
case definition: clinical evidence of AOM
(evidence of inflammation of tympanic
membrane and middle ear)
tympanocentesis: required in both studies at
baseline. Second tap desirable to obtain data on
MEF concentrations and promptness of
bacteriologic cure.
endpoints: both clinical and microbiologic
test of cure: not addressed; 4 weeks of follow-up
1977 FDA Guidance on AOM
(continued)
In the absence of culture of middle ear fluid,
no specific claim can be made regarding the
effectiveness of any anti-infective drug.
AOM in the 80s: What Happened
no new formal guidance on otitis from FDA
internal discussions on AOM centered on
requirement to perform tympanocentesis on
every child enrolled in a trial
procedure is difficult to do
too few are trained to do it
its hampering enrollment in trials
it costs too much
Is there a better way to design AOM trials,
and know whether a drug works or not?
1992 Points-to-Consider Document
on AOM
number of trials: two suggested
- clinical only study (no tympanocentesis at baseline) to
establish equivalence to an approved product
- one clinical/microbiologic study with tympanocentesis at
baseline
case definition: should be rigid
tympanocentesis: strongly encouraged in those
patients judged to be therapeutic failures
endpoints: clinical; clinical and microbiologic
test of cure: not specifically addressed
1992 Points-to-Consider Document
on AOM (continued)
The open micro study should establish
acceptable microbial and clinical outcome
in at least 25 patients with H. influenzae, in
at least 25 patients with S. pneumoniae, and
in at least 15 patients with M. catarrhalis.
1992 IDSA/FDA Guidelines on
AOM
number of trials: two suggested
- a micro study (100 patients)
- a comparative clinical trial (tap optional); double-blind
case definition: clinical criteria listed
tympanocentesis: tap required in those patients
who are not clinical successes (failure, relapse,
recurrence)
endpoints: clinical; clinical and microbiologic
test-of-cure: 1-2 weeks after completion of therapy
1997 & 1998 FDA Draft Guidance on AOM
number of trials: two suggested
- a micro study, non-comparative; increase numbers
- a comparative clinical trial
case definition: tighten, tighten, tighten
tympanocentesis: repeat tap at study day 3-5 as
critical measure of treatment efficacy; perform
tympanocentesis in all failures
endpoints: primary efficacy endpoints are clinical
cure at TOC and pathogen eradication.
1998 AOM Draft Guidance:
Advisory Committee Recommendations
With regard to the microbiologic endpoint:
1998: Tympanocentesis obtained at the on-therapy
visit should not be considered evidence of
documented eradication. Rather, a negative
culture result may represent antimicrobial
suppression.
2001: Tympanocentesis obtained on therapy should
be the primary microbiologic endpoint.
1998 AOM Draft Guidance:
Advisory Committee Recommendations 1998
(continued)
Enroll more patients < 2 years of age.
Gain much more experience with penicillin-
resistant organisms.
1998 AOM Draft Guidance:
Advisory Committee Recommendations
Increase the number of patients under 24
months of age
Enroll patients with ruptured TMs, history
of recurrent otitis, antibiotic prophylaxis
Include patients with recent AOM who have
failed a course of antibiotics
2001 AC Recommendations
Timing of assessment of clinical outcome:
primary endpoint should be end of therapy visit ;
late follow-up visit is an important secondary
endpoint
Would encourage follow-up as secondary with taps
for failures
2001 AC Recommendations
(continued)
Most informative tap:
Consensus was taps on therapy and at the
time of failure were needed
Cure rates should be bacteria specific such
that the cure rate exceeds the spontaneous
cure rate by some arbitrary amount, say 10
or 20%
2001 AC Recommendations (continued)
Clinical only and Micro studies:
Why would we want to do clinical only studies
anymore for this indication? No meaningful
efficacy data
With high spontaneous cure rate, what information
will be gained from them?
Dont see how can do efficacy studies without
knowing the organism
Therefore, tighten up inclusion criteria for clinical
only study
Clinical case definition for AOM
Experience tells us, AOM can be a difficult
call
Even when the inclusion criteria are tight,
and case report forms document presence of
specific objective criteria, there is a broad
range of culture-positive results for
investigators, from low to high
Back to the Future
clinical case definition: How strict is strict?
trial design considerations: tympanocentesis for
one and all?
baseline/on-therapy/at time of failure
endpoints and timing of assessments:
clinical-only studies
micro-based studies
non-inferiority v. superiority designs
placebo-controlled trials
spectrum of disease:
severity, chronicity
distinct patient populations
Back to the Future
Question of the day:
What do we need to know,
what constitutes substantial evidence,
that a novel antimicrobial drug works and
is safe for children with otitis media?
Potrebbero piacerti anche
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (400)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (345)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- Ortho - Prostho RelationshipDocumento40 pagineOrtho - Prostho RelationshipAshis Biswas100% (1)
- Case Studies in Nursing FundamentalsDocumento393 pagineCase Studies in Nursing FundamentalsBeverly Lavilla100% (1)
- CBAHI PresentationDocumento23 pagineCBAHI PresentationPrince Jhessie L. AbellaNessuna valutazione finora
- Haad Previous QPDocumento30 pagineHaad Previous QPArshad100% (1)
- Pathophysiology of IBDDocumento11 paginePathophysiology of IBDOktarina Heni SunandarNessuna valutazione finora
- FamorcaDocumento137 pagineFamorcaStephanie Villanueva Advincula88% (32)
- A Concise Guide To Endodontic Procedures (9783662437292)Documento198 pagineA Concise Guide To Endodontic Procedures (9783662437292)ava parham100% (2)
- Dermotologist PDFDocumento2 pagineDermotologist PDFankitNessuna valutazione finora
- KLP 5 - Nutrition Intake and MedicationDocumento4 pagineKLP 5 - Nutrition Intake and MedicationdzakyNessuna valutazione finora
- Moebius Syndrome: A Guide To UnderstandingDocumento8 pagineMoebius Syndrome: A Guide To Understandingterima kasihNessuna valutazione finora
- Clinical Research AssociateDocumento3 pagineClinical Research Associateapi-77229440Nessuna valutazione finora
- Haematolgy MDT WebDocumento2 pagineHaematolgy MDT WebbfdboiiNessuna valutazione finora
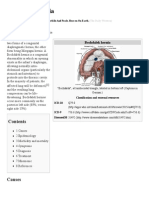
- Bochdalek Hernia - Wikipedia, The Free Encyclopedia PDFDocumento5 pagineBochdalek Hernia - Wikipedia, The Free Encyclopedia PDFMilda InayahNessuna valutazione finora
- In Memoriam Allan Cott, M.D. 1910-1993: Journal of Orthomolecular Medicine. He WasDocumento4 pagineIn Memoriam Allan Cott, M.D. 1910-1993: Journal of Orthomolecular Medicine. He WasSchwab RealHumanNessuna valutazione finora
- Research On Social Work Practice: PurposeDocumento10 pagineResearch On Social Work Practice: PurposeMAHESH KOUJALAGINessuna valutazione finora
- An Evidence-Based System For The Classification and ClinicalDocumento9 pagineAn Evidence-Based System For The Classification and ClinicalAna Maria Montoya GomezNessuna valutazione finora
- Jogi - Basic Ophthalmology, 4th Edition (Ussama Maqbool)Documento7 pagineJogi - Basic Ophthalmology, 4th Edition (Ussama Maqbool)pipikafiyaNessuna valutazione finora
- Quality Control of CT Image Using American College of Radiology (ACR) nd/4.0/)Documento8 pagineQuality Control of CT Image Using American College of Radiology (ACR) nd/4.0/)Md. Ashikur RahmanNessuna valutazione finora
- Abbott Alinity S FactSheetDocumento1 paginaAbbott Alinity S FactSheetInayat UllahNessuna valutazione finora
- Indian JOurnal of PediatricsDocumento8 pagineIndian JOurnal of Pediatricsliska ramdanawatiNessuna valutazione finora
- Convention PosterDocumento1 paginaConvention PosterShailesh RNessuna valutazione finora
- Pe103-Course-Guide-And-Module-Sem. 2022-2023Documento55 paginePe103-Course-Guide-And-Module-Sem. 2022-2023Thomas Danjo ManulatNessuna valutazione finora
- WEEK 8-PoemsDocumento5 pagineWEEK 8-Poemsjommel vargasNessuna valutazione finora
- PubLSIS II Survey Finding Report June 2018Documento620 paginePubLSIS II Survey Finding Report June 2018Mao udangNessuna valutazione finora
- Failure To Thrive With NotesDocumento45 pagineFailure To Thrive With NotesJason Jimmy Lee PillayNessuna valutazione finora
- QuestionnaireDocumento10 pagineQuestionnaireLyrad MagpantayNessuna valutazione finora
- Stress Testing of Drug SubstancesDocumento29 pagineStress Testing of Drug SubstancesMr. HIMANSHU PALIWALNessuna valutazione finora
- Ministry of Health Begins Recruitment For Barbados Nursing JobsDocumento2 pagineMinistry of Health Begins Recruitment For Barbados Nursing JobsKweku ZurekNessuna valutazione finora
- Jurnal SR 3Documento12 pagineJurnal SR 3Zaina Khairunisa AsyaNessuna valutazione finora
- PolioDocumento4 paginePolioShambhu NathNessuna valutazione finora