Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Heterogeneous Catalysis Reaction Kinetics
Caricato da
Fhan Sani SeowDescrizione originale:
Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Heterogeneous Catalysis Reaction Kinetics
Caricato da
Fhan Sani SeowCopyright:
Formati disponibili
REACTION KINETICS OF
HETEROGENEOUS CATALYSIS
BKF3472
Chemical Reaction Engineering
II
1
TOPIC OUTCOME
Define a catalyst, a catalytic mechanism and a rate limit
step.
Describe the steps in a catalytic mechanism and how one
goes about deriving a rate law and a mechanism and rate
limiting step consistent with the experimental data.
Use Regression to discriminate between reaction rate laws
and mechanisms.
Size isothermal reactors for reactions with Langmuir-
Hinshelwood kinetics.
Discuss the different types of catalyst deactivation and the
reactor types and describe schemes that can help offset the
deactivation.
Analyze catalyst decay and conversion for CSTRs and
PFRs with temperature-time trajectories, moving bed
reactors, and straight through transport reactors.
2
WHAT IS CATALYST?
Definition of catalyst
A substance that affects the rate of a reaction but
comes out from the process unchanged
History
More than 2000 years ago; in wine, cheese and
bread; Berzelius works expanded by Ostwal.
Small amount of catalyst is used.
3
WHAT IS CATALYST?
Efficiency depends on activity, properties & life
of the catalyst
Examples:
Ammonia synthesis Promoted iron
SO
2
oxidation Vanadium Pentaoxide
Cracking Silica, alumina
Dehydrogenation Platinum, Molybdenum
4
Every catalytic reaction is a sequence of elementary steps,
in which reactant molecules bind to the catalyst, where
they react, after which the product detaches from the
catalyst, liberating the latter for the next cycle.
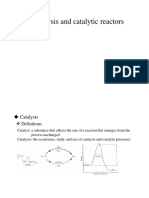
5
Potential energy diagram of a heterogeneous catalytic
reaction, with gaseous reactants and products and a solid
catalyst. Note that the uncatalyzed reaction has to overcome a
substantial energy barrier, whereas the barriers in the catalytic
route are much lower.
6
Classification:
Homogenous catalysis
Heterogeneous catalysis
Catalysts are generally used to:
Speedup reactions
Change the operating temperature level
Influence the product distribution
Catalyst affect yield and selectivity
Changes only the rate of reaction; it does not affect the
equilibrium.
7
Homogeneous:
Catalytic:
At 600K the ratio of catalytic to homogeneous rate is
1.44x10
11
Example: Boudart compared the homogeneous versus
catalytic rates of ethylene hydrogenation.
2
43000
exp 10
27
H
p
RT
r
|
.
|
\
|
=
2
13000
exp 10 2
27
H
p
RT
x r
|
.
|
\
|
=
8
WHAT IS CATALYST?
Alters the rate of reaction
Highly selective
Does it participate in the reaction ??
How does it change the rate ?? Offers an
alternate path with low E.
Does it affect AH
R
, AG
R
, and Eq. constant ??
Does it affect yield & selectivity ??
Does it initiate a reaction ??
9
HETEROGENEOUS CATALYSIS
10
HETEROGENEOUS CATALYTIC PROCESS
Involve more than one phase; usually the
catalyst is a solid and the reactants and
products are in liquid or gaseous form.
Examples:
AlCl
3
used in alkylation and dealkylation.
Pt used in isomerization reactions,
hydrogenation and dehydrogenation reactions.
11
Ostwald Process
Hot Pt wire
over NH
3
solution
Pt-Rh catalysts used
in Ostwald process
4NH
3
(g) + 5O
2
(g) 4NO (g) + 6H
2
O (g)
Pt catalyst
2NO (g) + O
2
(g) 2NO
2
(g)
2NO
2
(g) + H
2
O (l) HNO
2
(aq) + HNO
3
(aq)
13.6
Catalytic Converters
CO + Unburned Hydrocarbons + O
2
CO
2
+ H
2
O
catalytic
converter
2NO + 2NO
2
2N
2
+ 3O
2
catalytic
converter
POROUS STRUCTURE
FINE PORES ---> AREA NEEDED FOR THE HIGH RATE OF
REACTION
e.g SILICA-ALUMINA CRACKING CATALYST HAS A PORE
VOLUME OF 0.6 cm
3
/g (pore radius 4 nm) ---> 300 m
2
/g
MOLECULAR SIEVE --> small pores admit small molecules
but prevent large molecules
e.g Clay, zeolite (natural); aluminosilicates (synthetic)
CATALYST PROPERTIES
14
CATALYST PROPERTIES
SUPPORTED CATALYSTS
active material dispersed over a less active substance
UNSUPPORTED CATALYSTS
mainly promoters that increase activity
DEACTIVATION decline of activity as time progress
Aging (gradual change in surface crystal structure)
Poisoning or Fouling (deposit of foreign material) e.g
coking
15
Promoter: is an additive which has no catalytic
properties of its own but enhances the activity of a
catalyst
Promoter results in:
Increase of available surface area
Stabilization against crystal growth and
sintering
Improvement of mechanical strength
Examples: Alumina, Asbestos
CATALYST PROPERTIES
16
Carrier: principally serve as a framework on which
catalyst is deposited - no catalytic properties of its
own
Carrier results in:
Highly porous nature - increase of available
surface area
Improve stability
Improves the heat transfer characteristics
Examples: Alumina, Asbestos, Carborundum, Iron
oxide, Manganese, Activated carbon, Zinc oxide
CATALYST PROPERTIES
17
Accelerator: are substances which can be added to
a reacting system to maintain the activity of a
catalyst by nullifying the effects of poisons
Poisons: substances which reduce the activity of a
catalyst. They are not deliberately added but are
unavoidably deposited during the reaction.
Examples: Sulfur, Lead, Metal ions such as Hg,
Pd, Bi, Sn, Cu, Fe etc.
CATALYST PROPERTIES
18
Inhibitor: substances added to the catalyst during its
manufacture to reduce its activity.
Coking/Fouling: deposition of carbonaceous material
on the surface of the catalyst - Common to
reactions involving hydrocarbons
CATALYST PROPERTIES
19
Activity: of a catalyst depends on the texture and
electronic structure. Activity of a catalyst can be
explained by:
Active centers on the surface of the catalyst
Geometry of surface
Electronic structure
Formation of surface intermediates
CATALYST PROPERTIES
20
Active site: is a point on the catalyst surface that can
form strong chemical bonds with an adsorbed
atom/molecule. These sites are unsaturated atoms
in the solid resulting from:
Surface irregularities
Dislocations
Edges of crystals
Cracks along grain boundaries
CATALYST PROPERTIES
21
REACTION KINETICS
22
REACTION KINETICS
Chemical Plant
Reactor-the heart of
the chemical plant
Reactor Design
23
REACTION KINETICS
Definition: Study of the rate at which
chemical process occurs and the reaction
mechanism.
Usage: Used to size the reactor; used to
determine the reactor dynamics
25
26
In 2007
REACTION MECHANISM
27
1. Bulk Diffusion of reacting molecules to the surface of the catalyst
2. Pore Diffusion of reacting molecules into the interior pores of the catalyst
3. Adsorption of reactants (chemisorption) on the surface of the catalyst
4. Reaction on the surface of the catalyst between adsorbed molecules
5. Desorption of products
6. Pore Diffusion of product molecules to the surface of the catalyst
7. Bulk Diffusion of product molecules
MECHANISM OF HETEROGENEOUS CATALYSIS
Steps 3,4 and 5 are important
28
Pore and film resistances in a catalyst particle
29
Diffusion:
MECHANISM OF HETEROGENEOUS CATALYSIS
RATE LIMITING STEP (RDS)
30
Rate-Determining Step (rds)
In a kinetics scheme involving more than one step, it may be that
one change occurs much faster or much slower than the others
(as determined by relative magnitudes of rate constants).
In such a case, the overall rate, may be determined almost
entirely by the slowest step, called the rate-determining step (rds).
RDS
31
RDS
32
33
RDS
Type of Limitation Variation of Reaction Rate with:
Velocity Particle Size Temperature
External diffusion / / /
Internal diffusion X / /
Surface reaction X X /
34
/ dependent X independent
- Diffusion controlled reactions are few. Diffusion limitation could be
avoided by adjusting the fluid velocity and catalyst particle size.
- 75% of the reactions which are not controlled by diffusion are
controlled by surface reaction.
- Chemisorption of reactants and desorption of product could also be
rate controlling in a few cases.
SYNTHESIZING A RATE LAW
35
ACTIVE SITE
Reactions are not catalyzed over the entire surface but only
at certain active sites or centers that result from unsaturated
atoms in the surface.
An active site is a point on the surface that can form strong
chemical bonds with an adsorbed atom or molecule.
From the reaction system of AB
SYNTHESIZING A RATE LAW
37
SYNTHESIZING RATE LAW
Example: Decomposition of cumene to form
benzene and propylene
Cumene (C) Benzene (B) + Propylene (P)
What would be the difference in rate law if the
reaction is limited by different step? (refer to
pg. 676 onwards)
38
IRREVERSIBLE SURFACE REACTION LIMITED
RATE LAW
Single site
Dual Site
Eley-Rideal
39
Algorithm to synthesize a rate law
Select a mechanism
Assume a rate-limiting step
Find the expression for concentration of the absorbed
species C
iS
Write a site balance
Derive the rate law
Compare with data
40
SYNTHESIZING A RATE LAW
Consider,
41
SYNTHESIZING A RATE LAW
From the experimental studies, it was found that the rate law is
I I N N
P
I
N
N
P K P K
K
P
P k
r
+ +
(
=
1
'
What is the mechanism? Please guess..
Adsorption
Surface Reaction
Desorption
Reaction Mechanism
42
SYNTHESIZING A RATE LAW
Is the surface reaction
rate limiting?
43
Assume, surface reaction is a limiting step,
Thus
Then,
(
S
S I
S N S S
K
C
C k r
44
SYNTHESIZING A RATE LAW
Substituting for C
N-S
, C
I-S
, and C
V
into C
T
= C
V
(1 + K
N
P
N
+ K
I
P
I
) :
Site balance:
SYNTHESIZING A RATE LAW
45
Linearizing the Initial Rate:
SYNTHESIZING A RATE LAW
46
SYNTHESIZING A RATE LAW
47
Heterogeneous Data Analysis for Reactor
Design
Steps invloved :
Developing an algebraic rate law consistent with
experimental observations
Find mechanism and rate-limiting step
Analyze the rate law
Design catalytic reactor to achieve desired X
48
DESIGN EQUATION
Catalyst weight W substitute V (reactor
volume)
Batch : N
AO
dX/dt = -r'
A
W
Packed-bed (tubular) F
AO
dX/dW = -r'
A
CSTR : W = F
AO
X / -r'
A
(less frequently used)
49
50
STEP 1: DEDUCE RATE LAW FROM EXPERIMENTAL DATA
51
STEP 2: FINDING THE MECHANISM CONSISTENT
WITH EXPERIMENTAL OBSERVATION
Which
step is the
rate
limiting
step?
52
STEP 3: EVALUATION OF RATE LAW PARAMETERS
USING REGRESSION ANALYSIS (POLYMATH)
Nonlinear least square program
(Example 10.2)
Model
Find k, K
B
and K
T
53
STEP 4: REACTOR DESIGN
Example 10.3
54
REACTOR DESIGN- GROUP WORK
Assuming Benzene and Toluene are weakly adsorbed on the catalyst
surface, determine the catalyst weight required to achieve 68% conversion.
Gro
up
No.
Question 1 Answer Q1 Question 2
1 Pressure drop
parameter, alpha= 2
x 10
-5
kg
-1
429.30 kg
What is the exact catalyst weight if
the assumption is not valid.
2 Pressure drop
parameter, alpha= 4
x 10
-5
kg
-1
431.15 kg
What is the exact catalyst weight if
the assumption is not valid.
3 Pressure drop
parameter, alpha= 6
x 10
-5
kg
-1
433.08kg
What is the exact catalyst weight if
the assumption is not valid.
4 Pressure drop
parameter, alpha=
9.8x10
-5
kg
-1
437
What is the exact catalyst weight if
the assumption is not valid.
55
REACTOR DESIGN- GROUP WORK
56
Assuming Benzene and Toluene are weakly adsorbed on the catalyst surface,
determine the catalyst weight required to achieve 68% conversion.
Gr
ou
p
No.
Question 1 Answer Q1 Question 2
5 Toluene molar flow
rate, F= 30
mol/min
260
What is the exact catalyst weight if the
assumption is not valid.
6 Toluene molar flow
rate, F= 40
mol/min
347.8966kg
What is the exact catalyst weight if the
assumption is not valid.
7 Toluene molar flow
rate, F= 70
mol/min
618
What is the exact catalyst weight if the
assumption is not valid.
REACTOR DESIGN- GROUP WORK
57
Assuming Benzene and Toluene are weakly adsorbed on the catalyst surface,
determine the catalyst weight required to achieve 68% conversion.
Gr
ou
p
No.
Question 1 Answer Q1 Question 2
8 Initial pressure= 30
atm
791
What is the exact catalyst weight if the
assumption is not valid.
9 Initial pressure= 50
atm
277.3415kg
What is the exact catalyst weight if the
assumption is not valid.
10 Initial pressure= 60
atm
192
What is the exact catalyst weight if the
assumption is not valid.
CATALYST DEACTIVATION
Common problem loss of catalytic activity
Catalyst activity defined by a(t)
a(t) = -r'
A
(t) / -r'
A
(t=0)
The rate of catalyst decay, r
d
can be expressed:
) ...... , ( ) ( ) (
) , 0 ( ) (
'
' '
P B A A
A A
C C C fn T k t a r
fresh t r t a r
=
= =
( ) | | ( )
2
decay, order 2nd For
decay, order 1st For
) ...... , (
a p(a)
a p(a)
C C C h T k t a p
dt
da
r
P B A d d
=
=
= =
58
TYPES OF CATALYST DECAY
1.) Sintering
2.) Coking
3.) Poisoning
4.) Slow Decay Temperature-Time Trajectories
5.) Moderate Decay Moving Bed
6.) Rapid Decay
Straight-Through Transport
Reactors (STTR)
Common law/situation
59
DECAY RATE LAW
Refer Table 10-7
60
EXAMPLE
Consider A B
Reaction information,
Isothermal, liquid phase
batch reactor,
catalyst due to decaying 2nd order.
W=20 kg, k=45 liter/kg h, k
d
=180 h
-1
,V=200 liter.
Find the X at t=2 hours
61
Design
Equation
Solution
W r
dt
dX
N
A AO
' =
A A
C t a k r ) ( ' ' =
t k
t a
d
+
=
1
1
) (
) 1 ( ) 1 ( X
V
N
X C C
AO
AO A
= =
Reaction rate
law
Decay Law
Stoichiometry
62
Combining
) 1 )( ( ' X t a k
V
W
dt
dX
=
t k
dt
V
Wk
X
dX
d
+
=
1
'
) 1 (
} }
+
=
t k
dt
V
Wk
X
dX
d
1
'
) 1 (
) 1 ln(
'
1
1
ln t k
Vk
Wk
X
d
d
+ =
Integrate
Solving
136 . 0
) 2 180 1 ln(
180 200
/ 45 20
1
1
ln
1
1
=
+
=
X
h h
h liter
hr kg liter kg
X
63
CATALYST DECAY IN REACTORS
Consider
Example 10-6
Example 10-7
Example 10-8
64
65
66
CATALYST DEACTIVATION- GROUP WORK
G1-G4
Based on the previous group work, assuming that the reaction is performed in a
moving bed reactor with catalyst moving rate of 10 kg/min, there is no pressure
drop, deactivation occurs according to the 1
st
order decay with the kd= 0,007 min
-
1
. Determine the weight of catalyst needed to achieve 68%, Answer: 523kg
G5-G7
Based on the previous group work, assuming that the reaction is performed in a moving
bed reactor with catalyst moving rate of 10 kg/min, there is no pressure drop,
deactivation occurs according to the zero order decay with the kd= 0,007 min
-1
.
Determine the weight of catalyst needed to achieve 68%, A: 282, 391, 775
G8-G10
Based on the previous group work, assuming that the reaction is performed in a moving
bed reactor with catalyst moving rate of 10 kg/min, there is no pressure drop,
deactivation occurs according to the 2
nd
order decay with the kd= 0,007 min
-1
. Determine
the weight of catalyst needed to achieve 68%, A: 1003, 302, 203
67
END
68
Potrebbero piacerti anche
- Spectrophotometric Determination of The Pka of Bromothymol BlueDocumento7 pagineSpectrophotometric Determination of The Pka of Bromothymol BlueSantanu Chowdhury50% (2)
- Alternative Processes For The Production of Styrene 1995 Applied Catalysis A GeneralDocumento21 pagineAlternative Processes For The Production of Styrene 1995 Applied Catalysis A GeneralAntonioLadeiraNessuna valutazione finora
- Catalysis and Catalytic Reactions: A. Sarath BabuDocumento77 pagineCatalysis and Catalytic Reactions: A. Sarath Babuazmigalaxy8955Nessuna valutazione finora
- Hydrogenation of Ethylene On Cu CatalystDocumento7 pagineHydrogenation of Ethylene On Cu CatalystHillman WiraNessuna valutazione finora
- 06 Chapter 37 (Complete)Documento61 pagine06 Chapter 37 (Complete)Jian JieNessuna valutazione finora
- Industrial Catalyst AssignmentDocumento14 pagineIndustrial Catalyst AssignmentHarshitNessuna valutazione finora
- Chapter 1Documento74 pagineChapter 1PMNessuna valutazione finora
- 04 - Catalyst PreparationDocumento52 pagine04 - Catalyst PreparationArdhito SetiawanNessuna valutazione finora
- CRE Notes PDFDocumento61 pagineCRE Notes PDFKrunal ThakarNessuna valutazione finora
- Step Involved in Catalytic ReactionDocumento47 pagineStep Involved in Catalytic ReactionSaeikh Z. Hassan75% (4)
- Catalytic Kinetics PDFDocumento47 pagineCatalytic Kinetics PDFCorby TranNessuna valutazione finora
- Chen 2007Documento9 pagineChen 2007Arisya JulvianaNessuna valutazione finora
- Heterogeneous Reaction KineticDocumento48 pagineHeterogeneous Reaction KineticChristian NwekeNessuna valutazione finora
- Catalysis and Catalytic ReactorsDocumento59 pagineCatalysis and Catalytic ReactorssyedmuhammadtariqueNessuna valutazione finora
- L19 External DiffusionDocumento30 pagineL19 External DiffusionEga NabellaNessuna valutazione finora
- 2 - Sine Cosine AlgorithmDocumento20 pagine2 - Sine Cosine AlgorithmAchmad P. RifaiNessuna valutazione finora
- Kinetics of Homogeneous ReactionDocumento56 pagineKinetics of Homogeneous ReactionSahel SahraeeNessuna valutazione finora
- Steps in Heterogeneous Catalytic ReactionsDocumento18 pagineSteps in Heterogeneous Catalytic ReactionsUsman BlembengNessuna valutazione finora
- Hidratación de Oxido de Etileno Cinética PDFDocumento5 pagineHidratación de Oxido de Etileno Cinética PDFangieNessuna valutazione finora
- Principles of Catalysis: Part 1c: DiffusionDocumento29 paginePrinciples of Catalysis: Part 1c: DiffusionSyahminazuhan PipimontelNessuna valutazione finora
- CREII-Module-I - Lecture 4 PDFDocumento34 pagineCREII-Module-I - Lecture 4 PDFshubhamNessuna valutazione finora
- Role of Interfacial Tension in The Oil IndustryDocumento13 pagineRole of Interfacial Tension in The Oil IndustryashwinsrvNessuna valutazione finora
- Chemisorption PosterDocumento1 paginaChemisorption PostershcnmartinNessuna valutazione finora
- Acrylic Acid Plant Design Absorption Col PDFDocumento37 pagineAcrylic Acid Plant Design Absorption Col PDFJohn Patrick DagleNessuna valutazione finora
- ADSORPTION Lect 01Documento35 pagineADSORPTION Lect 01MarwahNessuna valutazione finora
- ChE426 Final Exam 2005Documento2 pagineChE426 Final Exam 2005احمد الدلالNessuna valutazione finora
- Catalytic Conversion of Glycerol To Value-Added Chemical Products PDFDocumento342 pagineCatalytic Conversion of Glycerol To Value-Added Chemical Products PDFGoklas WinnerNessuna valutazione finora
- Exp5 Determination of Heat of Solution FDocumento8 pagineExp5 Determination of Heat of Solution FJim100% (1)
- Lecture 5a CatalysisDocumento22 pagineLecture 5a CatalysisSandeep ChawlaNessuna valutazione finora
- MI6 Chief Alex Younger Full SpeechDocumento13 pagineMI6 Chief Alex Younger Full SpeechLatika M BourkeNessuna valutazione finora
- Surface Chemistry Adsorption)Documento134 pagineSurface Chemistry Adsorption)dimple joeNessuna valutazione finora
- Catalysts: Role and Function of The CatalystDocumento20 pagineCatalysts: Role and Function of The Catalystyussra amerNessuna valutazione finora
- CHEM 355 Surface Chemistry and Phase EquilibriaDocumento97 pagineCHEM 355 Surface Chemistry and Phase EquilibriaMarfo FredNessuna valutazione finora
- 3 - Catalysts and CatalysisDocumento25 pagine3 - Catalysts and Catalysisshan0214Nessuna valutazione finora
- Chapter 1 Reaction and Reactor FundamentalsDocumento28 pagineChapter 1 Reaction and Reactor FundamentalsAndy Tan WXNessuna valutazione finora
- General Introduction: Chapter Four ExtractionDocumento19 pagineGeneral Introduction: Chapter Four ExtractionMujahid HaddadNessuna valutazione finora
- Kinetics of Homogeneous Reactions Simple Reactor TypesDocumento9 pagineKinetics of Homogeneous Reactions Simple Reactor TypesNikai Hermawan AmrullahNessuna valutazione finora
- Propane To Acrylic AcidDocumento231 paginePropane To Acrylic AcidShahida Norizan100% (1)
- Catalytic Reaction EngineeringDocumento48 pagineCatalytic Reaction EngineeringM Deepika100% (1)
- 4 - Rate Laws and Stoichiometry - StuDocumento62 pagine4 - Rate Laws and Stoichiometry - StuTiệp MatícNessuna valutazione finora
- CREII-Module-I - Lecture 2 PDFDocumento28 pagineCREII-Module-I - Lecture 2 PDFshubhamNessuna valutazione finora
- InfluenzaVirusInfectionsHumans Jul13Documento2 pagineInfluenzaVirusInfectionsHumans Jul13dw_duchNessuna valutazione finora
- The Explosion at Dow Kings LynnDocumento19 pagineThe Explosion at Dow Kings LynnBurgosg ValeryNessuna valutazione finora
- Part III - CRE II LecturesDocumento59 paginePart III - CRE II LecturesArunPThomas100% (1)
- Enzyme Kinetics ExplainedDocumento43 pagineEnzyme Kinetics ExplainedBlessings Chawinga100% (1)
- 14 EquilibriumDocumento6 pagine14 EquilibriumAgam HanasichulaNessuna valutazione finora
- Advanced Reaction Engineering - 7Documento49 pagineAdvanced Reaction Engineering - 7Anonymous 4jVlYsNessuna valutazione finora
- Understanding Catalysis and Reactor DesignDocumento43 pagineUnderstanding Catalysis and Reactor DesignLê MinhNessuna valutazione finora
- How Catalysts Drive Chemical ReactionsDocumento86 pagineHow Catalysts Drive Chemical Reactionsdhan_azueloNessuna valutazione finora
- Adsorption PDFDocumento45 pagineAdsorption PDFKhantilBuchNessuna valutazione finora
- Rate LawsDocumento19 pagineRate LawsEli BerkowitzNessuna valutazione finora
- Tutorial 1 SolutionDocumento3 pagineTutorial 1 Solutionpleco4meNessuna valutazione finora
- Cre First Chapter Octave LevenspeilDocumento59 pagineCre First Chapter Octave LevenspeilManish PatilNessuna valutazione finora
- Lec 6 - Multiple ReactionsDocumento37 pagineLec 6 - Multiple ReactionskaurNessuna valutazione finora
- 1 Bioprocess Engineering CH 1Documento22 pagine1 Bioprocess Engineering CH 1Fasil ManNessuna valutazione finora
- Chapter 3 - Stoichiometry 20-2-2016 PDFDocumento63 pagineChapter 3 - Stoichiometry 20-2-2016 PDFSyukuri JaafarNessuna valutazione finora
- IMMOBILIZED ENZYME SYSTEMS: EFFECT OF pH AND TEMPERATUREDocumento60 pagineIMMOBILIZED ENZYME SYSTEMS: EFFECT OF pH AND TEMPERATURERalph EvidenteNessuna valutazione finora
- Chapter 1Documento74 pagineChapter 1Sasmilah KandsamyNessuna valutazione finora
- Catalysis and Catalytic Reactions: A. Sarath BabuDocumento77 pagineCatalysis and Catalytic Reactions: A. Sarath BabuvamsiakellaNessuna valutazione finora
- Catalysis and Catalytic ReactionsDocumento77 pagineCatalysis and Catalytic Reactionsdian_2108Nessuna valutazione finora
- Catalyst Lecture OverviewDocumento48 pagineCatalyst Lecture OverviewAquafina BaghdadNessuna valutazione finora
- Exp 9 Air Particulate SamplingDocumento6 pagineExp 9 Air Particulate SamplingFhan Sani SeowNessuna valutazione finora
- Exp 8 Membrane Treatment UnitDocumento1 paginaExp 8 Membrane Treatment UnitFhan Sani SeowNessuna valutazione finora
- COD Test Determines Organic PollutantsDocumento4 pagineCOD Test Determines Organic Pollutantskh!mNessuna valutazione finora
- Tissue Paper ManufacturingDocumento43 pagineTissue Paper ManufacturingFhan Sani Seow33% (6)
- Exp 5 Jar TestDocumento5 pagineExp 5 Jar TestFhan Sani SeowNessuna valutazione finora
- BoilerDocumento3 pagineBoilerFhan Sani SeowNessuna valutazione finora
- Exp 4 AasDocumento4 pagineExp 4 AasFhan Sani SeowNessuna valutazione finora
- Water Quality Parameters GuideDocumento58 pagineWater Quality Parameters GuideFhan Sani Seow100% (1)
- Water and Wastewater Quality SlideDocumento26 pagineWater and Wastewater Quality SlideFhan Sani SeowNessuna valutazione finora
- Rise and Fall of Rome CivilizationDocumento33 pagineRise and Fall of Rome CivilizationFhan Sani SeowNessuna valutazione finora
- Maleik AnhidritDocumento5 pagineMaleik AnhidritsakinnxNessuna valutazione finora
- Production of Isobutene From N-Butane Over PD Modified SAPOs and MeAPOsDocumento6 pagineProduction of Isobutene From N-Butane Over PD Modified SAPOs and MeAPOsFhan Sani SeowNessuna valutazione finora
- Chapter 4Documento23 pagineChapter 4adnanNessuna valutazione finora
- Report On Pandemic InfluenzaDocumento32 pagineReport On Pandemic InfluenzaFhan Sani SeowNessuna valutazione finora
- Sigmatropic and electrocyclic reactions overviewDocumento25 pagineSigmatropic and electrocyclic reactions overvieweliyachrisNessuna valutazione finora
- 2012EC 2 Semester 3 Quarter Chemistry Worksheet For Grade 11. I. Choose The Best Answer From The Given AlternativesDocumento5 pagine2012EC 2 Semester 3 Quarter Chemistry Worksheet For Grade 11. I. Choose The Best Answer From The Given AlternativesPatrix ParkerNessuna valutazione finora
- Aromatic Substitution MSc-2Documento122 pagineAromatic Substitution MSc-2BukhariNessuna valutazione finora
- Properties and Reactions of Haloalkanes: Bimolecular Nucleophilic SubstitutionDocumento48 pagineProperties and Reactions of Haloalkanes: Bimolecular Nucleophilic SubstitutionKunjal100% (1)
- As Level Chemistry 2.1.3 ALKENES: Answer All Questions Max 63 MarksDocumento16 pagineAs Level Chemistry 2.1.3 ALKENES: Answer All Questions Max 63 MarksAneesa KumarNessuna valutazione finora
- .In Ypage: Name Reactions (Organic Chemistry)Documento12 pagine.In Ypage: Name Reactions (Organic Chemistry)Sai SidharthNessuna valutazione finora
- Enzyme Structure, Classification and MechanismDocumento19 pagineEnzyme Structure, Classification and MechanismIslam SamirNessuna valutazione finora
- Enzyme Catalysis Lab Hypothesis: Exercise 2A: Test of Catalase ActivityDocumento6 pagineEnzyme Catalysis Lab Hypothesis: Exercise 2A: Test of Catalase ActivityMarques LoveNessuna valutazione finora
- Cre QuestionDocumento2 pagineCre QuestionChinu Routa100% (1)
- 03 MainDocumento3 pagine03 Mainjerc1324Nessuna valutazione finora
- Potent tobacco varieties and enantioselective synthesis of nicotineDocumento13 paginePotent tobacco varieties and enantioselective synthesis of nicotinePatricia Bianca Dela Cruz0% (1)
- Haloalkanes and Haloarenes (Que)Documento27 pagineHaloalkanes and Haloarenes (Que)Dhruv KuchhalNessuna valutazione finora
- Polymath Project Report FinalDocumento24 paginePolymath Project Report FinalDIAN NUR AIN BINTI ABD RAHIM A20MJ0019Nessuna valutazione finora
- Electrophilic Addition Part 1 EdexcelDocumento3 pagineElectrophilic Addition Part 1 EdexcelKevin The Chemistry TutorNessuna valutazione finora
- Group B2 - Batch - Stirring RateDocumento7 pagineGroup B2 - Batch - Stirring RateMimi Sharina HassanNessuna valutazione finora
- IIT-JEE 2021 Assignment on Alcohols, Phenols, Ethers and Carbonyl CompoundsDocumento8 pagineIIT-JEE 2021 Assignment on Alcohols, Phenols, Ethers and Carbonyl CompoundsAnurag RamachandranNessuna valutazione finora
- Chemical Kinetic - Dec2016 PDFDocumento137 pagineChemical Kinetic - Dec2016 PDFFaisal AzamNessuna valutazione finora
- The Sandmeyer Reaction: Replacement of The Diazonium Group by CL, BR, or CNDocumento2 pagineThe Sandmeyer Reaction: Replacement of The Diazonium Group by CL, BR, or CNUsman GhaniNessuna valutazione finora
- Polymer-Supported Catalysis in Synthetic Organic Chemistry, Clapham Et. Al.Documento26 paginePolymer-Supported Catalysis in Synthetic Organic Chemistry, Clapham Et. Al.Ivan Jose Acosta MoralesNessuna valutazione finora
- Kinetics of Consecutive Reactions First ReactionDocumento2 pagineKinetics of Consecutive Reactions First ReactionMariana Green100% (5)
- Enzyme Temperature Lab ReportDocumento5 pagineEnzyme Temperature Lab Reportapi-401166686Nessuna valutazione finora
- Chemical Kinetics PDFDocumento5 pagineChemical Kinetics PDFBrahmanand TiwariNessuna valutazione finora
- Topic 1: Siti Wahidah Binti Puasa PHONE NO: 03-55436327 011-32338927 Reference: Fogler 4 Edition, Levenspeil 3 EditionDocumento35 pagineTopic 1: Siti Wahidah Binti Puasa PHONE NO: 03-55436327 011-32338927 Reference: Fogler 4 Edition, Levenspeil 3 EditionJohnNessuna valutazione finora
- ExerciseDocumento30 pagineExerciseMahendra ShahNessuna valutazione finora
- OrganocatalysisDocumento42 pagineOrganocatalysisHifsa HussainNessuna valutazione finora
- G22 Serapion-Module 9 EnzymesDocumento3 pagineG22 Serapion-Module 9 Enzymestherese angelieNessuna valutazione finora
- Sirte University: Plug Flow Reactor (PFR)Documento6 pagineSirte University: Plug Flow Reactor (PFR)Sliman Al TayaNessuna valutazione finora
- ORG 3 Palladium Catalysis The Suzuki Reaction LMDocumento6 pagineORG 3 Palladium Catalysis The Suzuki Reaction LMVictor OdoyoNessuna valutazione finora
- Csir Net Gate Advanced Organic Chemistry PDFDocumento15 pagineCsir Net Gate Advanced Organic Chemistry PDFdhanapalNessuna valutazione finora