Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Complex Titration Methods
Caricato da
Girma SelaleDescrizione originale:
Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Complex Titration Methods
Caricato da
Girma SelaleCopyright:
Formati disponibili
Chapter 17 Complexation Reactions and Titrations
Complex-formation reactions are widely used in analytical chemistry. One of the first uses of these reagents was for titrating cations. In addition, many complexes are colored or absorb ultraviolet radiation; the formation of these complexes is often the basis for spectrophotometric determinations. Some complexes are sparingly soluble and can be used in gravimetric analysis. Complexes are also widely used for extracting cations from one solvent to another and for dissolving insoluble precipitates. The most useful complex forming reagents are organic compounds that contain several electron donor groups that form multiple covalent bonds with metal ions.
FORMING COMPLEXES Most metal ions react with electron-pair donors to form coordination compounds or complexes. The donor species, or ligand is an ion or a molecule that forms a covalent bond with a cation or a neutral metal atom by donating a pair of electrons that are then shared by the two. The number of covalent bonds that a cation tends to form with electron donors is its coordination number. Typical values for coordination numbers are two, four, and six. The species formed as a result of coordination can be electrically positive, neutral, or negative.
A ligand that has a single donor group, such as ammonia, is called unidentate(single-toothed), whereas one such as glycine, which has two groups available for covalent bonding, is called bidenate. Tridentate, tetradentate, pentadentate, and hexadentate chelating agents are also known. Another important type of complex, a macrocycle, is formed between a metal ion and a cyclic organic compound. The selectivity of a ligand for one metal ion over another relates to the stability of the complexes formed. The higher the formation constant of a metal-ligand complex, the better the selectivity of the ligand for the metal relative to similar complexes formed with other metals.
Producing Soluble Compelxes Complexation reactions involve a metal ion M reacting with a ligand L to form a complex ML. M+L ML Complexation reactions occur in a stepwise fashion, and the reaction above is often followed by additional reactions: ML + L ML2 ML2 + L ML3 MLn-1 + L MLn Unidentate ligands invariably add in a series of steps. With multidentate ligands, the maximum coordination number of the cation may be satisfied with only one or a few added ligands.
continued
The equilibrium constants for complex formation reactions are generally written as formation constants. M + 2L
M + 3L M + nL
ML2
ML3 MLn
ML2 2 2 K1K 2 M L
ML3 3 K1K 2 K 3 3 M L
MLn n n K 1 K 2.... Kn M L
The overall formation constants are products of the stepwise formation constants for the individual steps leading to the product.
Forming Insoluble Species
The addition of ligands to a metal ion may result in insoluble species, such as the familiar nickeldimethylglyoxime precipitate. In many cases, the intermediate uncharged complexes in the stepwise formation scheme may be sparingly soluble, whereas the addition of more ligand molecules may result in soluble species. AgCl is insoluble, but addition of large excess of Cl- produces soluble AgCl2-, AgCl32-, and AgCl43-.
continued
In contrast to complexation equilibria, which are most often treated as formation reactions, solubility equilibria are usually treated as dissociation reactions
MxAy(s) xMy+(aq) + yAx-(aq)
Ksp = [My+]x[Ax-]y where, Ksp = solubility product. Hence, for BiI3, the solubility product is written Ksp = [Bi3+][I-]3. The formation of soluble complexes can be used to control the concentration of free metal ions in solution and thus control their reactivity.
TITRATION WITH INORGANIC COMPLEXING AGENTS Complexation reactions have many uses in analytical chemistry, but their classical application is in complexometric titrations. Here, a metal ion reacts with a suitable ligand to form a complex, and the equivalence point is determined by an indicator or a suitable instrumental method. The formation of soluble inorganic complexes is not widely used for titration but the formation of precipitates is the basis for many important determinations.
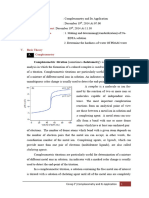
Complexation Titrations The progress of a complexometric titration is generally illustrated by a titration curve, which is usually a plot of pM = -log[M] as a function of the volume of titrant added. Most often in complexometric titrations the ligand is the titrant and the metal ion the analyte, although occasionally the reverse is true. Many precipitation titrations use the metal ion as the titrant. Most simple inorganic ligands are unidentate, which can lead to low complex stability and indistinct titration end points.
continued
As titrants, multidentate ligands, particularly those having four or six donor groups, have two advantages over their unidentate counterparts. First, they generally react more completely with cations and thus provide sharper end points. Second, they ordinarily react with metal ions in a single-step process, whereas complex formation with unidentate ligands usually involves two or more intermediate species.
Precipitation Titratons (Chapter 13)
Precipitation titrimetry, which is based on reactions that yield ionic compounds of limited solubility, is one of the oldest analytical techniques. The slow rate of formation of most precipitates, however, limits the number of precipitating agents that can be used in titrations to a handful. The most widely used and important precipitating reagent, silver nitrate, which is used for the determination of the halogens, the halogen-like anions. Titrations with silver nitrate are sometimes called argentometric titrations.
The Shapes of Titration Curves Titration curves for precipitation reactions are derived in a completely analogous way to the methods described for titrations involving strong acids and strong bases. P-functions are derived for the preequivalence-point region, the postequivalence point region, and the equivalence point for a typical precipitation titraton. Most indicators for argentometric titrations respond to changes in the concentration of silver ions. As a consequence, titraton curves for precipitation reactions usually consist of a plot of pAg versus volume of AgNO3.
End Point for Argentometric Titrations
Three types of end points are: (1) chemical, (2) potentiometric, (3) amperometric. Potentiometric end points are obtained by measuring the potential. To obtain an amperometric end point, the current generated between a pair of silver microelectrodes is measured and plotted as a function of reagent volume. The chemical end point consists of a color change or the appearance or disappearance of turbidity. The requirements are (1) the color change should occur over a limited range in the p-function, and (2) the color change should take place within the steep portion of the titration curve.
Formation of a Colored Precipitate The Mohr Method Sodium chromate can serve as an indicator for the argentometric determination of chloride, bromide, and cyanide ions by reacting with silver ion to form a brick-red silver chromate (Ag2CrO4) precipitate in the equivalencepoint region. The reactions involved in the determination of chloride and bromide (X-) are titration reaction: Ag+ + Xindicator reaction: 2Ag+ + CrO42AgX(s) [white] Ag2CrO4(s) [red]
The solubility of silver chromate is several times grater than that of silver chloride or silver bromide.
Adsorption Indicators: The Fajans Method
An adsorption indicator is an organic compound that tends to be adsorbed onto the surface of the solid in a precipitation titration. Ideally, the adsorption occurs near the equivalence point and results not only in a color change but also in a transfer of color from the solution to the solid (or the reverse). Fluorescein is a typical adsorption indicator useful for the titration of chloride ion with silver nitrate. In aqueous solution, fluorescein partially dissociates into hydronium ions and negatively charged fluoresceinate ion that are yellow-green. The fluoresceinate ion forms an intensely red silver salt. Titrations involving adsorption indicators are rapid, accurate, and reliable.
The Volhard Method (Colored Complex)
In the Volhard method, silver ions are titrated with a standard solution of thiocyanate ion: Ag+ + SCNAgSCN(s) Iron (III) serves as the indicator. The solution turns red with the first slight excess of thiocyanate ion: Fe(SCN)2+ Kf Fe 3+ SCN - red The titration must be carried out in acidic solution to prevent precipitation of iron (III) as the hydrated oxide. Fe3+ + SCN-
Fe( SCN )2+ 105 10 3 .
continued
The most important application of the Volhard method is for the indirect determination of halide ions. A measured excess of standard silver nitrate solution is added to the sample, and the excess silver ion is determined by back-titration with a standard thiocyanate solution.
ORGANIC COMPLEXING AGENTS (Chapter 17) Many different organic complexing agents have become important in analytical chemistry because of their inherent sensitivity and potential selectivity in reacting with metal ions. Such reagents are particularly useful in precipitating metal and in extracting metal from one solvent to another. The most useful organic reagents form chelate complexes with metal ions.
Reagents for Precipitating Metals
One important type of reaction involving an organic complexing agent is that in which an insoluble, uncharged complex is formed. Usually, it is necessary to consider stepwise formation of soluble species in addition to the formation of the insoluble species. Thus, a metal ion Mn+ reacts with a complexing agent Xto form MX(n-1)+, MX2(n-2)+, MXn-1+, and MXn(soln). Mn+ + nXMXn(soln)
MXn n - n K1 K 2.... Kn n+ M X
MXn(solid) MXn(soln) Keq = [MXn] Solubility product expression is: Ksp = [Mn+][X-]n = Keq / n
Forming Soluble Complexes for Extractions Many organic reagents are useful in converting metal ions into form that can be readily extracted from water into an immiscible organic phase. Extraction are widely used to separate metals of interest from potential interfering ions and for achieving a concentrating effect by extracting into a phase of smaller volume. Extractions are applicable to much smaller amounts of metals than precipitations, and they avoid problems associated with coprecipitation.
Ethylenediaminetetraacetic Acid (EDTA)
Ethylenediaminetetraacetic acid [also called (ethylenedinitrilo)tetraacetic acid], which is commonly shortened to EDTA, is the most widely used complexometric titrant. Fully protonated EDTA has the structure
The EDTA molecule has six potential sites for bonding a metal ion: the four carboxyl groups and the two amino groups, each of the latter with an unshared pair of electrons. Thus, EDTA is a hexadentate ligand.
EDTA Is a Tetrabasic Acid
The dissociation constants for the acidic groups in EDTA are K1 = 1.02 X 10-2, K2 = 2.14 X 10-3, K3 = 6.92 X 10-7, and K4 = 5.50 X 10-11 . It is of interest that the first two constants are of the same order of magnitude, which suggests that the two protons involved dissociate from opposite ends of the long molecule. As a consequence of their physical separation, the negative charge created by the first dissociation does not greatly affect the removal of the second proton. The various EDTA species are often abbreviated H4Y, H3Y-, H2Y2-, HY3-, and Y4-.
Reagents for EDTA Titrations
The free acid H4Y and the dihydrate of the sodium salt, Na2H2Y.2H2O, are commercially available in reagent quality. Under normal atmospheric conditions, the dihydrate, Na2H2Y.2H2O, contains 0.3% moisture in excess of the stoichiometric amount. This excess is sufficiently reproducible to permit use of a corrected weight of the salt in the direct preparation of a standard solution. The pure dihydrate can be prepared by drying at 80oC for several days in an atmosphere of 50% relative humidity.
Nitrilotriacetic acid (NTA)
The Nature of EDTA Complexes with Metal Ions
Solutions of EDTA are valuable as titrants because the reagent combines with metal ions in a 1:1 ratio regardless of the charge on the cation. Ag+ + Y4AgY3Al3+ + Y4AlYEDTA is a remarkable reagent not only because it forms chelates with all cation but also because most of these chelates are sufficiently stable for titrations. This great stability undoubtedly results from the several complexing sites within the molecule that give rise to a cagelike structure in which the cation is effectively surrounded and isolated from solvent molecules. The ability of EDTA to complex metals is responsible for its widespread use as a preservative in foods and in biological samples.
Indicators for EDTA Titrations Indicators are organic dyes that form colored chelates with metal ions in a pM range that is characteristic of the particular cation and dye. The complexes are often intensely colored and are discernible to the eye at concentrations in the range of 10-6 to 10-7 M. Eriochrome Black T is a typical metal-ion indicator used in the titration of several common cations. H2O + H2InHIn2- + H3O+ K1 = 5 X 10-7 red blue H2O + HIn2blue In3- + H3O+ orange K2 = 2.8 X 10-12
The acids and their conjugate bases have different colors.
continued
The metal complexes of Eriochrome Black T are generally red, as is H2In-. Thus, for metal-ion detection, it is necessary to adjust the pH to 7 or above so that the blue form of the species, HIn2-, predominates in the absence of a metal ion. Until the equivalence point in a titration, the indicator complexes the excess metal ion so that the solution is red. With the first slight excess of EDTA, the solution turns blue as a consequence of the reaction MIn- + HY3HIn2- + MY2red blue
Titration Methods Employing EDTA Direct Titration: Many of the metals in the periodic table can be determined by titration with standard EDTA solution. Some methods are based on indicators that respond to the analyte itself, whereas others are based on an added metal ion. Methods Based on Indicators for an Added metal Ion: In case where a good, direct indicator for the analyte is unavailable, a small amount of a metal ion for which a good indicator is available can be added. The metal ion must form a complex that is less stable than the analyte complex.
continued
Potentionmetric Methods: Potential measurements can be used for end-point detection in the EDTA titration of those metal ion for which specific ion electrodes are available.
Spectrophotometric Methods: Measurement of UV/visible absorption can also be used to determine the end points of titrations. In these cases, an instrument responds to the color change in the titration rather than relying on a visual determination of the end point.
continued
Back-Titration Methods: Back-titrations are useful for the determination of cations that form stable EDTA complexes and for which a satisfactory indicator is not available; the determination of thallium is an extreme example. The method is also useful for cations such as Cr(III) and Co(III) that react only slowly with EDTA. A measured excess of standard EDTA solution is added to the analyte solution. After the reaction is judged complete, the excess EDTA is back-titrated with a standard magnesium or zinc ion solution to an Eriochrome Black T or Calmagite end point.
continued
Displacement methods: In displacement titrations, an unmeasured excess of a solution containing the magnesium or zinc complex of EDTA is introduced into the analyte solution. If the analyte forms a more stable complex than that of magnesium or zinc, the following displacement reaction occurs: MgY2- + M2+ MY2- + Mg2+ where M2+ represents the analyte cation. The liberated Mg2+ or, in some cases Zn2+ is then titrated with a standard EDTA solution. Displacement titrations are used when no indicator for an analyte is available.
Potrebbero piacerti anche
- Unit 4 or Chapter 17Documento73 pagineUnit 4 or Chapter 17Danica Rose ZapanzaNessuna valutazione finora
- Chem 28 Post Lab 2Documento39 pagineChem 28 Post Lab 2juneNessuna valutazione finora
- Complexometric Titrations - PPT 1Documento26 pagineComplexometric Titrations - PPT 1Khairi Mustafa Salem67% (3)
- Complexation and Precipitation Reactions and TitrationsDocumento53 pagineComplexation and Precipitation Reactions and TitrationsDivya TripathyNessuna valutazione finora
- Ligand Subsitution On Carbonatopentamine Cobalt (III) NitrateDocumento40 pagineLigand Subsitution On Carbonatopentamine Cobalt (III) Nitratevijaybenadict100% (2)
- Oxidation-Reduction Reactions: Key Concepts, Examples, and Balancing EquationsDocumento28 pagineOxidation-Reduction Reactions: Key Concepts, Examples, and Balancing EquationsSachin KumarNessuna valutazione finora
- Complexometric Titrations by Gunja ChaturvediDocumento16 pagineComplexometric Titrations by Gunja ChaturvediGunja Chaturvedi100% (3)
- Formation Org CHP 2,, Stabilities of Coordination CompoundsDocumento19 pagineFormation Org CHP 2,, Stabilities of Coordination CompoundsErkyihun DamtieNessuna valutazione finora
- Session 6 - Analytical Chem - Complexation and Precipitation Part 2Documento22 pagineSession 6 - Analytical Chem - Complexation and Precipitation Part 2MehdiNessuna valutazione finora
- Mr. Deepak Pradhan: Asst. Prof. Dept. of Pharmaceutical Chemistry Ggscop, YnrDocumento41 pagineMr. Deepak Pradhan: Asst. Prof. Dept. of Pharmaceutical Chemistry Ggscop, YnrDeepak Pradhan100% (2)
- Transition MetalsDocumento15 pagineTransition MetalsNana Yaa Agyeiwaa Osei-AmoakoNessuna valutazione finora
- Experiment 3 2Documento6 pagineExperiment 3 2Kenth Roger A. MaquilingNessuna valutazione finora
- Utility of Complex Metric TitrationDocumento8 pagineUtility of Complex Metric Titrationcyper zoonNessuna valutazione finora
- Sayed Hashem..443Documento21 pagineSayed Hashem..443Bad BoyNessuna valutazione finora
- Quantitative Analysis of Cobalt and Copper SolutionDocumento10 pagineQuantitative Analysis of Cobalt and Copper SolutionDaniel Arul NesanNessuna valutazione finora
- SCH 1201 - Inorganic Chemistry Ii - Transition Metal ChemistryDocumento305 pagineSCH 1201 - Inorganic Chemistry Ii - Transition Metal ChemistrysanelisofuturemoyoNessuna valutazione finora
- JAE 1991 ReviewDocumento10 pagineJAE 1991 ReviewCan ERTANNessuna valutazione finora
- Redox Reactions NotesDocumento17 pagineRedox Reactions NotesTejas SinghNessuna valutazione finora
- 55 (2013) 12764-12766Documento3 pagine55 (2013) 12764-12766Juan FernandezNessuna valutazione finora
- FUll NOTES-Transition Metal ChemistryDocumento9 pagineFUll NOTES-Transition Metal ChemistryRomario Dallaz HudsonNessuna valutazione finora
- Organometallic ReactionDocumento33 pagineOrganometallic ReactionIsma KaniaNessuna valutazione finora
- Complexation TitrationDocumento20 pagineComplexation TitrationHardev Singh0% (1)
- 08 - Chapter 1Documento40 pagine08 - Chapter 1Girmaye HaileNessuna valutazione finora
- Migratory InsertionDocumento13 pagineMigratory InsertionNitaEkawatiNessuna valutazione finora
- Oxidative AdditionDocumento7 pagineOxidative AdditionMuhammad Hassan ZiaNessuna valutazione finora
- 5.3. Transition ElementsDocumento12 pagine5.3. Transition ElementsTahmina MaryamNessuna valutazione finora
- Redox Titration Applications PDFDocumento15 pagineRedox Titration Applications PDFAbdelrhman Abooda100% (2)
- Corrosion Control and PreventionDocumento34 pagineCorrosion Control and PreventionGrace Pentinio100% (1)
- Coordination Chemistry PrimerDocumento2 pagineCoordination Chemistry PrimerNaveen KamatNessuna valutazione finora
- MetalsDocumento39 pagineMetalsAditya ChudasamaNessuna valutazione finora
- Importance of C-C Cross Coupling Reactions in Pharmaceutical ChemistryDocumento67 pagineImportance of C-C Cross Coupling Reactions in Pharmaceutical ChemistryAnonymous vRpzQ2BLNessuna valutazione finora
- Instrumental Analysis Techniques: Chemiluminescence and BioluminescenceDocumento30 pagineInstrumental Analysis Techniques: Chemiluminescence and BioluminescenceSozdar ArgoshiNessuna valutazione finora
- Redox Reactions: Classical Idea of Redox ReactionDocumento10 pagineRedox Reactions: Classical Idea of Redox ReactionRohan ThomasNessuna valutazione finora
- Corrosion Of Metals ExplainedDocumento40 pagineCorrosion Of Metals ExplainedMobashir AliNessuna valutazione finora
- 04-Electrochemical Kinetics of CorrosionDocumento40 pagine04-Electrochemical Kinetics of Corrosionmubsan100% (1)
- Stoichiometry Oxidation NumbersDocumento13 pagineStoichiometry Oxidation NumbersSantosh KashidNessuna valutazione finora
- Aqueous Inorganic ChemistryDocumento40 pagineAqueous Inorganic ChemistryRamazan AshirkhanNessuna valutazione finora
- Class 11 Chemistry Notes 2023-24 8. Redox ReactionsDocumento40 pagineClass 11 Chemistry Notes 2023-24 8. Redox ReactionsAyushi Shah100% (1)
- Corrected Theoretical Basis of Analysis - Complexometric TitrationsDocumento19 pagineCorrected Theoretical Basis of Analysis - Complexometric TitrationsRajeswari RajiNessuna valutazione finora
- Stoichiometry Mole-II (XI)Documento29 pagineStoichiometry Mole-II (XI)Raju SinghNessuna valutazione finora
- Module 1 - Electrochemical EnergyDocumento129 pagineModule 1 - Electrochemical EnergyknightruzelNessuna valutazione finora
- Complexometric TitrationDocumento4 pagineComplexometric TitrationWendell Kim LlanetaNessuna valutazione finora
- Complexometry Determination Hardness ofDocumento30 pagineComplexometry Determination Hardness ofMichael CarloNessuna valutazione finora
- Complexometric TitrationDocumento29 pagineComplexometric TitrationLive Happy100% (1)
- 3830 Lecture Notes Part4 - 2008 - RedoxDocumento18 pagine3830 Lecture Notes Part4 - 2008 - RedoxKola PattabhiNessuna valutazione finora
- IR Spectroscopy Reveals C=O Bond StrengthDocumento29 pagineIR Spectroscopy Reveals C=O Bond StrengthSniper ArcheryNessuna valutazione finora
- Rahman S. Z. Saleem: Department of Chemistry & Chemical Engineering, SBASSE Lahore University of Management SciencesDocumento22 pagineRahman S. Z. Saleem: Department of Chemistry & Chemical Engineering, SBASSE Lahore University of Management Scienceshazyhazy9977Nessuna valutazione finora
- Organometallics - Part 2Documento33 pagineOrganometallics - Part 2Rohan Bhupen Shah ae22b052Nessuna valutazione finora
- Redox Reactions Class 11 Notes Chemistry: - OxidationDocumento5 pagineRedox Reactions Class 11 Notes Chemistry: - Oxidation11 A Prasann JamaleNessuna valutazione finora
- Mole Concept Motion ClassDocumento15 pagineMole Concept Motion ClassJames FrancisNessuna valutazione finora
- PDFDocumento155 paginePDFHifza shairwani100% (1)
- Inorganic Chemistry 4: Oxidative Addition and Reductive EliminationDocumento25 pagineInorganic Chemistry 4: Oxidative Addition and Reductive EliminationRafi NovalNessuna valutazione finora
- Fourth International Conference on Non-Aqueous Solutions: Vienna 1974Da EverandFourth International Conference on Non-Aqueous Solutions: Vienna 1974V. GutmannNessuna valutazione finora
- Reductive EliminationDocumento14 pagineReductive EliminationAɞdȗl NąvêêdNessuna valutazione finora
- Non-Aqueous Solutions – 5: Plenary and Section Lectures Presented at the Fifth International Conference on Non-Aqueous Solutions, Leeds, England, 5-9 July 1976Da EverandNon-Aqueous Solutions – 5: Plenary and Section Lectures Presented at the Fifth International Conference on Non-Aqueous Solutions, Leeds, England, 5-9 July 1976J. B. GillNessuna valutazione finora
- Chemistry: a QuickStudy Laminated Reference GuideDa EverandChemistry: a QuickStudy Laminated Reference GuideValutazione: 5 su 5 stelle5/5 (1)
- Selected Constants: Oxidation–Reduction Potentials of Inorganic Substances in Aqueous SolutionDa EverandSelected Constants: Oxidation–Reduction Potentials of Inorganic Substances in Aqueous SolutionNessuna valutazione finora
- Advances in Organometallic Chemistry and Catalysis: The Silver / Gold Jubilee International Conference on Organometallic Chemistry Celebratory BookDa EverandAdvances in Organometallic Chemistry and Catalysis: The Silver / Gold Jubilee International Conference on Organometallic Chemistry Celebratory BookArmando J. L. PombeiroValutazione: 5 su 5 stelle5/5 (1)
- Practice Makes Perfect in Chemistry: Oxidation-ReductionDa EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionValutazione: 5 su 5 stelle5/5 (1)
- Ligand Design in Metal Chemistry: Reactivity and CatalysisDa EverandLigand Design in Metal Chemistry: Reactivity and CatalysisMark StradiottoNessuna valutazione finora
- F AC 21 08 PotentiometryDocumento64 pagineF AC 21 08 PotentiometryGirma SelaleNessuna valutazione finora
- Redox Hand OutDocumento4 pagineRedox Hand OutGirma SelaleNessuna valutazione finora
- Chapter 25Documento20 pagineChapter 25vishwanathzNessuna valutazione finora
- Best Fruit 2013 (Procedure) 890Documento6 pagineBest Fruit 2013 (Procedure) 890Girma SelaleNessuna valutazione finora
- Solutions and ConcentrationDocumento11 pagineSolutions and ConcentrationGirma Selale100% (1)
- Statistics For Analytical Chemistry (Girma Selale)Documento46 pagineStatistics For Analytical Chemistry (Girma Selale)Girma SelaleNessuna valutazione finora
- Static Hand OutDocumento8 pagineStatic Hand OutGirma SelaleNessuna valutazione finora
- Redox Hand OutDocumento4 pagineRedox Hand OutGirma SelaleNessuna valutazione finora
- Cours Acide-Base en English PDFDocumento22 pagineCours Acide-Base en English PDFالغزيزال الحسن EL GHZIZAL HassaneNessuna valutazione finora
- Complexometric Titration 1Documento14 pagineComplexometric Titration 1Girma Selale0% (1)
- Complex Titration MethodsDocumento46 pagineComplex Titration MethodsGirma Selale100% (1)
- LSAP 423 Tech Data 25kVA-40KVA - 3PH 400VDocumento1 paginaLSAP 423 Tech Data 25kVA-40KVA - 3PH 400Vrooies13Nessuna valutazione finora
- KOREADocumento124 pagineKOREAchilla himmudNessuna valutazione finora
- DEWA Electrical Installation Regulations Section 1 OverviewDocumento123 pagineDEWA Electrical Installation Regulations Section 1 Overviewsiva_nagesh_280% (5)
- System Bus in Computer Architecture: Goran Wnis Hama AliDocumento34 pagineSystem Bus in Computer Architecture: Goran Wnis Hama AliGoran WnisNessuna valutazione finora
- Enbrighten Scoring Rubric - Five ScoresDocumento1 paginaEnbrighten Scoring Rubric - Five Scoresapi-256301743Nessuna valutazione finora
- Cytogenectics Reading ListDocumento2 pagineCytogenectics Reading ListHassan GillNessuna valutazione finora
- Hazop Recommendation Checked by FlowserveDocumento2 pagineHazop Recommendation Checked by FlowserveKareem RasmyNessuna valutazione finora
- English 2.2 FPT PolytechnicDocumento10 pagineEnglish 2.2 FPT PolytechnicKieu Mai Trang (FPL HCM)0% (1)
- Health Benefits of Kidney BeansDocumento17 pagineHealth Benefits of Kidney BeansShyneAneeshNessuna valutazione finora
- Armitage Tutorial for Cyber Attack ManagementDocumento54 pagineArmitage Tutorial for Cyber Attack Managementworkmumbai3870Nessuna valutazione finora
- Ultra Slimpak G448-0002: Bridge Input Field Configurable IsolatorDocumento4 pagineUltra Slimpak G448-0002: Bridge Input Field Configurable IsolatorVladimirNessuna valutazione finora
- Zombie Exodus Safe Haven GuideDocumento148 pagineZombie Exodus Safe Haven GuidejigglepopperNessuna valutazione finora
- Srimanta Shankardev: Early LifeDocumento3 pagineSrimanta Shankardev: Early LifeAnusuya BaruahNessuna valutazione finora
- PTW QuestionareDocumento63 paginePTW QuestionareIshtiaq Ahmad100% (2)
- Automorphic Representations and L-Functions For The General Linear Group - Volume 2cDocumento210 pagineAutomorphic Representations and L-Functions For The General Linear Group - Volume 2cluisufspaiandreNessuna valutazione finora
- VV016042 Service Manual OS4 PDFDocumento141 pagineVV016042 Service Manual OS4 PDFCamilo Andres Uribe Lopez100% (1)
- ATB Farmacología 2Documento194 pagineATB Farmacología 2Ligia CappuzzelloNessuna valutazione finora
- Epidemiological Cutoff Values For Antifungal Susceptibility TestingDocumento36 pagineEpidemiological Cutoff Values For Antifungal Susceptibility Testingdadrrui100% (1)
- Solution Proposal For SGF - BomDocumento2 pagineSolution Proposal For SGF - BomABHISHEK ADHIKARYNessuna valutazione finora
- Analog To Digital Conversion (ADC)Documento62 pagineAnalog To Digital Conversion (ADC)Asin PillaiNessuna valutazione finora
- Radial Drill Catalog-110620Documento14 pagineRadial Drill Catalog-110620Anto SiminNessuna valutazione finora
- A Comparison of Fuel Cell Testing Protocols PDFDocumento7 pagineA Comparison of Fuel Cell Testing Protocols PDFDimitrios TsiplakidesNessuna valutazione finora
- Schedule FinalDocumento6 pagineSchedule FinalJamora ManilynNessuna valutazione finora
- Design of Helical Antennas For 433 MHZ Radio Telemetry Ground Station and Uav (Unmanned Aerial Vehicle)Documento7 pagineDesign of Helical Antennas For 433 MHZ Radio Telemetry Ground Station and Uav (Unmanned Aerial Vehicle)Tiara Nira SariNessuna valutazione finora
- Single-phase half-bridge inverter modes and componentsDocumento18 pagineSingle-phase half-bridge inverter modes and components03 Anton P JacksonNessuna valutazione finora
- BCMEDocumento9 pagineBCMEVenkateshwaran VenkyNessuna valutazione finora
- Mapúa Welding Shop PracticeDocumento7 pagineMapúa Welding Shop PracticeJay EmNessuna valutazione finora
- Acl Data Analytics EbookDocumento14 pagineAcl Data Analytics Ebookcassiemanok01Nessuna valutazione finora
- Localized Commercial LeafletDocumento14 pagineLocalized Commercial LeafletJohn Kim CarandangNessuna valutazione finora
- Impacts of DecarbonizationDocumento2 pagineImpacts of DecarbonizationCM SoongNessuna valutazione finora