Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Heat Transfer Hazira1
Caricato da
Ashish JoshiCopyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Heat Transfer Hazira1
Caricato da
Ashish JoshiCopyright:
Formati disponibili
Heat transfer
Dr. Anupama Sharma,
Associate Professor,
UICET, Panjab University, Chandigarh
2
Introduction to Heat Transfer
Heat transfer is the transfer of heat due to a temperature difference with different
Mechanisms: conduction, convection, and radiation. Conduction refers to heat
transfer that occurs across a stationary solid or fluid in which a temperature gradient
exists. Convection refers to the heat transfer that occurs across a moving fluid in
which a temperature gradient exists. Radiation refers to the heat transfer between
two surfaces at different temperatures separated by a medium transparent to the
electromagnetic waves emitted by the surfaces.
Heat Transfer
Heat always moves from a warmer place
to a cooler place.
Hot objects in a cooler room will cool to
room temperature.
Cold objects in a warmer room will heat up
to room temperature.
Understanding Heat
Transfer, Conduction,
Convection and Radiation
Heat Transfer Methods
Heat transfers in three ways:
Conduction
Convection
Radiation
Poor conductors are good insulators
Wood, wool, straw, paper, cork, and Styrofoam are all
poor conductors of heat. But all of these objects are
good insulators because they delay the transfer of
heat.
A Styrofoam cup will keep coffee hot because it
doesnt allow much heat to be transferred out of the
coffee and into the cup.
Air is a poor conductor of heat; heat is transferred
through air at a very slow rate.
Snow is also a poor conductor and a good insulator.
Snow slows the escape of heat from the earths
surface. It prevents the heat from escaping too rapidly.
A blanket is a good insulator because it slows
the transfer of your body heat to your
surroundings. A blanket does not provide you
with heat; it just delays the transfer of heat from
your body to other materials.
No insulator can completely prevent heat from
escaping. An insulator just reduces the rate at
which the heat is transferred.
Heat is the only thing that can be transferred,
cold cannot be transferred.
1.) If you hold one end of a metal bar
against a piece of ice, the end in your
hand will soon become cold. Does cold
flow from the ice to your hand?
2.) Why does snow last the longest on
the roof of a well-insulated house?
3.) You can stick your hand into a hot
pizza oven for several seconds without
harm, whereas youd never touch the
metal insides for even a second. Why?
Conduction
When you heat a metal strip at one end, the heat
travels to the other end.
As you heat the metal, the particles vibrate, these
vibrations make the adjacent particles vibrate, and so on
and so on, the vibrations are passed along the metal and
so is the heat. We call this?
Conduction
Conduction
Molecules in a warm substance have a lot of
kinetic energy (they collide more frequently).
Molecules in cool substances do not have a lot of
kinetic energy.
When heat is transferred from a warmer
substance to a cooler substance, energy of
molecules is also transferred. The molecules of
each substance interact with each other. The fast
moving molecules of the warm substance bump
into the slower moving molecules of the cool
substance, increasing the speed of the molecules
(and ultimately the temperature) of the cool
substance.
Conduction
The conduction of heat from a warmer
substance to a cooler substance means
that there is an interaction between
molecules.
Metals are good conductors of heat
because heat flows quickly through
them.
Which is a better conductor of heat:
metal or wood?
Metal feels colder because it is a better conductor.
The metal takes more heat energy out of your
hand than the wood does, making the temperature
of your hand lower.
Metals are different
The outer e______ of metal atoms
drift, and are free to move.
When the metal is
heated, this sea of
electrons gain k_____
energy and transfer it
throughout the metal.
Insulators, such as w___ and p____, do not
have this sea of electrons which is why they
do not conduct heat as well as metals.
lectrons
inetic
ood lastic
Why does metal feel colder than wood, if they
are both at the same temperature?
Metal is a conductor, wood is an insulator. Metal
conducts the heat away from your hands. Wood
does not conduct the heat away from your hands as
well as the metal, so the wood feels warmer than
the metal.
Convection
What happens to the particles in a liquid or a
gas when you heat them?
The particles spread out and
become less dense.
This effects fluid movement.
What is a fluid? A liquid or gas.
Fluid movement
Cooler, more d____, fluids
sink through w_____, less
dense fluids.
In effect, warmer liquids and
gases r___ up.
Cooler liquids and gases s___.
ense
armer
ise
ink
Water movement
Hot water
rises
Cooler
water sinks
Convection
current
Cools at the
surface
Why is it windy at the seaside?
Cold air sinks
Where is the
freezer
compartment
put in a fridge?
Freezer
compartment
It is put at the
top, because
cool air sinks,
so it cools the
food on the
way down.
It is warmer
at the
bottom, so
this warmer
air rises and
a convection
current is
set up.
Q: In the living room, the heating unit is placed in the floor but the
the refrigerator has a top-mounted cooling coil. Why?
A: Air warmed by the baseboard heating unit is pushed to the top of
the room by the cooler and denser air. Air cooled by the cooling coil
sinks to the bottom of the refrigerator.
The third method of heat transfer
How does heat energy get
from the Sun to the Earth?
There are no particles
between the Sun and the
Earth so it CANNOT
travel by conduction or
by convection.
?
RADIATION
Emission experiment
Four containers were filled with warm water. Which
container would have the warmest water after ten minutes?
Shiny metal
Dull metal
Dull black
Shiny black
The __________ container would be the warmest after ten
minutes because its shiny surface reflects heat _______ back
into the container so less is lost. The ________ container
would be the coolest because it is the best at _______ heat
radiation.
shiny metal
radiation
dull black
emitting
Absorption experiment
Four containers were placed equidistant from a heater. Which
container would have the warmest water after ten minutes?
The __________ container would be the warmest after ten
minutes because its surface absorbs heat _______ the best.
The _________ container would be the coolest because it is
the poorest at __________ heat radiation.
dull black
radiation
shiny metal
absorbing
Shiny metal
Dull metal
Dull black
Shiny black
The StefanBOLTZMANN
Law Of Radiation
The rate at which an object emits radiant energy is
proportional to the fourth power of its absolute temperature.
This is known as Stefans law and is expressed as follows,
where is the Stefan-Boltzmann constant, = 5.67 10
-8
W/m
2
.K
4
.
.
4
eAT P
t
Q
o = =
The factor e is called the emissivity, which is a number between
0 and 1. Perfect radiators have a value of 1 for e. A is the surface
area and T is the temperature of the radiator in Kelvin.
Convection questions
Why are boilers placed beneath hot water
tanks in peoples homes?
Hot water rises.
So when the boiler heats the water, and the hot water
rises, the water tank is filled with hot water.
Why does hot air rise and cold air sink?
Cool air is more dense than warm air, so the
cool air falls through the warm air.
Steady heat conduction
Rate of
heat transfer
into the wall
Rate of
heat transfer
out of the wall
Rate of change
of the energy
of the wall
-
=
Zero for steady
operation
0
0
wall
in out
dE
Q Q
dt
=
= =
Fouriers Law
Fouriers law of heat conduction for the wall can be expressed as:
,
(W)
cond wall
dT
Q kA
dx
=
Integrating the above equation and rearranging yields:
1 2
,
(W)
cond wall
T T
Q kA
L
=
Thermal Conductivities
Substance Thermal
Conductivity: k
W / (m K)
Gold 291
Glass 0.84
Water 0.60
Wood 0.10
Air 0.023
Metals have high
thermal conductivity,
most electrical insulators
also have low thermal
conductivity.
Air is a great insulator,
except that large air
spaces allow heat flow
by convection.
The thermal conductivities of some common
materials are given in Fig. 2.1-3 and Table 2.1-1.
The Thermal Diffusivity
The thermal diffusivity, , is defined as
v
C
k
o =
Where and C
v
are the density and specific heat of the material, respectively.
Thermal resistance network
Heat conduction through a plane wall can be rearranged as:
Where R
wall
is the conduction resistance expressed as:
1 2
,
(W)
cond wall
wall
T T
Q
R
=
( C/W)
wall
L
R
kA
=
30
Fouriers law of conduction One-dimensional
Consider the conduction of heat through a slab of thickness L. The lower and upper
surfaces are kept at a constant temperature T
1
and T
2
, respectively. A steady-state
temperature profile T(y) is established in the slab.
Consider two surface in slab separated with a infinitesimal distance dy. Due to
temperature gradient generated in the slab, heat flow from the surface y to the surface
y+dy. A heat flux is defined as the amount of heat transferred per unit area per unit time,
and can be expressed as
dy
dT
k q
y
=
where k is the thermal conductivity of the medium. This equation is Fouriers law
of conduction for one-dimensional heat conduction in the y-direction. The mks units of
the heat flux and the thermal conductivity are W/m
2
and Wm
-1
K
-1
, respectively.
Consider the long cylindrical layer
Assumptions:
the two surfaces of the cylindrical layer are maintained at constant
temperatures T
1
and T
2
,
no heat generation,
constant thermal conductivity,
one-dimensional heat conduction.
Fouriers law of heat conduction
Heat conduction in cylinders
,
(W)
cond cyl
dT
Q kA
dr
=
Separating the variables and integrating from r=r
1
, where
T(r
1
)=T
1
, to r=r
2
, where T(r
2
)=T
2
Substituting A =2prL and performing the integrations give
Since the heat transfer rate is constant
2 2
1 1
,
r T
cond cyl
r r T T
Q
dr kdT
A
= =
=
} }
( )
1 2
,
2 1
2
ln /
cond cyl
T T
Q Lk
r r
t
=
1 2
, cond cyl
cyl
T T
Q
R
=
Heat conduction in cylinders
37
Conduction through cylindrical composite wall
Steady heat transfer through multilayered cylindrical or spherical
shells can be handled just like multilayered plane.
The steady heat transfer rate through a three-layered composite
cylinder of length L with convection on both sides is expressed by:
( )
( ) ( ) ( )
( )
,1 ,1 ,3 ,3 ,2
2 1 3 2 4 3
1 1 1 2 3 2 2
ln / ln / ln /
1 1
2 2 2 2 2
total conv cyl cyl cyl conv
R R R R R R
r r r r r r
r L h Lk Lk Lk r L h t t t t t
= + + + + =
= + + + +
Multilayered cylinders
Critical radius of insulation
Adding more insulation to a wall or to the attic always
decreases heat transfer.
Adding insulation to a cylindrical pipe or a spherical
shell,however, is a different matter.
Adding insulation increases the conduction resistance of
the insulation layer but decreases the convection
resistance of the surface because of the increase in the
outer surface area for convection.
The heat transfer from the pipe may increase or decrease,
depending on which effect dominates.
The variation of the heat transfer rate with the outer radius of the
insulation r
2
is shown in the figure. The value of r
2
at which
reaches a maximum is determined by:
Performing the differentiation and
solving for r
2
yields
Thus, insulating the pipe may
actually increase the rate of heat
transfer instead of decreasing it.
2
0
dQ
dr
=
,
(m)
cr cylinder
k
r
h
=
41
Thermal boundary layer
Consider a fluid of uniform temperature T
approaching a flat plate of constant
temperature T
s
in the direction parallel to the plate. At the solid/liquid interface the
fluid temperature is T
s
since the local fluid particles achieve thermal equilibrium at
the interface. The fluid temperature T in the region near the plate is affected by the
plate, varying from T
s
at the surface to T
in the main stream. This region is called
the thermal boundary layer.
The three-dimensional solid
Volume element dV is
centered at (x,y,z).
System surface, O
z
x
y
(x,y,z)
= Energy generation
rate, W/m
3
.
2Az
2Ay
2Ax
q
-
42
ENGR 370: Heat & Mass
Transfer/Conduction 1
z z z
q A q
''
=
y y y
q A q
''
=
x x x
q A q
''
=
y
z
x
Differential
volume element
43
ENGR 370: Heat & Mass
Transfer/Conduction 1
=
Rate of
change
of energy
in AV
+
Sum of heat
flows
across faces
of AV
Rate of
energy
generation
in AV
This is the First Law of Thermodynamics for the
closed system that is our volume element.
The energy balance
44
ENGR 370: Heat & Mass
Transfer/Conduction 1
Heat Diffusion Equation
(2.13)
Equation (2.13) is the general equation of heat
conduction for three-dimensional Cartesian
coordinate systems.
( )
( )
( ) ( )
T
CT k T
t x x
T T
k T k T q
y x z z
c c c
c c c
c c c c
c c c c
-
| |
=
|
\ .
| | | |
+ + +
| |
\ . \ .
45
ENGR 370: Heat & Mass
Transfer/Conduction 1
Heat Equation
The Heat Equation
A differential equation whose solution provides the temperature distribution in a
stationary medium.
Based on applying conservation of energy to a differential control volume
through which energy transfer is exclusively by conduction.
Cartesian Coordinates:
Net transfer of thermal energy into the
control volume (inflow-outflow)
p
T T T T
k k k q c
x x y y z z t
| | c c c c c c c | | | |
+ + + =
| | |
c c c c c c c
\ . \ .
\ .
(2.17)
Thermal energy
generation
Change in thermal
energy storage
46
ENGR 370: Heat & Mass
Transfer/Conduction 1
Heat Flux Components
(2.22)
T T T
q k i k j k k
r r z |
c c c
''
=
c c c
r
q
''
q
|
''
z
q
''
Cylindrical Coordinates:
( )
, , T r z |
sin
T T T
q k i k j k k
r r r u u |
c c c
'' =
c c c
(2.25)
r
q
'' q
u
''
q
|
''
Spherical Coordinates:
( )
, , T r | u
Cartesian Coordinates:
( )
, , T x y z
T T T
q k i k j k k
x y z
c c c
'' =
c c c
x
q
''
y
q
''
z
q
''
(2.3)
47
ENGR 370: Heat & Mass
Transfer/Conduction 1
Heat Flux Components (cont.)
In angular coordinates , the temperature gradient is still
based on temperature change over a length scale and hence has
units of C/m and not C/deg.
( )
or , | | u
Heat rate for one-dimensional, radial conduction in a cylinder or sphere:
Cylinder
2
r r r r
q A q rLq t
'' ''
= =
or,
2
r r r r
q A q rq t
' ' '' ''
= =
Sphere
2
4
r r r r
q A q r q t '' '' = =
48
ENGR 370: Heat & Mass
Transfer/Conduction 1
Heat Equation (Radial Systems)
2
1 1
p
T T T T
kr k k q c
r r r z z t
r
| |
| | c c c c c c c
| | | |
+ + + =
| | |
c c c c c c c
\ . \ .
\ .
(2.24)
Spherical Coordinates:
Cylindrical Coordinates:
2
2 2 2 2
1 1 1
sin
sin sin
p
T T T T
kr k k q c
r r t
r r r
u
| | u u
u u
| | c c c c c c c
| | | |
+ + + =
| | |
c c c c c c c
\ . \ .
\ .
(2.27)
49
ENGR 370: Heat & Mass
Transfer/Conduction 1
Q hot Q cold
T
h
T
i,wall
T
o,wall
T
c
Region I : Hot Liquid-
Solid Convection
NEWTONS LAW OF
CCOLING
dq
x
= h
h
. T
h
T
iw
( )
.dA
Region II : Conduction
Across Copper Wall
FOURIERS LAW
dq
x
= k.
dT
dr
Region III: Solid
Cold Liquid
Convection
NEWTONS LAW OF
CCOLING
dq
x
= h
c
. T
ow
T
c
( )
.dA
THERMAL
BOUNDARY LAYER
Energy moves from
hot fluid to a surface
by convection,
through the wall by
conduction, and then
by convection from
the surface to the
cold fluid.
Region I : Hot Liquid
Solid Convection
T
h
T
iw
=
q
x
h
h
.A
i
q
x
= h
hot
. T
h
T
iw
( )
.A
Region II :
Conduction Across
Copper Wall
q
x
=
k
copper
.2tL
ln
r
o
r
i
T
o,wall
T
i,wall
=
q
x
.ln
r
o
r
i
|
\
|
.
|
k
copper
.2tL
Region III : Solid
Cold Liquid
Convection
T
o,wall
T
c
=
q
x
h
c
.A
o
q
x
= h
c
T
o,wall
T
c
( )
A
o
+
T
h
T
c
= q
x
1
h
h
.A
i
+
ln
r
o
r
i
|
\
|
.
|
k
copper
.2tL
+
1
h
c
.A
o
(
(
(
(
(
q
x
=U.A. T
h
T
c
( )
1
1
.
ln .
.
(
(
(
(
(
+
|
|
.
|
\
|
+ =
cold i copper
i
o
o
i hot
o
h r k
r
r
r
r h
r
U
U = The Overall Heat Transfer Coefficient [W/m.K]
T
h
T
c
=
q
x
R
1
+ R
2
+ R
3
U =
1
A.ER
r
o
r
i
Thermal contact resistance (I)
In reality surfaces have some roughness.
When two surfaces are pressed against each other, the peaks
form good material contact but the valleys form voids filled with
air.
As a result, an interface contains
numerous air gaps of varying sizes
that act as insulation because of the
low thermal conductivity of air.
Thus, an interface offers some
resistance to heat transfer, which
is termed the thermal contact
resistance, R
c
.
The value of thermal contact resistance depends on the
surface roughness,
material properties,
temperature and pressure at the interface,
type of fluid trapped at the interface.
Thermal contact resistance is observed to decrease with
decreasing surface roughness and increasing interface
pressure.
The thermal contact resistance can be minimized by
applying a
thermally conducting liquid called a thermal grease.
Thermal contact resistance
Generalized thermal resistance
network
The thermal resistance concept can be used to solve steady heat
transfer problems that involve parallel layers or combined series-
parallel arrangements.
The total heat transfer of two parallel layers
( )
1 2 1 2
1 2 1 2
1 2 1 2
1 1 T T T T
Q Q Q T T
R R R R
| |
= + = + = +
|
\ .
1
total
R
1 2
1 2 1 2
1 1 1
=
total
total
R R
R
R R R R R
| |
= +
|
+
\ .
Heat Transfer Coefficients
System h (W/m
2
K)
Air (1 bar, free convection) 6-30
Air (1 bar, forced convection) 10-200
Water (free convection) 500-1000
Water (Forced convection) 600-8000
Boiling water 2500-100000
Condensing Steam 2500-70000
Heat transfer from finned
surfaces
Newtons law of cooling
Two ways to increase the rate of heat transfer:
increasing the heat transfer coefficient,
increase the surface area fins
( )
conv s s
Q hA T T
=
Types of fins
Circular-rod fin
Rectangular fin
Radial fin
Fin equation
Under steady conditions, the energy balance
on this volume element can be expressed as
Where
Substituting and dividing by Dx, we obtain
, , cond x cond x x conv
Q Q Q
+A
= +
( )( )
conv
Q h p x T T
= A
( )
, ,
0
cond x x cond x
Q Q
hp T T
x
+A
+ =
A
Rate of
heat conduction
into the element
Rate of
heat conduction
from the element
Rate of heat
convection from
the element
= -
Taking the limit as Ax 0 gives
From Fouriers law of heat conduction we have
Substitution of (B) into (A) gives
( )
0
cond
dQ
hp T T
dx
+ =
cond c
dT
Q kA
dx
=
( )
0
c
d dT
kA hp T T
dx dx
| |
=
|
\ .
(A)
(B)
For constant cross section and constant thermal conductivity
Where, equation is a linear, homogeneous, second-order
differential equation with constant coefficients.
The general solution of (A) is
C
1
and C
2
are constants whose values are to be determined
from the boundary conditions at the base and at the tip of the
fin.
2
2
2
0
d
m
dx
u
u =
;
c
hp
T T m
kA
u
= =
1 2
( )
mx mx
x C e C e u
= +
(A)
Several boundary conditions are typically employed:
At the fin base
Specified temperature boundary condition, expressed as:
q(0)= q
b
= T
b
-T
At the fin tip
1. Specified temperature
2. Infinitely Long Fin
3. Adiabatic tip
4. Convection (and
combined convection
and radiation).
Boundary conditions
For a sufficiently long fin the temperature at the fin tip approaches the ambient
temperature
Boundary condition: q(L)=T(L)-T
=0
When x so does e
mx
C
1
=0
@ x=0: e
mx
=1 C
2
= q
b
The temperature distribution:
heat transfer from the entire fin
/
( )
c
x hp kA
mx
b
T x T
e e
T T
= =
( )
0
c c b
x
dT
Q kA hpkA T T
dx
=
= =
Infinitely long fin (T
fin
tip
=T)
Adiabatic tip
Boundary condition at fin tip:
After some manipulations, the temperature
distribution:
heat transfer from the entire fin
0
x L
d
dx
u
=
=
( )
cosh
( )
cosh
b
m L x
T x T
T T mL
( )
0
tanh
c c b
x
dT
Q kA hpkA T T mL
dx
=
= =
Convection from fin tip
(or Combined Convection and Radiation)
A practical way of accounting for the heat loss from the fin tip is to replace
the fin length L in the relation for the insulated tip case by a
corrected length defined as
L
c
=L+A
c
/p
For rectangular and cylindrical fins L
c
is
L
c,rectangular
=L+t/2
L
c,cylindrical
=L+D/4
To maximize the heat transfer from a fin the temperature of
the fin should be uniform (maximized) at the base value of
T
b
In reality, the temperature drops along the fin, and thus the
heat transfer from the fin is less. To account for the effect
we define a fin efficiency
or
,max
fin
fin
fin
Q
Q
q = =
Actual heat transfer rate from the fin
Ideal heat transfer rate from the fin
if the entire fin were at base temperature
,max
( )
fin fin fin fin fin b
Q Q hA T T q q
= =
Fin efficiency
Fin efficiency
For constant cross section of very long fins:
For constant cross section with adiabatic tip:
( )
( )
,
,max
1 1
fin c b
c
long fin
fin fin b
Q hpkA T T
kA
Q hA T T L hp mL
q
= = = =
( )
( )
,
,max
tanh
tanh
fin c b
adiabatic fin
fin fin b
Q hpkA T T aL
Q hA T T
mL
mL
q
= =
=
Fin efficiency (extra)
Fin efficiency (extra)
Fin effectiveness
The performance of the fins is judged on the basis of the
enhancement in heat transfer relative to the no-fin case.
The performance of fins is expressed in terms of the fin
effectiveness e
fin
defined as
( )
fin fin
fin
no fin b b
Q Q
Q hA T T
c
= = =
Heat transfer rate
from the surface of
area A
b
Heat transfer rate from
the fin of base area A
b
Remarks regarding fin effectiveness
The thermal conductivity k of the fin material should be as
high as possible. It is no coincidence that fins are made
from metals.
The ratio of the perimeter to the cross-sectional area of
the fin p/A
c
should be as high as possible.
The use of fins is most effective in applications involving
a low convection heat transfer coefficient.
The use of fins is more easily justified when the medium
is a gas instead of a liquid and the heat transfer is by
natural convection instead of by forced convection.
Overall effectiveness
An overall effectiveness for a finned surface is
defined as the ratio of the total heat transfer from
the finned surface to the heat transfer from the
same surface if there were no fins.
( )
,
fin
fin overall
no fin
unfin fin fin
no fin
Q
Q
h A A
hA
c
q
=
+
=
Proper length of a fin
An important step in the design of a fin is the determination of
the appropriate length of the fin once the fin material and the
fin cross section are specified.
The temperature drops along the
fin exponentially and asymptotically
approaches the ambient temperature
at some length.
Fins in thermal resistance
network
Many problems encountered in practice are two- or three-
dimensional and involve rather complicated geometries
for which no simple solutions are available.
An important class of heat transfer problems for which
simple solutions are obtained encompasses those
involving two surfaces maintained at constant
temperatures T
1
and T
2
.
The steady rate of heat transfer between these two
surfaces is expressed as
Q=Sk(T
1
=T
2
)
S is the conduction shape factor, which has the
dimension of length.
Heat transfer in common
configurations
Where to find S
Try This
In a double pipe heat exchanger, a reaction mixture
having C
pm
=2.87 kJ/kg.K is flowing at rate of 7260 kg/h
and is to be cooled from 377.6 K to 344.3 K. Cooling
water at 288.8 K is available and the flow rate is 4536
kg/h. The overall U
o
is 653 W/m2.K. Calculate:
a) the outlet water temperature
b) the area A
o
of the exchanger for countercurrent flow
c) the area A
o
of the exchanger for co-current flow
(Answers: a) outlet temp. : 325.2 K, b) area A
o
: 5.43 m
2
c) area A
o
:6.46 m
2
)
The Physical mechanism of the dimensionless numbers
Nusselt numbers
It is the ratio of convection heat
transfer rate to the conduction heat
transfer rate. Consider an internal flow
in a channel of height L and the
temperatures at the lower and upper
surfaces are T
1
& T
2
, respectively.
The convection heat transfer rate is
The conduction heat transfer rate is
The ratio
u
T
1
T
2
L
) (
2 1
T T hA Q
conv
=
) (
2 1
T T
L
kA
Q
cond
=
k
hL
L
kA
hA
Q
Q
Nu
cond
conv
L
= = =
The Reynolds number
It is the ratio of inertia force to viscous force of the moving fluid
- Inertia force
- The viscous force
- The ratio
is called kinematic viscosity of the fluid.
3 2 2 2 2
2
( )
i
L L
F ma L L L u
s s
= = = =
2 2
v
u u
F A L L uL
y L
t
A
= = = =
A
Re
L
uL uL uL
= = =
=
The Prandtl number
It is the ratio of the momentum diffusivity to the thermal diffusivity. It
controls how fast the heat diffuses in a medium. It has the form
The momentum diffusivity is the kinematic viscosity and it controls
the rate of diffusion of momentum in a fluid medium. The ratio of the
two is called Prandtl number.
Pr
p
p
c
k
k
c
u
o
= = =
p
k
c
o
=
Dr. aziye Balku 80
k
C
p
= Pr
Pr<<1 heat diffuses very quickly in liquid metals, tbl thicker
Pr>>1 heat diffuses very slowly in oils relative to momentum, tbl
thinner than vbl
k
C
heat of y dif fusivit molecular
momentum of y dif fusivit molecular
p
o
v
= = = Pr
Reynolds Analogy
It can be shown that, under specific conditions (no external
pressure gradient and Prandtle number equals to one), the
momentum and heat transfers can be related. The momentum
transfer of fluid passing a flat plate can be characterized by the
skin friction coefficient, C
f
. The heat transfer between the plate
and the flow can be characterized by the Nusselt number, Nu.
They can be related as:
St
Nu
k
hL
Nu
V
Nu C
f
w
f
=
= =
= = =
2
C
: becomes analogy The
Pr Re VCp
h
St : St number Stanton Define
. ,
2
1
C where ,
2
Re
f
2
f
t
Reynolds Analogy (cont.)
The Reynolds analogy related the flow parameters to the thermal
parameters. If a given flow field can be determined, the heat
transfer characteristics can be found by using the Reynolds
analogy.
A modified Reynolds analogy has been obtained to take into
consideration of the fact that Prandtle number Pr is usually not
equal to one:
60. Pr 0.6 for , Pr
2
3 / 2
< < = St
C
f
Note: The Reynolds analogy should only be used when the
pressure gradient is zero. However, in turbulent flow, this
condition is not that important. Therefore, the analogy can be
applied even when the pressure gradient is nonzero for turbulent
flows.
Reynolds Analogy (cont.)
To obtain the averaged convection coefficient, we can integrate
the local coefficient along the plate.
3 / 1 2 / 1
0
2 / 1
2 / 1 3 / 1
0
Pr Re 664 . 0
) ( Pr ) ( 332 . 0
1
x
x
f
x
x
k
x h
u N
x
dx
v
U
x
k
dx h
x
h
= =
= =
} }
4. Dittus-Boelter expression
for fully developed turbulent flow in smooth tubes
n
Pr Re Nu
8 . 0
023 . 0 =
=
3 . 0
4 . 0
n
For heating of the fluid
For cooling of the fluid
w b
w b
t t
t t
>
<
Characteristic length, inside diameter
Reference temperature,
) (
2
1
" '
b b b
t t t + =
Applicability
5 4
10 2 . 1 Re 10 s s
100 6 . 0 s s Pr
60 / > d L
Fully developed
C t t C t t C t t
o
w b
o
w b
o
w b
10 oil , 30 ~ 20 water , 50 gas s s s
Moderate temperature difference
Example : Water is heated from 25.3 to 34.6 in a tube with a diameter
of d=20mm and a length of 5m, the velocity is u=2m/s. Calculate the
convection heat transfer coefficient.
Solution
1Forced convection in tube
2Reference temperature
( ) C 30
2
6 . 34 3 . 25
2
1
o " '
=
+
= + =
b b b
t t T
Physical properties:
k=0.618W/(m.K), v=0.80510
-6
m
2
/s,
Pr=5.42, =995.7kg/m
3
, c
p
=4.17 kJ/kg
3Calculate dimensionless group and choose equation
4 4
6
10 10 97 . 4
10 805 . 0
02 . 0 2
Re
> =
= =
v
ud
n
Nu Pr Re 023 . 0
8 . 0
= For heating of the fluid n=0.4
(4) Calculation and correction
( ) 5 . 258 42 . 5 10 97 . 4 023 . 0
4 . 0
8 . 0
4
= = Nu
W/(m.K) 7987
02 . 0
618 . 0 5 . 258
=
= =
d
Nuk
h
Check whether the parameters are in the range of application
60 250
02 . 0
5
> = =
d
L
( )
( ) W
t t c
d
u q
b b p
24285 3 . 25 6 . 34 10 174 . 4
4
02 . 0
2 7 . 995
4
3
2
' "
2
=
=
=
t
t
( ) =
b w
t t hA q
C 68 . 39
5 02 . 0 7895
24285
30
o
=
+ =
+ = + =
t
tDL h
q
t
hA
q
t t
b b b
C 20 68 . 9 30 68 . 39
o
< = =
b w
t t
6. Convection in ducts
characteristic dimension: hydraulic (equivalent) diameter D
H
.
P
A
D
H
4
=
Athe cross-sectional area of the flow
P the wetted perimeter
Annular tube
( )
( )
1 2
1 2
2
1
2
2
d d
d d
d d
D
H
=
+
=
t
t
Rectangular tube
( ) b a
ab
b a
ab
D
H
+
=
+
=
2
2
4
|
.
|
\
|
=
= =
1
4
2
2 1
2
2 1
d
s s
d
d D
d P d s s A
H
t
t
t
This method suitable for many cases
There are some notable exceptions where the method does not work
Tube bank
88
For non-round pipes,
the hydraulic diameter
D
h
= 4A
c
/P
A
c
= cross-section area
P = wetted perimeter
HYDRAULIC DIAMETER
Sieder Tate Correlation
14 . 0
3 / 1 8 . 0
(Pr) (Re) 027 . 0
|
|
.
|
\
|
=
s
Nu
0.7Pr 16,700
Re10,000
L/D 10, all properties are evaluated at bulk temperature
Can be used for large temperature differences between
surface and bulk
7. Heat transfer in laminar tube flow
Information of fully developed laminar in ducts by Shah and Londun
6-3 Flow across cylinders and spheres
Separation point at
0
0
=
c
c
= y
y
u
Drag force
2
2
=
u
A C F
D D
A -- frontal area of the body
is the product of diameter
and length
2. Choice of equations for cross flow over cylinders
3. Noncircular cylinders
17) - (6 Pr Re
3 / 1
f
n
f
C
k
hd
Nu = =
Reference temperature (T
w
+T
)/2
Characteristic length d
The constant C and n are given in Table 6-2
17) - (6 Pr Re
3 / 1
f
n
f
C
k
hd
Nu = =
Table
Re C n
0.4-4 0.989 0.33
4-40 0.911 0.385
40-4,000 0.683 0.465
4,000-40,000 0.193 0.618
40,000-400,000 0.0266 0.805
1. cross flow outside the pipes
d
2
x
2
x
1
zigzag
alignment
d
2
x
2
x
1
straight
alignment
In this situation, the affecting factors to h are the arrangement,
distance and row number of the pipes in addition to Re and Pr.
h under forced convective flow outside pipe
6-4 Flow across tube banks
line - in staggered
h h >
10 or more rows of tubes in
the direction of flow
Reference temperature
2 / ) (
+ = t t t
w f
Characteristic length d
Re is based on the u
max
17) - (6 Pr Re
3 / 1
f
n
f
C
k
hd
Nu = =
t) arrangemen line - (in )] /( [
max
d S S u u
n n
=
For staggered area flow minimum is for )] /( [
max p n n
S d S S u u =
If not
] ) 2 / [(
) 2 / (
2 / 1 2 2
max
d S S
S u
u
p n
n
+
=
1. cross flow outside pipes 4 . 0
Pr Re Nu
n
Cc =
4 . 0
2
Pr Re
n
C
d
k
h c =
applied for: Re = 50007000x
1
/d
2
= 1.2~5.0, x
2
/d
2
= 1.2~5.0.
defined T: average of inlet and outlet fluid
character size: outer diameter of pipe, d
2
velocity: taken at the smallest flow area, i. e., the highest velocity.
row No
straight zigzag
C
n n
1 0.60 0.171 0.6 0.171 For x
1
/d
2
= 1.2~3
C =1 +0.1 x
1
/d
2
For x
1
/d
2
> 3 ,
C = 1.3
2 0.65 0.151 0.6 0.228
3 0.65 0.161 0.6 0.290
4 0.65 0.151 0.6 0.290
or
2 2
2
2
4
4
e
t d
d
d
t
t
| |
|
\ .
=
straight
alignment
2 2
2
2
3
4
2 4
e
t d
d
d
t
t
| |
|
\ .
=
zigzag
alignment
Equivalent diameter, d
e
Fluid velocity, u
where D inner diameter of shellm
l distance between two bafflesm
2
1
d
A Dl
t
| |
=
|
\ .
u = q
v
/ A
d
2
d
2
straight zigzag
ratio of pipe area
Free Convection
A free convection flow field is a self-sustained flow driven by the
presence of a temperature gradient. (As opposed to a forced
convection flow where external means are used to provide the
flow.) As a result of the temperature difference, the density field
is not uniform also. Buoyancy will induce a flow current due to
the gravitational field and the variation in the density field. In
general, a free convection heat transfer is usually much smaller
compared to a forced convection heat transfer. It is therefore
important only when there is no external flow exists.
hot
cold
T| +
T+ |
Flow is unstable and a circulatory
pattern will be induced.
General Considerations (cont)
Free Boundary Flows
Occur in an extensive (in principle, infinite), quiescent (motionless
at locations far from the source of buoyancy) fluid.
Plumes and Buoyant Jets:
Free Convection Boundary Layers
Boundary layer flow on a heated or cooled surface induced
by buoyancy forces.
( )
s
T T
=
Vertical Plates
Vertical Plates
Free Convection Boundary Layer Development on a Heated Plate:
Ascending flow with the maximum velocity occurring in the boundary layer
and zero velocity at both the surface and outer edge.
How do conditions differ from those associated with forced convection?
How do conditions differ for a cooled plate ( )?
s
T T
<
Basic Definitions
Buoyancy effect:
Warm,
Surrounding fluid, cold,
Hot plate
Net force=(
- )gV
The density difference is due to the temperature difference and it can be
characterized by their volumetric thermal expansion coefficient, |:
1 1 1
( )
P
T T T T
T
|
|
c A
= ~ =
c A
A ~ A
Grashof Number and Rayleigh Number
Define Grashof number, Gr, as the ratio between the buoyancy force and the
viscous force:
3 3
2 2
( )
S
g T T L g TL
Gr
| |
v v
A
= =
Grashof number replaces the Reynolds number in the convection
correlation equation. In free convection, buoyancy driven flow
sometimes dominates the flow inertia, therefore, the Nusselt number is
a function of the Grashof number and the Prandtle number alone.
Nu=f(Gr, Pr). Reynolds number will be important if there is an
external flow.
In many instances, it is better to combine the Grashof number and the
Prandtl number to define a new parameter, the Rayleigh number,
Ra=GrPr. The most important use of the Rayleigh number is to
characterize the laminar to turbulence transition of a free convection
boundary layer flow.
For example, when Ra>10
9
, the vertical free convection boundary
layer flow over a flat plate becomes turbulent.
Pertinent Dimensionless Parameters
Grashof Number:
( )
3
2
Buoyancy Force
Viscous Force
s
L
g T T L
Gr
|
v
=
Rayleigh Number:
( )
3
Pr
s
L L
g T T L
Ra Gr
|
vo
= =
characteristic length of surface L
(a thermo thermal dynamic expansion coeffi property of the cien fl d) t ui |
1
|
c
| |
=
|
c \ .
p
T
( ) Perfect Gas: =1/ K T |
T
s
T
Vertical Plates (cont)
Empirical Heat Transfer Correlations
Laminar Flow
( )
9
10 :
L
Ra <
( )
1/ 4
4 / 9
9 /16
0.670
0.68
1 0.492/ Pr
L
L
Ra
Nu = +
(
+
All Conditions:
( )
2
1/ 6
4 / 9
9 /16
0.387
0.825
1 0.492/ Pr
L
L
Ra
Nu
= +
`
(
+
)
Example
Determine the rate of heat loss from a heated pipe as a result of natural (free)
convection.
T
s
=100C
T
=0C
D=0.1 m
Film temperature( T
f
): averaged boundary layer temperature T
f
=1/2(T
s
+T
)=50 C.
k
f
=0.03 W/m.K, Pr=0.7, v=210
-5
m
2
/s, |=1/T
f
=1/(273+50)=0.0031(1/K)
3 3
6
2 5 2
1/ 6
2
9/16 8/ 27
2
( ) (9.8)(0.0031)(100 0)(0.1)
Pr (0.7) 7.6 10 .
(2 10 )
0.387
{0.6 } 26.0 (equation 11.15 in Table 11.1)
[1 (0.559 / Pr) ]
0.03
(26) 7.8( / )
0.1
( ) (7.8)( )(
S
D
f
D
S
g T T L
Ra
Ra
Nu
k
h Nu W m K
D
q hA T T
|
v
t
= = =
= + =
+
= = =
= = 0.1)(1)(100 0) 244.9( )
Can be significant if the pipe are long.
W =
Horizontal Plates
Horizontal Plates
Buoyancy force is normal, instead of parallel, to the plate.
Flow and heat transfer depend on whether the plate is heated or cooled and
whether it is facing upward or downward.
Heated Surface Facing Upward or Cooled Surface Facing Downward
s
T T
>
s
T T
<
( )
1/ 4 4 7
0.54 10 10
L
L L
Nu Ra Ra = < <
( )
1/ 3 7 11
0.15 10 10
L
L L
Nu Ra Ra = < <
How does depend on L when
1/ 3
?
L L
Nu Ra h
Horizontal Plates (cont)
Heated Surface Facing Downward or Cooled Surface Facing Upward
s
T T
>
s
T T
<
( )
1/ 4 5 10
0.27 10 10
L
L L
Nu Ra Ra = < <
Why do these flow conditions yield smaller heat transfer rates than those
for a heated upper surface or cooled lower surface?
Problem: Solar Receiver (cont)
PROPERTIES: Table A-4, air (T
f
= 550 K): k = 0.0439 W/mK, v = 45.6 10
-6
m
2
/s, o =
66.7 10
-6
m
2
/s, Pr = 0.683, | = 1.82 10
-3
K
-1
.
ANALYSIS: (a) The total heat loss is
( )
4
rad conv s s s s
q q q A T hA T T co
= + = +
With Ra
L
= g| (T
s
- T
)L
3
/vo = 9.8 m/s
2
(1.82 10
-3
K
-1
) 500K (12m)
3
/(45.6 66.7 10
-12
m
4
/s
2
) = 5.07 10
12
, the Churchill and Chu correlation yields
( )
{ }
2
1/ 6
2 2 L
8/ 27
9/16
k 0.387Ra 0.0439W/ m K
h 0.825 0.825 42.4 6.83W/ m K
L 12m
1 0.492/ Pr
= + = + =
+
`
(
(
)
Hence, with A
s
= tDL = 264 m
2
( ) ( )
4 2 8 2 4 2 2
q 264m 0.2 5.67 10 W/ m K 800K 264m 6.83W/ m K 500K
= +
6 5 6
rad conv
q q q 1.23 10 W 9.01 10 W 2.13 10 W = + = + =
Boiling Heat Transfer
General Considerations (cont.)
Special Cases in Boiling
Pool Boiling:
Liquid motion is due to natural convection and bubble-induced mixing.
Saturated Boiling:
Liquid temperature is slightly larger than saturation temperature.
Forced Convection Boiling:
Fluid motion is induced by external means, as well as by bubble-induced mixing.
Subcooled Boiling:
Liquid temperature is less than saturation temperature.
Boiling Curve
The Boiling Curve
Reveals range of conditions associated with saturated pool boiling on a
plot.
s e
q T A ''
Little vapor formation.
Liquid motion is due principally to single-phase natural convection.
Free Convection Boiling
( )
5
e
T C A <
Onset of Nucleate Boiling -
( )
5
e
ONB T C A ~
Water at Atmospheric Pressure
Boiling Curve (cont.)
Nucleate Boiling
( )
5 30
e
T C A < <
Isolated Vapor Bubbles
( )
5 10
e
T C A < <
Liquid motion is strongly influenced by nucleation
of bubbles at the surface.
and increase sharply with increasing .
s e
h q T A ''
Heat transfer is principally due to contact of liquid
with the surface (single-phase convection) and not
to vaporization.
Jets and Columns
( )
10 30
e
T C A < <
Increasing number of nucleation sites causes
bubble interactions and coalescence into
jets and slugs.
Liquid/surface contact is impaired.
continues to increase with while h begins to decrease.
s
q''
e
T A
Isolated bubble Jets or colums
Boiling Curve (cont.)
Critical Heat Flux - CHF,
( )
max
30
e
q T C A '' ~
Maximum attainable heat flux in nucleate boiling.
2
max
1 MW/m for water at atmospheric pressure. q'' ~
Potential Burnout for Power-Controlled Heating
An increase in beyond causes the surface to be blanketed by vapor,
and the surface temperature can spontaneously achieve a value that potentially
exceeds its melting point
s
q''
max
q''
( )
1000 . A >
s
T C
If the surface survives the temperature shock, conditions are characterized
by film boiling.
Film Boiling
Heat transfer is by conduction and
radiation across the vapor blanket.
A reduction in follows the cooling
curve continuously to the Leidenfrost
point corresponding to the minimum
heat flux for film boiling.
s
q''
min
q''
Boiling Curve (cont.)
A reduction in below causes an abrupt reduction in surface
temperature to the nucleate boiling regime.
s
q''
min
q''
Transition Boiling for Temperature-Controlled Heating
Characterized by a continuous decay of with increasing
( )
max min
from to
s
q q q '' '' ''
.
e
T A
Surface conditions oscillate between nucleate and film boiling, but portion
of surface experiencing film boiling increases with .
e
T A
Also termed unstable or partial film boiling.
Transition
Jet
Film
Dimensionless groups in Boiling
on condensati boiling during absorbed heat latent Maximum
liquid by absorbed heat sensible Maximum
h
T C
Ja Number Jacob
fg
p
/
) ( _ =
A
=
force tension surface
force nal gravitatio L g
Number Bond
V L
=
=
o
2
) (
3 3
2 2
( )
S
g T T L g TL
Gr
| |
v v
A
= =
Correleations
Pool Boiling Correlations
Nucleate Boiling
Rohsenow Correlation
( )
,
3
1/ 2
,
Pr
p l
l v
l fg n
s f f
e
g l
s
c T
q
g
h
C h
A
o
| |
(
=
|
(
\
'
|
.
'
,
, Surface/Fluid Combination (Table 10.1)
s f
C n
Critical Heat Flux
( )
1/ 4
max 2
0.149
l v
fg v
v
g
q h
o
(
'' =
(
Correleations
Film Boiling
The cumulative (and coupled effects) of convection and radiation across
the vapor layer
4 / 3 4 / 3 1/ 3
conv rad
h h h h ~ +
( )
( )
1/ 4
3
l v fg
conv
D
v v v s sat
g h D
h D
Nu C
k k T T
v
' (
= =
(
Geometry
Cylinder(Hor.) 0.62
C
Sphere 0.67
( )
,
0.80
fg fg p v s sat
h h c T T ' = +
( )
4 4
s sat
rad
s sat
T T
h
T T
co
=
If ,
0.75
conv rad
conv rad
h h
h h h
>
~ +
Factors affecting boiling heat transfer
* temperature difference : At = t
w
t
s
, the boiling state is different
at different At .
* liquid properties: the properties that help bubbles to generate
and leave the surface are helpful for increasing heat flux
e. g., r and k , h and o , h
* operation pressureP , t
s
, and o , therefore, increase P at
same At will let h .
* heating surfacethe material and the roughness have strong
effects to boiling heat transfer.
h is higher on clean surface than it is polluted.
A rough surface tends to generate more bubbles , h .
Rough the metal surface by mechanical processing or
chemical corrosionh on the copper surface can be increased
up to 80%)
Add a little other chemicals in boiling liquid, such as alcohol,
to reduce o of the liquid h can be increased 20% 80%
stirring the liquid to help the bubbles leaving the heating
surface.
Enhancement of boiling process
Problem: Electronic Chip Cooling
Chip thermal conditions associated with cooling by immersion in a fluorocarbon.
KNOWN: Thickness and thermal conductivity of a silicon chip. Properties of saturated
fluorocarbon liquid
FIND: (a) Temperature at bottom surface of chip for a prescribed heat flux and for a flux that is
90% of CHF, (b) Effect of heat flux on chip surface temperatures; maximum allowable heat flux
for a surface temperature of 80
C.
Problem: Electronic Chip Cooling (cont)
ASSUMPTIONS: (1) Steady-state conditions, (2) Uniform heat flux and adiabatic sides, hence
one-dimensional conduction in chip, (3) Constant properties, (4) Nucleate boiling in liquid.
PROPERTIES: Saturated fluorocarbon (given):
p,
c = 1100 J/kgK, h
fg
= 84,400 J/kg, =
1619.2 kg/m
3
,
v
= 13.4 kg/m
3
, o = 8.1 10
-3
kg/s
2
, = 440 10
-6
kg/ms, Pr = 9.01.
ANALYSIS: (a) Energy balances at the top and bottom surfaces yield
( )
o cond s o s
q q k T T L '' '' = = =
s
q''
; where T
s
and
s
q''
are related by the Rohsenow correlation,
( )
1/ 3
1/ 6
n
s,f fg
s
s sat
p, fg v
C h Pr
q
T T
c h g
o
| |
(
''
= |
(
|
(
\ .
Hence, for
s
q'' = 5 10
4
W/m
2
,
( )
1/ 3
1.7
4 2
s sat
6
0.005 84, 400J kg 9.01
5 10 W m
T T
1100J kg K
440 10 kg m s 84, 400J kg
| |
| =
|
\ .
( )
1/ 6
3 2
2 3
8.1 10 kg s
15.9 C
9.807m s 1619.2 13.4 kg m
(
( =
(
( )
s
T 15.9 57 C 72.9 C = + =
Problem: Electronic Chip Cooling (cont)
From the rate equation,
4 2
o
o s
s
q L 5 10 W m 0.0025m
T T 72.9 C 73.8 C
k 135W m K
''
= + = + =
For a heat flux which is 90% of the critical heat flux (C
1
= 0.9),
( )
( )
1/ 4
3 2 2 3
2
3
8.1 10 kg s 9.807m s 1619.2 13.4 kg m
13.4kg m
(
(
(
(
(
From the results of the previous calculation and the Rohsenow correlation, it follows that
( )
( )
1/ 3
1/ 3 4 2
e o
T 15.9 C q 5 10 W m 15.9 C 13.9 5 22.4 C '' A = = =
Hence, T
s
= 79.4C and
4 2
o
13.9 10 W m 0.0025m
T 79.4 C 82 C
135W m K
= + =
( )
1/ 4
v 3
o max fg v
2
v
g
q 0.9q 0.9 0.149h 0.9 0.149 84, 400J kg 13.4kg m
o
'' '' = = = (
(
4 2 4 2
o
q 0.9 15.5 10 W m 13.9 10 W m '' = =
(b) Parametric calculations for 0.2 s C
1
s 0.9 yield the following variations of
s o
T and T with q .
o
''
30000 60000 90000 120000 150000
Chip heat flux, qo''(W/m^2)
70
72
74
76
78
80
82
T
e
m
p
e
r
a
t
u
r
e
(
C
)
To
Ts
The chip surface temperatures, as well as the difference between temperatures, increase with
increasing heat flux. The maximum chip temperature is associated with the bottom surface, and
T
o
= 80C corresponds to
4 2
o,max
q 11.3 10 W m '' = <
which is 73% of CHF (
max
q''
= 15.5 10
4
W/m
2
).
COMMENTS: Many of todays VLSI chip designs involve heat fluxes well in excess of 15
W/cm
2
, in which case pool boiling in a fluorocarbon would not be an appropriate means of heat
dissipation.
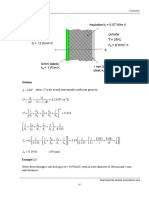
Problem: Quenching of Aluminum Sphere
Initial heat transfer coefficient for immersion of an
aluminum sphere in a saturated water bath at atmospheric
pressure and its temperature after immersion for 30 seconds.
KNOWN: A sphere (aluminum alloy 2024) with a uniform temperature of 500C and
emissivity of 0.25 is suddenly immersed in a saturated water bath maintained at atmospheric
pressure.
FIND: (a) The total heat transfer coefficient for the init ial condition; fraction of the total
coefficient contributed by radiation; and (b) Estimate the temperature of the sphere 30 s after
it has been immersed in the bath.
SCHEMATIC
Saturated water
T = 100 C
sat
o
10.5. Forced convection boiling
It is also called as two phase flow :
rapid change from the liquid to
vapor in the flow direction
Vapor + liquid flow
Boiling + Forced flow
Buoyancy effect + bulk motion
What are we interested in?
The Nu as a function of the distance from the
entrance
10.6 Condensation
If the temp. of the vapor is less than the saturation temperature
the vapor starts to condensate.
- a glass or cup with cold drink
- the mirror in the bathroom
Two types of condensation
Film condensation and dropwise condensation
Film condensation
The entire surface is coved by the condensed
liquid which flow continuously down on the
surface
The boundary layer can be defined.
The velocity at the surface is zero.
The velocity at the boundary b/w the liquid and
the vapor is not zero.
The vapor near the boundary flows as well.
The temperature monotonically decreases with y.
Condensation
Direct Contact Condensation
Nusselt Theory (Assumptions)
Average Nusselt Number (analytical solution)
( )
( )
1 4
3
0 943
/
.
L
l l v fg
L
l l l sat s
g h L
h L
Nu
k k T T
' (
= =
(
( )
( )
1 0 68
Jakob number
.
fg fg
p sat s
fg
h h Ja
c T T
Ja
h
' = +
Film Condensation on Radial Systems
A single tube or sphere:
( )
( )
1 4
3
/
l l l fg
D
l sat s
g k h
h C
T T D
u
' (
=
(
Tube: C =0.729 Sphere: C=0.826
( )
( )
1 4
3
0 729
/
,
.
l
l l fg
D N
l sat s
g k h
h
N T T D
u
( '
= (
(
A vertical tier of N tubes: figure (c)
Dropwise Condensation
Surface is covered by drops ranging from a few micrometers to agglomerations
visible to the naked eye.
Non-Wetting surface
(oil coating)
Wetting surface
Cohesion>Adhesion Cohesion < Adhesion
Which surface will induce the film condensation more easily?
Which condensation will have higher heat transfer coefficient?
Since the film condensation causes the insulation in the down stream the heat
transfer efficiency is less than the dropwise condensation
Special surface coating can be applied in order to induce the dropwise
condensation.
Dr. aziye Balku 137
RADIATION
Energy emitted by matter in the form of electromagnetic
waves(or photons) as a result of the changes in the
electronic configurations of the atoms or molecules
All bodies at a temperature above absolute zero emit thermal radiation
Does not require an intervening medium
Fastest (at the speed of light)
Possible also in vacuum
Example: energy of sun reaching the earth
Thermal radiation: form of radiation emitted by bodies
because of their temperature
different from other forms of electromagnetic radiation;
X-rays, gamma rays, microwaves, and television waves
that are not related with temperature
Electromagnetic Spectrum
Electromagnetic radiation is categorized
into types by their wavelengths.
The types of radiation and the respective
wavelength ranges are shown in Figure.
Radiation with shorter wavelengths are
more energetic, evident by the harmful
gamma and x-rays on the shorter end of
the spectrum.
Radio waves, which are used to carry radio
and TV signals, are much less energetic;
however, they can pass through walls with
no difficulty due to their long wavelengths.
The type of radiation emitted by a body
depends on its temperature.
In general, the hotter the object is, the
shorter the wavelengths of emitted
radiation, and the greater the amount.
A much hotter body, such as the sun
(~5800 K), emits the most radiation in the
visible range.
Introduction
Any matter with temperature above absolute zero (0 K)
emits electromagnetic radiation.
In a simplified picture, radiation comes from the
constantly changing electromagnetic fields of the
oscillating atoms.
Electromagnetic radiation can be visualized as waves
traveling at the speed of light.
The two prominent characters of the wave are the
wavelength () and frequency ().
The wavelength is the distance between crest to crest
on the wave.
The frequency is related to wavelength by the
following:
v
c
=
The amount of radiation emitted by a body depends
on its temperature, and is proportional to T
4
.
This relation shows that as the temperature of the
object increases, the amount of radiation emitted
increases very rapidly.
The emitted radiation will travel at the speed of light
until it is absorbed by another body.
The absorbing medium can be gas, liquid, or solid.
Radiation does not require a medium to pass
through.
This is demonstrated by solar radiation which pass
through interplanetary space to reach the earth.
Radiative Properties
When radiation strikes a surface, a portion of it
is reflected, and the rest enters the surface.
Of the portion that enters the surface, some
are absorbed by the material, and the
remaining radiation is transmitted through.
The ratio of reflected energy to the incident
energy is called reflectivity, .
Transmissivity () is defined as the fraction of
the incident energy that is transmitted through
the object.
Absorptivity () is defined as the fraction of the incident energy that is
absorbed by the object.
The three radiative properties all have values between zero and 1.
Furthermore, since the reflected, transmitted, and absorbed radiation
must add up to equal the incident energy, the following can be said
about the three properties:
o + t + = 1
Dr. aziye Balku 142
STEFAN-BOLTZMANN LAW
The maximum rate of radiation that can be emitted from a surface at an
absolute temperature is;
o Stefan-Boltzman constant
=5.6710
-8
W/m
2
.K
4
Black body: an idealized surface that emits radiation at this maximum rate
Black body radiation: radiation emitted by blackbodies
Real surfaces emit less radiation
c
1 = c
1 0 s s c For real bodies
For black bodies
Emissivity of the surface
4
max , S S emit
T A Q o =
-
4
S S emit
T A Q co =
-
Dr. aziye Balku 143
Radiation heat transfer between a surface
and the surfaces around it
Dr. aziye Balku 144
) (
4 4
surr S S rad
T T A Q =
-
co
) (
-
= T T A h Q
S S combined total
Combined heat transfer coefficient
includes effects of both convection
and radiation in such an example
and conduction heat transfer may
be neglected.
Emissivity
A black body is an ideal emitter.
The energy emitted by any real surface is less than
the energy emitted by a black body at the same
temperature.
At a defined temperature, a black body has the
highest monochromatic emissive power at all
wavelengths.
The ratio of the emissive power of real body E to the
blackbody emissive power E
b
at the same
temperature is the hemispherical emissivity of the
surface.
b
E
E
= c
The Emission Process
For gases and
semitransparent solids,
emission is a volumetric
phenomenon.
In most solids and liquids the
radiation emitted from interior
molecules is strongly absorbed by
adjoining molecules.
Only the surface molecules can
emit radiation.
Radiative Heat Transfer
Consider the heat transfer between two
surfaces, as shown in Figure.
What is the rate of heat transfer into
Surface B?
To find this, we will first look at the
emission from A to B.
Surface A emits radiation as described in
4
, A A A emitted A
T A Q o c =
This radiation is emitted in all directions, and only a fraction of it
will actually strike Surface B.
This fraction is called the shape factor, F.
The amount of radiation striking Surface B is therefore:
4
, A A A B A inceident B
T A F Q o c
=
The only portion of the incident radiation contributing to heating
Surface B is the absorbed portion, given by the absorptivity
B
:
4
, A A A B A B absorbed B
T A F Q o c o
=
Above equation is the amount of radiation gained by Surface B
from Surface A.
To find the net heat transfer rate at B, we must now subtract the
amount of radiation emitted by B:
4
, B B B emitted B
T A Q o c =
The net radiative heat transfer (gain) rate at Surface B is
emitted B absorbed B B
Q Q Q
, ,
=
4 4
B B B A A A B A B B
T A T A F Q o c o c o =
Shape Factors
Shape factor, F, is a
geometrical factor which is
determined by the shapes and
relative locations of two
surfaces.
Figure illustrates this for a
simple case of cylindrical
source and planar surface.
Both the cylinder and the plate
are infinite in length.
In this case, it is easy to see
that the shape factor is
reduced as the distance
between the source and plane
increases.
The shape factor for this
simple geometry is simply the
cone angle () divided by 2
Shape factors for other simple
geometries can be calculated
using basic theory of geometry.
For more complicated geometries,
the following two rules must be
applied to find shape factors
based on simple geometries.
The first is the summation rule.
1 2 2 2 1 1
= F A F A
This rule says that the shape factor from a surface (1) to
another (2) can be expressed as a sum of the shape
factors from (1) to (2a), and (1) to (2b).
The second rule is the reciprocity rule, which relates the
shape factors from (1) to (2) and that from (2) to (1) as
follows:
b a
F F F
2 1 2 1 2 1
+ =
Thus, if the shape factor from (1) to (2) is known, then the shape
factor from (2) to (1) can be found by:
1 2
1
2
2 1
= F
A
A
F
If surface (2) totally encloses the surface 1:
1
2 1
=
F
Planck Radiation Law
The primary law governing blackbody radiation is the Planck
Radiation Law.
This law governs the intensity of radiation emitted by unit
surface area into a fixed direction (solid angle) from the
blackbody as a function of wavelength for a fixed temperature.
The Planck Law can be expressed through the following
equation.
( )
1 1 2
5
2
1
1 2
,
= m sr Wm
e
hc
T I
kT
hc
b
h = 6.625 X 10
-27
erg-sec (Planck Constant)
K = 1.38 X 10
-16
erg/K (Boltzmann Constant)
C = Speed of light in vacuum
The behavior is illustrated in
the figure.
The Planck Law gives a
distribution that;
peaks at a certain wavelength,
the peak shifts to shorter
wavelengths for higher
temperatures, and
the area under the curve
grows rapidly with increasing
temperature.
Weins Displacement Law:
At any given wavelength, the black body
monochromatic emissive power increases with
temperature.
The wavelength
max
at which is a maximum
decreases as the temperature increases.
The wavelength at which the monochromatic
emissive power is a maximum is found by setting the
derivative of previous Equation with respect to .
( )
d
e
hc
d
d
T dE
kT
hc
=
1
1 2
,
5
2
mK T 8 . 2897
max
=
Wien law for three different stars
Dr. aziye Balku 157
Although there are three mechanisms
of heat transfer, a medium may involve
only two of them simultaneously
Heat transfer through a vacuum is by
radiation only
Solids: conduction and radiation
Fluids:
conduction and radiation (no motion)
convection and radiation (in motion)
conduction and convection (no
radiation)
Dr. aziye Balku 158
HEAT TRANSFER BETWEEN TWO
PLATES
rad cond total
Q Q Q
- - -
+ =
rad total
Q Q
- -
=
cond total
Q Q
- -
=
cond total
Q Q
- -
=
Potrebbero piacerti anche
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (119)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (399)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (587)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2219)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (344)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (890)
- Amanita Muscaria Herb of ImmortalityDocumento134 pagineAmanita Muscaria Herb of Immortalitycorbugabriel8448100% (4)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (73)
- From Creation To CreatorDocumento50 pagineFrom Creation To CreatorAmy Walton100% (8)
- Thickness of Rockwool InsulationDocumento21 pagineThickness of Rockwool InsulationjrfmlNessuna valutazione finora
- Design Calculations - Insulation Thickness REV-1Documento4 pagineDesign Calculations - Insulation Thickness REV-1delMAK100% (1)
- Solar OvenDocumento6 pagineSolar Ovenapi-322074467Nessuna valutazione finora
- BS en 196-9-2003 Heat of Hydration - Semi-Adiabatic MethodDocumento24 pagineBS en 196-9-2003 Heat of Hydration - Semi-Adiabatic MethodCarl HeNessuna valutazione finora
- Adsorption Column Design PDFDocumento58 pagineAdsorption Column Design PDFCharles John Catan100% (1)
- Drain Pipe Work of Indoor UnitDocumento24 pagineDrain Pipe Work of Indoor UnitMiguel AbuegNessuna valutazione finora
- Etag 002-1Documento75 pagineEtag 002-1DerRennerNessuna valutazione finora
- Gulf Per Lite Loose Fill Insulation TdsDocumento4 pagineGulf Per Lite Loose Fill Insulation TdsilieoniciucNessuna valutazione finora
- Production of 50 000 MT Per Year ButadieneDocumento311 pagineProduction of 50 000 MT Per Year ButadieneAshish Joshi100% (8)
- A BiofilmDocumento3 pagineA BiofilmAshish JoshiNessuna valutazione finora
- Engineering Mathematics AssignmentDocumento1 paginaEngineering Mathematics AssignmentAshish JoshiNessuna valutazione finora
- Why You and The Universe Are One - SFGateDocumento4 pagineWhy You and The Universe Are One - SFGateAshish JoshiNessuna valutazione finora
- Notice Board: Strategy For The Preparation (Mains Exam.)Documento3 pagineNotice Board: Strategy For The Preparation (Mains Exam.)Ashish JoshiNessuna valutazione finora
- 14 TH ReportDocumento99 pagine14 TH ReportSushil RajkumarpandianNessuna valutazione finora
- Pour Point Petro LabDocumento2 paginePour Point Petro LabAshish JoshiNessuna valutazione finora
- Project TDocumento3 pagineProject TAshish JoshiNessuna valutazione finora
- Pour Point Petro LabDocumento2 paginePour Point Petro LabAshish JoshiNessuna valutazione finora
- Joshi FluidizationDocumento8 pagineJoshi FluidizationAshish JoshiNessuna valutazione finora
- BXUV.P259 Fire Resistance Ratings - ANSI/UL 263Documento10 pagineBXUV.P259 Fire Resistance Ratings - ANSI/UL 263AlexNessuna valutazione finora
- 031 Vikotherm R2 Status May2014Documento59 pagine031 Vikotherm R2 Status May2014primz_nugrohoNessuna valutazione finora
- Aging of Polyurethane Foam Insulation inDocumento16 pagineAging of Polyurethane Foam Insulation invadim_k_69Nessuna valutazione finora
- Water Storage Tanks (M) - Rev 02-09-2017Documento4 pagineWater Storage Tanks (M) - Rev 02-09-2017arjun 11Nessuna valutazione finora
- TechnicalSheet ESDocumento1 paginaTechnicalSheet ESAndreja GjureskiNessuna valutazione finora
- Tarecpir Quick Guide Nov15 FINALDocumento16 pagineTarecpir Quick Guide Nov15 FINALdvtrascaNessuna valutazione finora
- Heaters Flexible in Design and Application To Fit Your Specific NeedsDocumento16 pagineHeaters Flexible in Design and Application To Fit Your Specific NeedskacemNessuna valutazione finora
- Module 1: Introduction To Heat TransferDocumento168 pagineModule 1: Introduction To Heat TransferMohit MittalNessuna valutazione finora
- CATALOG-TATEYAMA Low PDFDocumento8 pagineCATALOG-TATEYAMA Low PDFtri hantoroNessuna valutazione finora
- STS Qac Sop 013 Insulation ProcedureDocumento9 pagineSTS Qac Sop 013 Insulation Proceduremohd as shahiddin jafriNessuna valutazione finora
- Ebara Stainless Steel Centrifugal PumpDocumento3 pagineEbara Stainless Steel Centrifugal PumpHelda MhptNessuna valutazione finora
- Power Plant Bid Forms and Price SchedulesDocumento80 paginePower Plant Bid Forms and Price SchedulesAnonymous 7ZYHilDNessuna valutazione finora
- ThermodynamicsDocumento35 pagineThermodynamicsmaheshluintelNessuna valutazione finora
- C07. Heating and Ventilation RequirementsDocumento14 pagineC07. Heating and Ventilation RequirementsCzarSASNessuna valutazione finora
- Sustainable Energy Retrofit Plan For Enhancing Energy Efficiency of Residential Apartments in Arid Climate: Case of AfghanistanDocumento16 pagineSustainable Energy Retrofit Plan For Enhancing Energy Efficiency of Residential Apartments in Arid Climate: Case of AfghanistanmonirNessuna valutazione finora
- VAI-ME-SPC-111 Pipe Insulation Technical Specification - Rev ADocumento8 pagineVAI-ME-SPC-111 Pipe Insulation Technical Specification - Rev AAdvis100% (1)
- Heat Transfer - ExercisesDocumento4 pagineHeat Transfer - ExercisesKareem HamzaNessuna valutazione finora
- Riopipeline2019 1451 Ibp1451 19 Ultra Novel Flow As PDFDocumento11 pagineRiopipeline2019 1451 Ibp1451 19 Ultra Novel Flow As PDFMarcelo Varejão CasarinNessuna valutazione finora
- Glass and Insulating MaterialDocumento48 pagineGlass and Insulating MaterialShifat RashidNessuna valutazione finora
- Modes of Heat Transfer: O Q (Gate, Ies, Ias)Documento7 pagineModes of Heat Transfer: O Q (Gate, Ies, Ias)ankitNessuna valutazione finora
- Jablite Basement BoardDocumento7 pagineJablite Basement BoardalokNessuna valutazione finora
- Cervac Board HS&HS PlusDocumento2 pagineCervac Board HS&HS PlusCynthia MillerNessuna valutazione finora
- Baguio BOQ FormatDocumento176 pagineBaguio BOQ FormatronatabbuNessuna valutazione finora