Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
132 870 1 PB
Caricato da
Yunifianti ViviDescrizione originale:
Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
132 870 1 PB
Caricato da
Yunifianti ViviCopyright:
Formati disponibili
DOI: 10.3315/jdcr.2010.
1059
60
Generalized molluscum contagiosum in an HIV patient treated with diphencyprone
Leena Chularojanamontri, Papapit Tuchinda, Kanokvalai Kulthanan, Woraphong Manuskiatti
Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand.
Corresponding author:
Leena Chularojanamontri, M.D. 2 Prannok Road, Bangkoknoi, Bangkok 10700, Thailand E-mail: sileena@staff1.mahidol.ac.th
Abstract
Background: Diphencyprone is a universal contact immunotherapy. The mechanism of action is based on an induction of the delayed-type hypersensitivity. Diphencyprone has been used in various forms for treatments of recalcitrant and facial warts, and alopecia areata. However, this treatment modality has not been generally used in immunocompromised patients. Main observation: The present report demonstrated the efficacy of diphencyprone immunotherapy on the treatment of generalized molluscum contagiosum in a human immunodeficiency virus (HIV)-infected patient. Minimal and transient side effects including pruritus, postinflammatory hyperpigmentation and irritation were noted. Conclusion: Diphencyprone contact immunotherapy appears to be a possible alternative treatment of widespread molluscum contagiosum in immunocompromised patients.
Key words:
AIDS, diphencyprone, DPC, HIV, molluscum contagiosum, poxvirus, treatment, virus
Introduction
Cutaneous molluscum contagiosum is a common viral skin infection which is caused by a DNA virus from the poxviruses (Poxviridae) family. It is most frequently found in children. However, it is also found in adolescents by sexually transmission, which usually appears on the genital area. Extragenital molluscum contagiosum occurs almost exclusively in HIV-infected patients or immunocompromised patients. Basically, molluscum contagiosum in healthy people is a self limiting disease which can be left to heal naturally. Therapy is mandatory for accelerating the healing process and concerning about spreading to other areas of the body and to other people. Contradictorily, it behaves differently in HIV infected patients. The more immunodeficiency is progressed, the more common and resistance to therapy is increased.1 Many treatment options such as physical destruction of the lesions (i.e. cryotherapy, extraction and curettage), topical agents (i.e. those applied directly to the lesions) and systemic treatment (i.e. those affecting the whole body) have been used for molluscum contagiosum.1 However, there is no standard treatment for molluscum contagiosum. The choice of treatment depends on the extension and location of the lesions and cooperation of the patients. Our
patient presented with generalized molluscum contagiosum and HIV infection. Judging from our review, diphencyprone (DPC) which is a contact immunotherapy was decided upon for use in our patient. The mechanism of action is based on an induction of the delayed-type hypersensitivity. DPC has been used in various forms for treatments of recalcitrant and facial warts, and alopecia areata.2 However, this treatment modality has not been generally used in immunocompromised patients. The present report demonstrated the efficacy of DPC on the treatment of generalized molluscum contagiosum in an HIV patient.
Case report
A 32-year-old patient presented with widespread umbilicated papules on genital and extragenital area for 7 months. At that time, he did not have other cutaneous disorders of HIV infection such as oral hairy leukoplakia, oral thrush, papular pruritic eruption, Kaposi sarcoma. Skin biopsy was performed on the lesion of the trunk and demonstrated molluscum bodies. A through history revealed that patient was HIV positive. He had a CD4+ count of 47 cell/mm3 but an HIV RNA level was not investigated at a previous hospital.
J Dermatol Case Rep 2010 4, pp 60-62
61
Diphencyprone in molluscum contagiosum, Chularojanamontri et al.
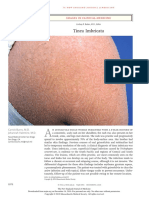
He has also received the antiretroviral therapy (lamivudine, tenofovir, efavirenz) and multidrug resistant therapy for tuberculosis (ethambutol, isoniazid, rifampicin, pyrazinamide, clarithromycin) for 7 months. His lesions did not improve when the patient was on the antiretroviral medications. The molluscum lesions were widespread on the eyelids, trunk and genital areas (Fig. 1). The total number of lesions was more than 100. Thus, the curettage, extraction or cryotherapy are cumbersome treatments especially on the eyelid area. Due to the widespread locations of the lesions, we decided to treat this patient with DPC. The instruction about treatment was conveyed to the patient including that he would be sensitized with 2% DPC on the left inner upper arm. He was instructed to keep this area dry for 6 hours. The desirable side-effects such as pruritus, erythema or allergic contact dermatitis, and undesirable side-effects, such as vesicle, dyspigmentation were explained to the patient. The left inner upper arm was chosen because of cosmetic concerns. The duration of treatment was 1-2 week intervals. After understanding the protocol of treatment, he decided to choose this method to treat his molluscum lesions. The patient was sensitized with 2% DPC on his left inner upper arm. After 2 weeks, a sensitization had occurred by showing the eczematous lesion on sensitized area. Then, his lesions were later treated with a concentration of 0.001%. Ten percent of the lesions were applied with DPC on each followup visit. The concentrations of DPC were gradually increased from 0.001% to 2.0%, depending on the patients tolerability. The frequency of application was 1-2 week intervals. All of the lesions on the eyelids cleared within 2 months of treatment. At that time, He had a CD4+ count of 76 cell/mm3. The other lesions on the trunk and the genitalia gradually decreased in size and resolved with consecutive applications of DPC (Fig. 2). Minimal and transient side effects, including pruritus, postinflammatory hyperpigmentation and irritation were noted.
Figure 1 Molluscum contagiosum lesions in the axilla.
Figure 2 Axilla after therapy with diphencyprone.
Discussion
The tri-ethyleneiminobenzoquinone (TEIB or trenimon) was the first topical contact sensitizer that was used to treat multiple superficial basal and squamous cell carcinomas in 1965.2 Unfortunately, it is carcinogenic, as just as the nitrogen mustard which had been used to treat cutaneous T-cell lymphoma. The other allergens such as poison ivy, nickel and formalin are unsuitable for topical immunotherapy because they can cross-react with other substances and common in environment.2 Therefore, the good contact sensitizer should be safe, lack of potential to induce cross-sensitization to other substances and not be a carcinogen. Nowadays, dinitrochlorobenzene (DNCB), squaric acid dibutyl ester (SADBE) and DPC are most common contact sensitizers. (SADBE is considerably more expensive than other agents (2).) There are many reports of their efficacy to treat benign and malignant dermatoses such as alopecia areata, viral warts, basal and squamous cell carcinoma and cutaneous T-cell lymphoma. DPC was first synthesized in 1959. It is prepared by bromination of dibenzylketone and then cyclized with base to
J Dermatol Case Rep 2010 4, pp 60-62
yield DPC.2 The standard solvent of DPC is acetone which is a strong Ultraviolet (UV) light absorber. UV radiation and heat cause degradation to DPC. Thus, the dilutions of DPC are usually collected in brown UV-opaque bottles and stored at room temperature. Basically, its shelf life is probably no longer than 6 months. It does not appear to cross-react with other chemicals and is not mutagenic in Ames assay. DPC is a universal contact sensitizer in which the response to treatment can occur in remote areas other than those of topical application. It has been widely used in alopecia areata and viral warts. Some studies reported successful treatment in molluscum contagiosum, vitiligo, primary and secondary malignant melanoma.2-7 The mechanisms of action include alterations in cytokine levels, nonspecific inflammation and reversal of CD4:CD8 ratio.8 The common side effects are generalized eczema, urticaria, local blistering and swelling and regional lymphadenopathy. The uncommon side effects are erythema multiforme, fever, palpitations, flulike symptoms, headache, vitiligo, and dyschromia in confetti.2,8-13 In HIV-seropositive patients, DNCB can be used
Diphencyprone in molluscum contagiosum, Chularojanamontri et al.
62
as an alternative treatment because it appears to have a beneficial clinical and immunologic outcome. A pilot study of DNCB and acquired immune deficiency syndrome (AIDS) was done in 1990.14,15 The immunologic profile of DNCB sensitized patients showed increasing of cytotoxic CD8 Tcells, natural killer cells and decreasing of HIV RNA levels.15 More important, the death rate in DNCB compliant group was lower than the non-compliant group.14 Traub and Margulis collected data on HIV-infected patients with topical DNCB in Brazil. After 21 months of treatment, 19 patients had significantly increased CD4 and CD8 T-cell counts and decreased parasitic infections.16 Our patient has an HIV infection and generalized molluscum contagiosum. Kang HS et al reported complete clearance of multiple molluscum contagiosum with DPC in 14 of 22 children (63.6%).3 Regard to DPC and HIV-infected patients, DPC sensitization also demonstrates diagnostic utility as a functional measure of immune competence but offers limited data about clinical efficacy.17 Following from these rationales, DPC has been tried in our patient, although there has not been any previous report from PubMed database for the DPC usage in treatment of molluscum contagiosum in an HIV patient. Good efficacy and minimal side effects were noted in our patient.
4. Aghaei S, Ardekani GS. Topical immunotherapy with diphenylcyclopropenone in vitiligo: A preliminary experience. Indian J Dermatol Venereol Leprol. 2008; 74: 628-631. 5. Damian DL, Thompson JF. Treatment of extensive cutaneous metastatic melanoma with topical diphencyprone. J Am Acad Dermatol. 2007; 56: 869-871. 6. Yoshino T, Asada H, Ando Y, Fujii H, Yamaguchi Y, Yoshikawa K, Itami S. Impaired responses of peripheral blood mononuclear cells to T-cell stimulants in alopecia areata patients with a poor response to topical therapy. Br J Dermatol. 2001; 145: 415-421. 7. Orecchia G, Douville H, Santagostino L,Rabbiosi G. Treatment of Multiple Relapsing Warts with Diphenciprone. Dermatologica. 1988; 177: 225-231. 8. Van der Steen P, Van de Kerkhof P, Der Kinderen D, van Vlijmen I, Happle R. Clinical and immnohistochemical responses of plantar warts to topical immunotherapy with diphenylcyclopropenone. J Dermatol. 1991; 18: 330-333. 9. Gordon PM, Aldrige RD, McVittie E, Hunter JA. Topical diphencyprone for alopecia areata: evaluation of 48 cases after 30 months follow-up. Br J Dermatol. 1996; 134: 869-871. 10. Hatzis J, Georgiotouo K, Kostakis P, Anastasiadis G, Tosca A, Varelzidis A, Straigos J. Treatment of alopecia areata with diphencyprone. Austral J Dermatol. 1988; 29: 33-36. 11. Lane PR, Hogan DJ. Diphencyprone [Letter]. J Am Acad Dermatol. 1988; 19: 364-365. 12. Monk B. Induction of hair growth in alopecia totalis with diphencyprone sensitization. Clin Exp Dermatol. 1989; 14: 154-157. 13. Van der Steen P, Happle R. Dyschromia in confetti as a side effect of topical immunotherapy with diphencyclopropenone. Arch Dermatol. 1992; 128: 518-520. 14. Stricker RB, Goldberg B, Epstein WL. Topical immune modulation (TIM): a novel approach to the immunotherapy of systemic disease. Immunolo Lett. 1997; 59: 145-150.
Conclusion
Topical DPC is easy and inexpensive method that can produce complete or partial regression in generalized molluscum contagiosum. It should be considered as another option in immunocompetent and immunocompromised patients, when other therapies are contraindicated.
References
1. Van der Wouden JC, van der Sande R, van Suijlekom-Smit LWA, Berger M, Butler C, Koning S. Interventions for cutaneous molluscum contagiosum. Cochrane Database of Syst Rev. 2009; 7: CD004767. 2. Buckley DA, Du Vivier AW. The therapeutic use of topical contact sensitizers in benign dermatoses. Br J Dermatol. 2001; 145: 385-405. 3. Kang SH, Lee D, Hoon Park J, Cho SH, Lee SS, Park SW. Treatment of Molluscum Contagiosum with Topical Diphencyprone Therapy. Acta Derm Venereol. 2005; 85: 529-530.
15. Stricker RB, Zhu YS, Elswood BF, Dumlao C, Van Elk J, Berger TG, Tappero J, Epstein WL, Kiprov DD. Pilot study of topical dinitrochlorobenzene (DNCB) in human immunodeficiency virus infection. Immuno Lett. 1993; 36: 1-6. 16. Traub A, Margulis SB, Stricker RB. Use of dinitrochlorobenzene (DNCB) as an immune modulator in HIV-positive patients: A pilot study from Brazil [Abstract PB0304]. Presented at: International Conference on AIDS. Irmandade da Santa Casa de Misericordia de Porto Alegre, Brazil: August 7-12, 1994. 17. Levis WR. Evaluation of delayed-type hypersensitivity in patients with suspected immunodeficiency. Am J Med. 2008; 12: e13.
J Dermatol Case Rep 2010 4, pp 60-62
Potrebbero piacerti anche
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- PDF 2Documento7 paginePDF 2Yunifianti ViviNessuna valutazione finora
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- 428 FullDocumento8 pagine428 FullYunifianti ViviNessuna valutazione finora
- Brain and BehaviorDocumento35 pagineBrain and BehaviorYunifianti ViviNessuna valutazione finora
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- Acute Kidney Injury Network - Definition of AKI 2007Documento8 pagineAcute Kidney Injury Network - Definition of AKI 2007Shreedhar JoshiNessuna valutazione finora
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- Selim Prestroke ACEDocumento7 pagineSelim Prestroke ACEYunifianti ViviNessuna valutazione finora
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (344)
- 10990106Documento8 pagine10990106Yunifianti ViviNessuna valutazione finora
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (399)
- Early Stroke Risk After Transient Ischemic Attack Among Individuals With Symptomatic Intracranial Artery StenosisDocumento5 pagineEarly Stroke Risk After Transient Ischemic Attack Among Individuals With Symptomatic Intracranial Artery StenosisYunifianti ViviNessuna valutazione finora
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- Migra inDocumento14 pagineMigra indoroty24Nessuna valutazione finora
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- JCN 2 126Documento8 pagineJCN 2 126Yunifianti ViviNessuna valutazione finora
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- Early Stroke Risk After Transient Ischemic Attack Among Individuals With Symptomatic Intracranial Artery StenosisDocumento5 pagineEarly Stroke Risk After Transient Ischemic Attack Among Individuals With Symptomatic Intracranial Artery StenosisYunifianti ViviNessuna valutazione finora
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Getting To The Right SiteDocumento17 pagineGetting To The Right SiteYunifianti ViviNessuna valutazione finora
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- Copy and Paste To Create A DocumentDocumento1 paginaCopy and Paste To Create A DocumentYunifianti ViviNessuna valutazione finora
- Severe Combined ImmunodeficiencyDocumento10 pagineSevere Combined ImmunodeficiencySUBHADEEP ADITYANessuna valutazione finora
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (73)
- Urosepsis: Oleh: DR - Yolan Novia Ulfah Pembimbing: Dr. Prabudi, SP.B (K) Onk, M.Kes, FICSDocumento30 pagineUrosepsis: Oleh: DR - Yolan Novia Ulfah Pembimbing: Dr. Prabudi, SP.B (K) Onk, M.Kes, FICSYolan Novia UlfahNessuna valutazione finora
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- TAREA 13 Safety Data Sheet For Quality SpecimensDocumento5 pagineTAREA 13 Safety Data Sheet For Quality SpecimensRosa CanahuiNessuna valutazione finora
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- Microbiology by CherkesDocumento600 pagineMicrobiology by CherkesГ. АлтангадасNessuna valutazione finora
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- Ca2 Prefinal Exam Pointers To ReviewDocumento3 pagineCa2 Prefinal Exam Pointers To ReviewlittlejynxNessuna valutazione finora
- Management of Tinea Corporis, Tinea CrurisDocumento10 pagineManagement of Tinea Corporis, Tinea CrurisRansidelenta Vistaprila ElmardaNessuna valutazione finora
- Imunologi Tumor: Syarifuddin WahidDocumento40 pagineImunologi Tumor: Syarifuddin WahidinhaNessuna valutazione finora
- Hiv/Aids: Prof. Dr. Ram Sharan Mehta Medical-Surgical Nursing DepartmentDocumento196 pagineHiv/Aids: Prof. Dr. Ram Sharan Mehta Medical-Surgical Nursing DepartmentDavid Sergio Salas VargasNessuna valutazione finora
- Reading Comprehension: HIVDocumento2 pagineReading Comprehension: HIVFlori75% (8)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- Spot DiagnosisDocumento77 pagineSpot DiagnosisRapid Medicine100% (3)
- Tonsillitis HelpDocumento8 pagineTonsillitis HelpGregory Allan CoNessuna valutazione finora
- Novel Proprietary Single-Domain Antibody Fragments: What Are Nanobodies?Documento18 pagineNovel Proprietary Single-Domain Antibody Fragments: What Are Nanobodies?InêsNessuna valutazione finora
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- Immunologic Tolerance AND AutoimmunityDocumento22 pagineImmunologic Tolerance AND AutoimmunityTenunBagusDariNttNessuna valutazione finora
- Tinea ImbricataDocumento1 paginaTinea ImbricataVirginia AneleyNessuna valutazione finora
- Approach To Child With Fever: Liew Qian YiDocumento33 pagineApproach To Child With Fever: Liew Qian YinavenNessuna valutazione finora
- Concept PaperDocumento1 paginaConcept PaperKurt NicolasNessuna valutazione finora
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (120)
- Greyson 2019Documento10 pagineGreyson 2019Ziha Zia Leonita FauziNessuna valutazione finora
- Chen & Zhang (2021) - Viral VectorsDocumento7 pagineChen & Zhang (2021) - Viral VectorsBrian ZárateNessuna valutazione finora
- Current Control Propaganda of Candida Infection Using Dragon FruitDocumento2 pagineCurrent Control Propaganda of Candida Infection Using Dragon FruitEishe Ann GaviolaNessuna valutazione finora
- Microbiology Manor2010Documento27 pagineMicrobiology Manor2010babyloves18Nessuna valutazione finora
- DysenteryDocumento9 pagineDysenteryRifki HerdaniNessuna valutazione finora
- Atopic DermatitisDocumento43 pagineAtopic DermatitisSaurav Arora100% (2)
- Dengue PPT YaarDocumento21 pagineDengue PPT YaarY ShouryaNessuna valutazione finora
- 15 Rhabdoviridae RabiesDocumento64 pagine15 Rhabdoviridae RabiesSadam IrshadNessuna valutazione finora
- Dr. Bhaskar Ganguly: M.V.Sc. Scholar, Animal Biotechnology Center, Cvasc, Gbpuat, Pantnagar - 263 145Documento18 pagineDr. Bhaskar Ganguly: M.V.Sc. Scholar, Animal Biotechnology Center, Cvasc, Gbpuat, Pantnagar - 263 145Bhaskar GangulyNessuna valutazione finora
- Congenital ToxoplasmosisDocumento64 pagineCongenital ToxoplasmosisFadila R. Lubis LubisNessuna valutazione finora
- Abbas Et Al. 2020 - Nature Immunology-2020-Abbas-TomaselloDocumento33 pagineAbbas Et Al. 2020 - Nature Immunology-2020-Abbas-TomaselloAchille BroggiNessuna valutazione finora
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- Drug Study of Ceftriaxone & RowatinexDocumento5 pagineDrug Study of Ceftriaxone & RowatinexLorina Lynne ApelacioNessuna valutazione finora
- 03 COVID Shimabukuro 508Documento39 pagine03 COVID Shimabukuro 508Kylie Betania Chávez VásquezNessuna valutazione finora
- LeukopeniaDocumento3 pagineLeukopeniaTanto SuriotoNessuna valutazione finora