Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Synthesis of Steroid Hormones
Caricato da
Ming ZhaoTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Synthesis of Steroid Hormones
Caricato da
Ming ZhaoCopyright:
Formati disponibili
Synthesis of Steroid Hormones
Overview of Steroids
Peptide Hormone vs. Steroid Hormone Synthesis
The Role of Cholesterol
Adrenal Steroids
Steroids from the Testis
Ovarian Steroids
Cortisol
Steroid Hormones
Steroid hormones: produced in the adrenal
cortex, testis, ovary, and some peripheral tissues
(adipose tissue, the brain!)
All steroid hormones share a typical (but not
identical) ring structure.
Steroid hormones
All steroid hormones are derived from
cholesterol and differ only in the ring structure
and side chains attached to it.
All steroid hormones are lipid soluble
Types of steroid hormones
Glucocorticoids; cortisol is the major
representative in most mammals
Mineralocorticoids; aldosterone being most
prominent
Androgens such as testosterone
Estrogens, including estradiol and estrone
Progestogens (also known a progestins) such
as progesterone
Steroid hormones
Are not packaged, but synthesized and immediately
released
Are all derived from the same parent compound:
Cholesterol
Enzymes which produce steroid hormones from
cholesterol are located in mitochondria and smooth
ER
Steroids are lipid soluble and thus are freely
permeable to membranes so are not stored in cells
Steroid hormones
Steroid hormones are not water soluble so have to
be carried in the blood complexed to specific binding
globulins.
Corticosteroid binding globulin carries cortisol
Sex steroid binding globulin carries testosterone and
estradiol
In some cases a steroid is secreted by one cell and is
converted to the active steroid by the target cell: an
example is androgen which secreted by the gonad
and converted into estrogen in the brain
Steroids can be transformed to active
steroid in target cell
Steroid Hormones
Steroid hormones are nonpolar (no net charge), and
can thus diffuse across lipid membranes (such as the
plasma membrane). They leave cells shortly after
synthesis.
phospholipid
Polar substances are water soluble (dissolve in water),
nonpolar substances are lipid soluble.
Functions of Steroid Hormones
Steroid hormones play important roles in:
- carbohydrate regulation (glucocorticoids)
- mineral balance (mineralocorticoids)
- reproductive functions (gonadal steroids)
Steroids also play roles in inflammatory
responses, stress responses, bone metabolism,
cardiovascular fitness, behavior, cognition, and
mood.
How does the synthesis of steroids differ
from that of peptide hormones?
While peptide hormones are encoded by specific genes, steroid
hormones are synthesized from the enzymatic modification of
cholesterol.
Thus, there is no gene which encodes aldosterone, for example.
As a result:
- There are far fewer different types of steroid hormones
than peptide hormones.
- Steroid structures are the same from species to species
- The regulation of steroidogenesis involves control of the
enzymes which modify cholesterol into the steroid hormone of
interest.
The Role of Cholesterol in Steroid Synthesis
The first enzymatic step in the production of ANY
steroid hormone begins with enzymatic
modification of cholesterol
Sources of Cholesterol for Steroid Synthesis
Cholesterol can be made within the cell from acetyl CoA
(de novo synthesis).
This is a multistep process, involving many enzymatic
reactions.
A key rate-limiting enzyme is HMG-CoA reductase.
There is negative feedback regulation of HMG-CoA
reductase activity by cholesterol, so that high
intracellular cholesterol inhibits de novo synthesis.
acetyl CoA HMG-CoA mevalonate cholesterol
HMG-CoA reductase
Sources of Cholesterol for Steroid Synthesis
Cholesterol is also taken up by the cell in the form of
low density lipoprotein (LDL).
- LDL is a complex composed of cholesterol,
phospholipids, triglycerides, and proteins (proteins
and phospholipids make LDL soluble in blood).
- LDL is taken into cells via LDL receptors, and broken
down into esterified cholesterol, and then free
cholesterol:
LDL
receptor
LDL esterified cholesterol free cholesterol
The amount of free cholesterol in the cell is
maintained relatively constant:
Source of Cholesterol for Steroid Synthesis
steroid
synthesis
free
cholesterol
level
esterified cholesterol level
cellular synthesis
of cholesterol
LDL
Cellular Localization of Cholesterol
Metabolism for Steroid Production
The first enzymatic step in steroid synthesis is the
conversion of cholesterol into pregnenolone.
The enzyme that catalyzes this reaction is located
in the inner mitochondrial membrane.
Steroidogenic Enzymes
Common name
"Old" name
Current name
Side-chain cleavage enzyme;
desmolase
P450
SCC
CYP11A1
3 beta-hydroxysteroid
dehydrogenase
3 beta-HSD
3 beta-HSD
17 alpha-hydroxylase/17,20 lyase
P450
C17
CYP17
21-hydroxylase
P450
C21
CYP21A2
11 beta-hydroxylase
P450
C11
CYP11B1
Aldosterone synthase
P450
C11AS
CYP11B2
Aromatase
P450
aro
CYP19
Steroid hormone synthesis
All steroid hormones are derived from cholesterol.
A series of enzymatic steps in the mitochondria
and ER of steroidogenic tissues convert
cholesterol into all of the other steroid hormones
and intermediates.
The rate-limiting step in this process is the
transport of free cholesterol from the cytoplasm
into mitochondria. This step is carried out by the
Steroidogenic Acute Regulatory Protein (StAR)
Steroid hormone synthesis
The cholesterol precursor comes from cholesterol
synthesized within the cell from acetate, from
cholesterol ester stores in intracellular lipid
droplets or from uptake of cholesterol-containing
low density lipoproteins.
Lipoproteins taken up from plasma are most
important when steroidogenic cells are chronically
stimulated.
cholesterol
Extracellular
lipoprotein
Cholesterol
pool
LH
ATP
cAMP
PKA+
Pregnenolone
Progesterone
Androstenedione
TESTOSTERONE
3bHSD
P450c17
17bHSD
acetate
Functions of Hormones
Derived from Cholesterol
Product Functions
Progesterone prepares uterus lining for
implantation of ovum
Glucocorticoids (cortisol)
(produced in adrenal cortex)
(catabolic steroid)
promote gluconeogenesis;
favor breakdown of fat and
protein (fuel mobilization);
anti-inflammatory
Mineralocorticoids
(aldosterone) (produced in
adrenal glands)
maintains blood volume and
blood pressure by increasing
sodium reabsorption by kidney
Androgens (strongest = testosterone)
(produced in testes primarily but weak
androgens in adrenal cortex) (anabolic
steroid)
development of male
secondary sex
characteristics; prevents
bone resorption
Estrogen
(produced in ovaries primarily but also
in adipose cells of males and females)
development of female
secondary sex
characteristics; prevents
bone resorption
Vitamin D (not a steroid hormone)
(produced in the skin in response to
UV light and processed to active form
in kidney)
intestinal calcium
absorption; promotes
bone formation; prevents
phosphate loss by
kidneys
Product Functions
Functions of Hormones
Derived from Cholesterol
activated to
turn
on pathways
Cholesterol
Pregnenolon
e
Progesterone
11-Deoxy-
cortisone
21-hydroxylase
Progesterone
Aldosterone
General pathways for the synthesis of aldosterone and
cortisol in the adrenal cortex
11-Deoxy-
cortisol
Progesterone
Cortisol
21-hydroxylase
Pathway for formation of androgens in the adrenal cortex.
Beware of the hype about taking DHEA
Cortisol made in same cells as androstenedione
Pregnenolone DHEA Androstenedione
Andro
Androstenedion
e
Estrone
(produced in both male
and female adipose cells)
Pathways for the synthesis of testosterone (testes) and the
estrogens estradiol (ovaries) and estrone (adipose cells)
Cholesterol
Pregnenolone Progesterone
Progesterone Androstenedione Testosterone
(pathway ends
here in testes)
Estradiol
(pathway continues
to here in ovaries)
In obese men, overproduction of estrogen in fat cells
can cause gynecomastia = excessive male breast
development
HO
Vitamin D
3
Diet
HO
OH
25(OH) D
3
Liver
25-OHase
OH
HO
OH
1,25(OH)
2
D
3
(active hormone form)
Kidney
1-OHase
HO
7
Provitamin D
3
(7-dehydrocholesterol:
Intermediate in cholesterol
synthesis)
UV from
sunlight
Skin
Photobiosynthesis of vitamin D
3
and its metabolism
Specific receptors in
intestine, bone, kidney
Ca:
Intestinal absorption
Renal reabsorption
PO
4
:
Intestinal absorption
Renal reabsorption
OHase =
hydroxylase
1,25-Dihydroxy Vitamin D3
1,25-dihydroxy Vitamin D3 is also derived
from cholesterol and is lipid soluble
Not really a vitamin as it can be synthesized
de novo
Acts as a true hormone
Adrenal Cortex: Steroid Hormone
Production
Aldosterone, sex hormones, cortisol
Synthesized from cholesterolsteroid ring
Adrenal Cortex: Steroid Hormone Production
Transport of Cholesterol
Cholesterol is lipid soluble, and mostly located
associated with the external mitochondrial membrane.
The conversion of cholesterol to steroids occurs in the
internal mitochondrial membrane.
Now, to see if you have been paying attention
How does cholesterol get from the external membrane
to the internal membrane?
Answer: Steroidogenic acute regulatory protein (StAR),
which transports cholesterol into the mitochondria,
moving it from the outer membrane to the inner
membrane.
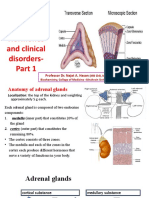
Adrenal Steroids
The adrenal glands are located immediately
superior to the kidneys.
There are three classes of adrenal steroids:
- mineralocorticoids,
- glucocorticoids, and
- androgens
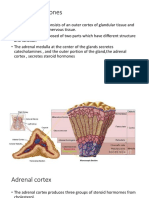
Organization of the Adrenal Gland
There is an adrenal cortex and adrenal medulla.
Steroids are made in three zones of the adrenal cortex:
mineralocorticoids: zona glomerulosa
glucocorticoids: zona fasciculata
androgens: zona reticularis
(Whats made in the adrenal medulla??)
Adrenal Steroidogenesis
The first enzymatic step is the conversion of cholesterol to
pregnenolone, which occurs in the mitochondria.
This reaction is carried out by the enzyme, cytochrome P450
side-chain cleavage (P450scc; also called desmolase, or
CYP11A1).
This is a rate limiting, nonreversible step in the initiation of
steroid biosynthesis.
This step occurs in adrenal, ovary, and testis.
Adrenal Steroidogenesis
Next, pregnenolone can be converted into three
different pathways, depending upon whether you
want to make mineralcorticoids, glucocorticoids, or
androgens:
pregnenolone 17a-hydroxypregnenolone dehydroepiandrosterone
progesterone androstenedione
glucocorticoids
mineralocorticoids (cortisol)
(aldosterone)
17a-hydroxylase
lyase
3b-hydroxysteroid dehydrogenase
21-hydroxylase
11b-hydroxylase
18 hydroxylase/oxidase
Adrenal Steroidogenesis
What determines which pathway is taken?
Each step of the pathway is regulated by a specific
enzyme.
Different zones of the adrenal cortex have different
relative activities of enzymes, resulting in different
chemical reactions taking place.
These enzymes are located in the smooth ER.
In the adrenal, you do NOT have to learn the names of these
enzymes. You DO have to understand what hormones are
produced, where they are produced, and why they are
produced there.
Production of Steroids in the Testis
The main steroid produced in the male is
testosterone, from the testis. In addition, the
testis makes some androstenedione,
dihydrotestosterone, and estradiol.
In the male, there is peripheral conversion of
testosterone to dihydrotestosterone (in androgen
target tissues, like muscle) and estradiol (mostly
in adipose tissue).
Organization of the Testis
The testis is organized into two main parts:
- seminiferous tubules: production of sperm cells,
location of Sertoli cells (stay tuned...)
- interstitial tissue: outside of the seminiferous
tubules; the steroidogenic cell is the Leydig cell
Function of Leydig Cells
Leydig cells: respond to luteinizing hormone (LH)
with steroid production (primarily testosterone).
Leydig cells are unusual in that they rely on de
novo synthesis of cholesterol more than other
cells (50%). Thus, only about 50% of cholesterol
used in steroid production is obtained from LDL.
The production of androgens from cholesterol is
identical to that in the adrenal, except that it
continues from androstenedione to testosterone.
Pathway of Testosterone Production
in the Testis
androstenedione
testosterone
17b-hydroxysteroid
oxidoreductase
Testosterone Metabolism
Testosterone can then be converted (mostly in
peripheral tissues) to:
- DHT (dihydrotestosterone) by 5a-reductase, or to
-estradiol (E2) by cytochrome P450 aromatase
Important Note
Since the enzymes/pathways for producing androgens
and estrogens are utilized in adrenal, testis, and ovary,
you will be expected to know the names of these
enzymes, and their role (example; know that 3b-HSD
converts pregnenolone into progesterone).
You are NOT responsible for drawing the structures of
these steroids. (You should recognize the typical ring
structure when you see it, however).
Also, know that LH stimulates Leydig cell
steroidogenesis.
Ovarian Steroidogenesis
The ovary produces estrogens (primarily
estradiol), progesterone, and androgens.
It relies largely on LDL as a source of cholesterol
for steroid synthesis (compare with testis).
Ovarian steroids are secreted primarily from
ovarian follicles and corpora lutea.
Ovarian Follicle
The follicle is the basic functional unit of the ovary.
It is composed of an oocyte, granulosa cells, and theca
cells.
When the follicle ruptures, it becomes a corpus luteum.
The Puzzle of Estrogen Production in the
Ovary
In the ovary, estradiol is formed from the conversion
of testosterone into estradiol by the enzyme
cytochrome P450 aromatase. This occurs in
granulosa cells.
However, granulosa cells do not have the enzyme
17a-hydroxylase/lyase, and thus cannot convert
progesterone into androgens.
Where do the androgens required for estrogen
production in granulosa cells come from?
The Two-Cell Theory of Estrogen Production
in the Ovary
Numerous studies have now shown that the
androgens required for aromatization come from the
neighboring theca cells:
theca cell granulosa cell
LH
LH receptor
cholesterol
androgens androgens
FSH
aromatase
estradiol
Other Steroid Production in the Ovary
After ovulation, the corpus luteum produces
progesterone and estradiol, to support the uterine
endometrium during pregnancy.
Progesterone is also produced from theca cells and
granulosa cells.
Regulation of Ovarian Steroidogenesis
The rate of estradiol production from follicles varies
greatly during the menstrual cycle.
Estradiol production is regulated by the effects of FSH on
P450 aromatase.
Similarly, LH and FSH influence the expression of P450scc
in granulosa cells. This increases production of which
gonadal steroid?
cholesterol pregnenolone progesterone
P450scc 3b-HSD
Cortisol Effects: Body Responses to Stress
Permissive effect on glucagon
Memory, learning and mood
Gluconeogenesis
Skeletal muscle breakdown
Lipolysis, calcium balance
Immune depression
Circadian rhythms
Cortisol Effects: Body Responses to Stress
Figure 23-4: Circadian rhythm of cortisol secretion
Control of Cortisol Secretion: Feedback Loops
External stimuli
Hypothalamic
Anterior Pituitary
Adrenal cortex
Tissues
Figure 23-3: The control pathway for cortisol
Cortisol: Role in Diseases and Medication
Use as immunosuppressant
Hyperimmune reactions (bee stings)
Serious side effects
Hypercortisolism (Cushing's syndrome)
Tumors (pituitary or adrenal)
Iatrogenic (physician caused)
Hypocortisolism (Addison's disease)
Steroid Hormones: Characteristics
Are made from cholesterol, are lipophilic & can
enter target cell
Are immediately released from cell after
synthesis
Interact with cytoplasmic or nuclear receptors
Activate DNA for protein synthesis
Are slower acting and have longer half-life than
peptide hormones
Examples: cortisol, estrogen & testosterone
Steroid Hormones: Review the Structure
Steroid Hormones: Molecular Action
Potrebbero piacerti anche
- What Women Want - What Men WantDocumento300 pagineWhat Women Want - What Men Wanta1954908100% (13)
- 010 - CAT-6015 - RH40E - Swing SystemDocumento24 pagine010 - CAT-6015 - RH40E - Swing SystemJHONNYS ALFONSO MANJARREZ GUTIERREZNessuna valutazione finora
- Chrysler Scan Tool Flash AvailabilityDocumento733 pagineChrysler Scan Tool Flash AvailabilityAlbert0% (1)
- A-level Biology Revision: Cheeky Revision ShortcutsDa EverandA-level Biology Revision: Cheeky Revision ShortcutsValutazione: 5 su 5 stelle5/5 (5)
- 2004 Polaris Parts ManualDocumento67 pagine2004 Polaris Parts ManualAngela TullochNessuna valutazione finora
- SteroidsDocumento12 pagineSteroidsSwapnil DabholkarNessuna valutazione finora
- Anatomy of Upper LimbDocumento112 pagineAnatomy of Upper LimbUzma KhanNessuna valutazione finora
- Central Nervous SystemDocumento25 pagineCentral Nervous System786waqar786100% (1)
- Endocrine System 2Documento50 pagineEndocrine System 2park jongseongNessuna valutazione finora
- GE 250 Engines CatalogueDocumento2 pagineGE 250 Engines Cataloguesarvin silvarajo100% (1)
- Fallout PNP 4.0 Character SheetDocumento9 pagineFallout PNP 4.0 Character SheetKulakNessuna valutazione finora
- Basics of Knee Arthroscopy: For The BeginnersDocumento40 pagineBasics of Knee Arthroscopy: For The BeginnersPedro Zambrano100% (1)
- Cholesterol Synthesis, Transport, and ExcretionDocumento37 pagineCholesterol Synthesis, Transport, and ExcretionEdo Pramana Putra100% (2)
- Steroid Hormone-SynthesisDocumento40 pagineSteroid Hormone-SynthesisAbubakar SuleimanNessuna valutazione finora
- Steroid SynthesisDocumento54 pagineSteroid SynthesisMohammed Mansour AbdullahNessuna valutazione finora
- Synthesis of Steroid HormonesDocumento54 pagineSynthesis of Steroid HormonesfanyazharNessuna valutazione finora
- Steroid HormDocumento40 pagineSteroid HormleNessuna valutazione finora
- 3-Adrenal Glands and Its Hormones-MWDocumento103 pagine3-Adrenal Glands and Its Hormones-MWAbditsion DhiisaniiNessuna valutazione finora
- 1 - Biokimia ReproduksiDocumento56 pagine1 - Biokimia ReproduksiTarissa Kartika EdwinNessuna valutazione finora
- 10 The Adrenal Gland Aldosterone2017 1Documento27 pagine10 The Adrenal Gland Aldosterone2017 1Hassan AhmedNessuna valutazione finora
- Anatomy and Physiology of Adrenal GlandsDocumento4 pagineAnatomy and Physiology of Adrenal Glandsxxxcamzxxx100% (3)
- GonadsDocumento26 pagineGonadsserenityaroundNessuna valutazione finora
- 10 The Adrenal Gland Aldosterone2017 1Documento27 pagine10 The Adrenal Gland Aldosterone2017 1Tariq Jamil KoraiNessuna valutazione finora
- Adrenal GlandDocumento32 pagineAdrenal GlandAlok KumarNessuna valutazione finora
- Cholesterol: by Arooj Fatima Roll Number:MCHF18M028Documento23 pagineCholesterol: by Arooj Fatima Roll Number:MCHF18M028ماہ نور فاطمہNessuna valutazione finora
- Adrecorticol Hormones and AntagonistsDocumento59 pagineAdrecorticol Hormones and AntagonistsFavourNessuna valutazione finora
- Cholesterol SynthesisDocumento19 pagineCholesterol Synthesisbrian mgabiNessuna valutazione finora
- Steroid HormonesDocumento16 pagineSteroid HormonesRishiNessuna valutazione finora
- SteroidsDocumento22 pagineSteroidsrashid_ahmed_rajputNessuna valutazione finora
- Endocrinologi Part 2 2015Documento74 pagineEndocrinologi Part 2 2015Annisa Tria FadillaNessuna valutazione finora
- 3010 Adrenal and Parathyroid HormonesDocumento21 pagine3010 Adrenal and Parathyroid HormonesDenesha KaurNessuna valutazione finora
- Lipids NotesDocumento5 pagineLipids NotesnofacejackNessuna valutazione finora
- Cholesterol: Synthesis and MetabolismDocumento45 pagineCholesterol: Synthesis and MetabolismSoffa ShmuelNessuna valutazione finora
- Coordination of Organ Functioning by Steroid Hormones. Tissue HormonesDocumento47 pagineCoordination of Organ Functioning by Steroid Hormones. Tissue HormonesPurwa RaneNessuna valutazione finora
- Biosynthesisofsteroidhormone Repro Kki08Documento24 pagineBiosynthesisofsteroidhormone Repro Kki08REG A44Safira Ulfi YusniaNessuna valutazione finora
- Adrenal Gland1Documento27 pagineAdrenal Gland1Rasel IslamNessuna valutazione finora
- Endocrine System Part 2Documento44 pagineEndocrine System Part 2Tharushi PereraNessuna valutazione finora
- Adrenal Gland: Structure, Hormones and Clinical Disorders-: Professor Dr. Najat A. HasanDocumento18 pagineAdrenal Gland: Structure, Hormones and Clinical Disorders-: Professor Dr. Najat A. HasanDrFarah Emad Ali100% (1)
- Endokrinologi: Zulkhah NoorDocumento121 pagineEndokrinologi: Zulkhah NoorShafiraNessuna valutazione finora
- Chapter 5 Endocrinology 1Documento53 pagineChapter 5 Endocrinology 1Abubakar JallohNessuna valutazione finora
- Adrenocortical HormonesDocumento77 pagineAdrenocortical HormonesMaxamed Faarax XaashiNessuna valutazione finora
- Adrenal Cortex HormonesDocumento21 pagineAdrenal Cortex HormonesAaa JjjjNessuna valutazione finora
- BCH 206 CholesterolDocumento3 pagineBCH 206 CholesterolNewtonNessuna valutazione finora
- Lec 6 Fed State PDFDocumento52 pagineLec 6 Fed State PDFrajeshNessuna valutazione finora
- Endocrine SystemDocumento15 pagineEndocrine SystemEcho MoralesNessuna valutazione finora
- Bio Chem M.phil PresentationDocumento37 pagineBio Chem M.phil Presentationknaina507Nessuna valutazione finora
- 1.adrenal Gland AnatomyDocumento60 pagine1.adrenal Gland AnatomyAstha ShresthaNessuna valutazione finora
- 9 Male Sex HormonesDocumento22 pagine9 Male Sex HormonesRana AbdullahNessuna valutazione finora
- Adrenal HormonesDocumento62 pagineAdrenal HormonesM.PRASAD NAIDUNessuna valutazione finora
- Regulation of Fatty Acid Synthesis and Steroid Hormone SynthesisDocumento35 pagineRegulation of Fatty Acid Synthesis and Steroid Hormone SynthesisKundaNessuna valutazione finora
- Hormon AdrenalDocumento76 pagineHormon AdrenalgitafazaNessuna valutazione finora
- Steroids AND TerpenesDocumento88 pagineSteroids AND Terpenesbuttercuplady_18763Nessuna valutazione finora
- Module 22 HormonesDocumento23 pagineModule 22 HormonesCrystal ManguneNessuna valutazione finora
- Introduction - Functions of Aldosterone and GlucocorticoidsDocumento19 pagineIntroduction - Functions of Aldosterone and GlucocorticoidssabaNessuna valutazione finora
- Biosynthesis of CholesterolDocumento20 pagineBiosynthesis of CholesterolAboubakar Moalim Mahad moh'dNessuna valutazione finora
- Group 2 Endocrine System Questions 5 - 7Documento35 pagineGroup 2 Endocrine System Questions 5 - 7Marcus KaglNessuna valutazione finora
- Synthesis and Function of Steroid HormonesDocumento36 pagineSynthesis and Function of Steroid HormonesIdris LubisNessuna valutazione finora
- Metabolism of CholesterolDocumento17 pagineMetabolism of CholesterolAvhishek PatelNessuna valutazione finora
- Cholesterol Synthesis, Transport, and Excretion: Abdul Salam M Sofro Faculty of Medicine YARSI UniversityDocumento39 pagineCholesterol Synthesis, Transport, and Excretion: Abdul Salam M Sofro Faculty of Medicine YARSI UniversitySofie Hanafiah NuruddhuhaNessuna valutazione finora
- Structure and FunctionsDocumento4 pagineStructure and FunctionsErnesto RodríguezNessuna valutazione finora
- Lipid Metabolism Cholesterol Derived Products & Bile AcidDocumento22 pagineLipid Metabolism Cholesterol Derived Products & Bile Acidvivek sNessuna valutazione finora
- Lecture 35 Cholesterol and Steroids - AHDocumento18 pagineLecture 35 Cholesterol and Steroids - AHغالية الزهرانيNessuna valutazione finora
- Derivatives of CholesterolDocumento13 pagineDerivatives of Cholesterolpratyay gangulyNessuna valutazione finora
- Endocrine Glands and HormonesDocumento46 pagineEndocrine Glands and Hormonesfisherp1Nessuna valutazione finora
- Adrenal GlandDocumento50 pagineAdrenal GlandAnita YadavNessuna valutazione finora
- Hormones of The GonadsDocumento17 pagineHormones of The GonadsSophia AgenyiNessuna valutazione finora
- Adrenal GlandDocumento36 pagineAdrenal Glandpranutan739Nessuna valutazione finora
- Lipid Metabolism - (V) - Cholesterol and LipoproteinsDocumento35 pagineLipid Metabolism - (V) - Cholesterol and Lipoproteinslightning proNessuna valutazione finora
- 2 - Motivations of InnovationDocumento2 pagine2 - Motivations of InnovationMing ZhaoNessuna valutazione finora
- Testing DiagramDocumento1 paginaTesting DiagramMing ZhaoNessuna valutazione finora
- Testing DiagramDocumento1 paginaTesting DiagramMing ZhaoNessuna valutazione finora
- 4 - Disruptive Trend ResponseDocumento1 pagina4 - Disruptive Trend ResponseMing ZhaoNessuna valutazione finora
- Instructors Manual PDFDocumento298 pagineInstructors Manual PDFMing ZhaoNessuna valutazione finora
- 2 EcosystemsDocumento2 pagine2 EcosystemsMing ZhaoNessuna valutazione finora
- 1 - Hind SwarajDocumento4 pagine1 - Hind SwarajMing ZhaoNessuna valutazione finora
- LEHE6HF2343Documento13 pagineLEHE6HF2343Ming ZhaoNessuna valutazione finora
- Partek TutorialDocumento28 paginePartek TutorialMing ZhaoNessuna valutazione finora
- Energy DarwinismDocumento132 pagineEnergy DarwinismMing ZhaoNessuna valutazione finora
- Disruptive InnovationsDocumento72 pagineDisruptive InnovationsMing ZhaoNessuna valutazione finora
- Summer Handbook NIHDocumento45 pagineSummer Handbook NIHMing ZhaoNessuna valutazione finora
- Vox Populi ArticleDocumento48 pagineVox Populi ArticleMing ZhaoNessuna valutazione finora
- The Future of EmploymentDocumento72 pagineThe Future of EmploymentAlfredo Jalife RahmeNessuna valutazione finora
- Manual For Ming'S Program Files 9/1/14Documento6 pagineManual For Ming'S Program Files 9/1/14Ming ZhaoNessuna valutazione finora
- SummaryDocumento4.756 pagineSummaryMing ZhaoNessuna valutazione finora
- What Do You Wish To Do in The Rest of Your Time at Columbia? What Is One Thing You Would Redo About The Last Four Years?Documento12 pagineWhat Do You Wish To Do in The Rest of Your Time at Columbia? What Is One Thing You Would Redo About The Last Four Years?Ming ZhaoNessuna valutazione finora
- What Is Applied MathDocumento2 pagineWhat Is Applied MathMing ZhaoNessuna valutazione finora
- What Is Applied MathDocumento2 pagineWhat Is Applied MathMing ZhaoNessuna valutazione finora
- Sustainability 02 01055Documento25 pagineSustainability 02 01055Ming ZhaoNessuna valutazione finora
- Gandhi PaperDocumento5 pagineGandhi PaperMing ZhaoNessuna valutazione finora
- Sdev Gis Assignment 1Documento2 pagineSdev Gis Assignment 1Ming ZhaoNessuna valutazione finora
- HW 6 DiaryDocumento5 pagineHW 6 DiaryMing ZhaoNessuna valutazione finora
- Written Matlab 3Documento2 pagineWritten Matlab 3Ming ZhaoNessuna valutazione finora
- 5-27 - 5-30Documento1 pagina5-27 - 5-30Ming ZhaoNessuna valutazione finora
- Ming Zhao Writing Sample Social Robotics ReviewDocumento5 pagineMing Zhao Writing Sample Social Robotics ReviewMing ZhaoNessuna valutazione finora
- Schedule Fall 2012Documento1 paginaSchedule Fall 2012Ming ZhaoNessuna valutazione finora
- Ntroduction TO Sychology: Spring 2014Documento51 pagineNtroduction TO Sychology: Spring 2014Ming ZhaoNessuna valutazione finora
- Midterm NotesDocumento1 paginaMidterm NotesMing ZhaoNessuna valutazione finora
- Perk Chart PDFDocumento6 paginePerk Chart PDFAlkarsilverbladeNessuna valutazione finora
- Trans Atm AvanzaDocumento21 pagineTrans Atm AvanzasutiknoNessuna valutazione finora
- DVDs Musicales - Rock, Jazz, BluesDocumento28 pagineDVDs Musicales - Rock, Jazz, BluesgatochaletNessuna valutazione finora
- Tennis Industry MagazineDocumento64 pagineTennis Industry MagazineLiya DavidovNessuna valutazione finora
- A Health and Safety Guideline For Your Workplace - Manual Materials HandlingDocumento6 pagineA Health and Safety Guideline For Your Workplace - Manual Materials HandlingJames UgoNessuna valutazione finora
- Unit 4: Games and Sports: West Visayas State University - 2020Documento45 pagineUnit 4: Games and Sports: West Visayas State University - 2020Allysa Marie SilbolNessuna valutazione finora
- BGD - Manual EN v1.3 - REF 1657881885Hh2A5kXAh2 3Documento44 pagineBGD - Manual EN v1.3 - REF 1657881885Hh2A5kXAh2 3Eduardo SSNessuna valutazione finora
- DecathlonDocumento2 pagineDecathlonNeenaNessuna valutazione finora
- Feats For Skills - GM BinderDocumento3 pagineFeats For Skills - GM BinderJay McKearnNessuna valutazione finora
- DCP l6600 PDFDocumento54 pagineDCP l6600 PDFStefanGarnetNessuna valutazione finora
- Cement - Mortar: Apparatus For Lime Testing Reactivity Air Content Meter 1 Litre CapacityDocumento1 paginaCement - Mortar: Apparatus For Lime Testing Reactivity Air Content Meter 1 Litre CapacityLESO IndustrialNessuna valutazione finora
- Running Jumping Javelin Throw Shotput Long Jump Sports Events InformationDocumento3 pagineRunning Jumping Javelin Throw Shotput Long Jump Sports Events InformationJasvinder SinghNessuna valutazione finora
- Pump AdaptorsDocumento2 paginePump AdaptorsDragan LazicNessuna valutazione finora
- Cotton Seed Basic Lot DispDocumento18 pagineCotton Seed Basic Lot DispSAWERA TEXTILES PVT LTDNessuna valutazione finora
- Soporte de Cardan 05Documento15 pagineSoporte de Cardan 05jyapias_1Nessuna valutazione finora
- 1 Parameter Floor (1-20)Documento4 pagine1 Parameter Floor (1-20)Papan SarkarNessuna valutazione finora
- IKC 4 Rule BookDocumento24 pagineIKC 4 Rule BookJee RyNessuna valutazione finora
- Código Descrição Preço Ash / LuporiniDocumento5 pagineCódigo Descrição Preço Ash / LuporiniHomero TeixeiraNessuna valutazione finora
- Sompo - Authorised WorkshopsDocumento2 pagineSompo - Authorised WorkshopsWan Ah-LunNessuna valutazione finora
- Your Bentley Bentayga V8: PresentingDocumento9 pagineYour Bentley Bentayga V8: PresentingThomas SeiferthNessuna valutazione finora
- Senarai Nama PemainDocumento1 paginaSenarai Nama PemaineddyputriNessuna valutazione finora
- Stratego : The Classic Game of Battlefield StrategyDocumento8 pagineStratego : The Classic Game of Battlefield Strategylionheart!Nessuna valutazione finora