Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Cu Damascene
Caricato da
jeren1228Descrizione originale:
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Cu Damascene
Caricato da
jeren1228Copyright:
Formati disponibili
Muhammad Khan Min Sung Kim October 19, 2011
Damascene Process and Chemical Mechanical Planarization
The constant demand to scale down transistors and improve device performance has led to material as well as process changes in the formation of IC interconnect. Traditionally, aluminum has been used to form the IC interconnects. The process involved subtractive etching of blanket aluminum as defined by the patterned photo resist. However, the scaling and performance demands have led to transition from Aluminum to Copper interconnects. The primary motivation behind the introduction of copper for forming interconnects is the advantages that copper offers over Aluminum. The table 1 below gives a comparison between Aluminum and Copper properties. Aluminum Copper 2.65-cm 1.68-cm 238W/m-K 394W/m-K Table 1: Comparing Aluminum and Copper properties [3]
Electrical Resistivity Thermal Conductivity
The table 1 shows that the electrical resistivity of Copper is much smaller than Aluminum. The smaller resistivity translates into smaller line resistances, which in turn translates into smaller RC delay. Smaller RC delay means the speed performance of the devices increases. Secondly as, resistivity decreases, Joule Heating also decreases. Joule Heating, Q = J2 So as resistivity decreases, Q decreases. This means that for the same amount of Joule Heating, we can have larger current densities, J. Larger values of current density means that we can have smaller areas, i.e. we can scale down the devices even more. Thirdly copper has larger activation energy than aluminum. Electromigration, a critical reliability issue, is largely dependent on the activation energy of the metal. Electromigration mean-time-to-failure (MTF) is directly proportional to the exponent of activation energy, EA of the metal. MTF = A(J-n)eEa/kT Since copper has higher activation energy than aluminum, it is more resistant to electromigration failures [3]. There are a few challenges associated with using copper for interconnects. Copper is difficult to etch using conventional etching techniques. Copper does not produce a volatile by-product during etching. For example, when chlorine gas is used to etch copper, it forms a chloride that does not readily evaporate. The chloride molecule sticks to the surface and prevents the underlying copper from etching. Another problem with copper is that it quickly diffuses into oxides and silicon, thereby causing junction spiking and shorts. Unlike aluminum, copper quickly oxidizes in air and does not form a protective oxide layer. Thus copper is less resistive to corrosion/oxidation. In order to address these challenges, IBM introduced a unique additive processing technique to form copper IC interconnects, commonly known as Damascene process. The name Damascene originates in Damascus, the capital of modern Syria. Damascene process is reminiscent of the metal inlay techniques used in the Middle East since the middle ages [2]. In Damascene process, copper is planarized, using Chemical Mechanical Planaraztion technique, rather than being etched. Secondly in order to prevent copper contamination into oxide or silicon, a barrier layer is deposited before copper deposition. Typically, Tantalum, Tantalum nitride, or Titanium Nitride is used as barrier layer. The Damascene process is an additive process. Firstly, the dielectric is deposited, and then dielectric is etched according to the defined photoresist pattern. Next, a barrier layer is deposited to prevent junction spiking. After that a thin seed layer of copper is deposited conformally over the surface. The seed layer is important, because without the seed layer the copper cannot be electrochemically deposited. After depositing
the seed layer, the structure is electroplated with copper. Final step is to planarize the excess copper. The process steps are shown in figure 1 below:
A variation of Damascene process is Dual Damascene process. In this process, the vias and the metal lines are created by etching holes and trenches in the dielectric, after which copper is deposited in a single step. In Dual Damascene process, one photo/etch step is needed for making holes for vias in the dielectric and another photo/etch step is needed for making trenches for metal lines. These two photo/etch steps can be performed in any order, i.e. via first then trench or trench first then via. The process steps are shown in figure 2 below:
Fig. 2 : Dual Damascene Process [2] Chemical Mechanical Planarization (CMP) is a process of smoothing wafer surfaces with the combination of chemical and mechanical forces [8]. The main reason for using a hybrid of chemical etching and free abrasive polishing is because mechanical grinding alone causes too much damage to the wafer surface, while chemical etching alone does not promote good planarization of surface. This process is generally used to remove silicon oxide, poly silicon, and metal surfaces and to provide plane surfaces. There are several components involved in CMP. First, there is platen, which is a rotating base that holds all the components on top. Abrasive sponge-like pad covers platen and rotates with the platen to
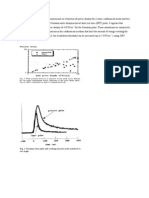
mechanically polish the surface of wafer. Wafer carrier is composed of retaining ring, backing film, carrier housing, and back press vacuum. A wafer is held upside down by retaining ring on a backing film. The retaining ring keeps the wafer in correct horizontal position for even polishing. Both the pad and wafer carrier are then counter rotated while slurry containing both abrasives and reactive chemicals is passed underneath. The following figure illustrates the design of CMP.
Figure 3 Illustration of Chemical Mechanical Planarization Machine [5]. CMP is achieved through applying load force provided by back press vacuum on the back of the wafer. When the pressure is applied on the wafer, the higher surfaces are polished away to level the surface layer. Also, most chemical reactions are isotropic and etch different crystal planes with different speed. CMP involves both chemical and mechanical effects at the same time. Some variables, such as temperature of the system, back pressure, velocity of the rotation, and slurry flow rate, play important roles in the quality of planarization. Typical process conditions are temperature from 10 C to 70 C, back pressure from 2 psi to 7 psi, velocity from 20 rpm to 80 rpm, and slurry flow rate from 100 mL/min to 200 mL/min. Typical removal rate for silicon oxide layer is approximately 2800/min and for metal is approximately 3500 /min.
The advantages of CMP are good selectivity, reduction of resist thickness variation, increased resolution of photolithographic process, improved step coverage of subsequent layer deposition, and multi-level interconnection. CMP encourages no lapping, which means that it will only polish the selected material and stop polishing at non-selective layer, whereas different planarization techniques may remove different materials at the same rate. Also, because CMP smoothes the surface layer, this accounts for reduction of resist thickness variation. Any higher surfaces are polished away to level the surface layer. CMP is essential for multi-layer chips. Neat planarization will improve step coverage of subsequent layer deposition, such as metal copper or aluminum, thus it maximizes the interconnection between the surface layers. This will promote multi-layer chips to be faster and more reliable because any bad connection between layers will be minimized. CMP also increases the resolution of photolithographic process by reducing the depth of focus. In order to project a photolithographic beam onto the surface of a wafer, the roughness of the surface of the wafer must be less than half of the depth of focus of the wafer. Smoothing the surface layer will reduce the depth of focus, therefore, improving the resolution of photolithography. Nevertheless, there are some limitations seen by CMP. Dishing and erosion occurs due to local planarization in which some areas of the wafer polish faster than the others. Dishing results in a well-like shape. CMP is also subjected to stress cracking, scratching, and corrosive attacking from slurry chemicals. The wafer is very weak and thin; as a result, over pressuring the wafer will lead to stress cracking or scratching the surface of the wafer. CMP process is also time-consuming and expensive.
Works Cited [1] Richard et al., Demystifying Chipmaking, Elsevier, 2005. [2] San Jose University Engineering Department, Copper Deposition, [Online], Available: http://www.engr.sjsu.edu/ sgleixner/mate166/LectureNotes/Copper%20and%20Damascene_S.pdf [Accessed: 17 Oct. 2011] [3] Michael Quirk and Julian Serda, Semiconductor Manufacturing Technology, 1st ed., Prentice Hall, 2000. [4] Robert Doering and Yoshio Nishi, Eds., Handbook of Semiconductor Manufacturing Technology, 2nd ed., CRC Press, 2007. [5] Alpsitec SARL. Alpsitec is represented by Crystec Technology Trading GmbH, http://www.crystec.com/alpovere.htm. [6] Joshua Chien, University of California Berkeley, CA, Chemical Mechanical Planarization. [Microsoft PowerPoint]. Berkeley, CA: Rohm & Haas. [7] Jeffrey Rockwell and Yuzhuo Li, Chemical Mechanical Polishing, 2000, http://www.files.chem.vt.edu/confchem/2000/a/rockwell/rockwell.htm. [8] Wikipedia, Chemical-mechanical planarization, 30 June 2011, http://en.wikipedia.org/wiki/Chemical-mechanical_planarization.
Potrebbero piacerti anche
- Touareg Supplementary HeatingDocumento32 pagineTouareg Supplementary HeatingAnonymous t1FCvPRSaNessuna valutazione finora
- 3electrical & Information System - ENGLISG-G9165Documento48 pagine3electrical & Information System - ENGLISG-G9165George Jhonson100% (4)
- Painting InspectionDocumento62 paginePainting InspectionbezzelNessuna valutazione finora
- Electroplating of PlasticsDocumento4 pagineElectroplating of PlasticsislammughalNessuna valutazione finora
- ElectropolishingDocumento6 pagineElectropolishingnagurvali65Nessuna valutazione finora
- InTech-Welding of Aluminum Alloys PDFDocumento25 pagineInTech-Welding of Aluminum Alloys PDFCortesar ManuNessuna valutazione finora
- Cast Steel-Sic Composites As Wear Resistant Materials: Dejan ČikaraDocumento5 pagineCast Steel-Sic Composites As Wear Resistant Materials: Dejan ČikaraAna MijatovicNessuna valutazione finora
- Electroplating of PlasticsDocumento13 pagineElectroplating of PlasticsVON KAISER BARRIDONessuna valutazione finora
- Instrument Data Sheets FormatsDocumento165 pagineInstrument Data Sheets Formatsrathnam.pm100% (1)
- Ceramic To Metal Joining ReportDocumento9 pagineCeramic To Metal Joining ReportmadangkNessuna valutazione finora
- Developments in Thermal Desalination Processes PDFDocumento14 pagineDevelopments in Thermal Desalination Processes PDFG.DNessuna valutazione finora
- RippleTankSE Key PDFDocumento10 pagineRippleTankSE Key PDFYesi Setyo Ningrum100% (1)
- Aspects of Lead Acid Battery Technology 9 GridsDocumento65 pagineAspects of Lead Acid Battery Technology 9 GridstjkiddNessuna valutazione finora
- DPR - AHP-PPP ShivSankul, Kolhapur 26.11.2018 PDFDocumento148 pagineDPR - AHP-PPP ShivSankul, Kolhapur 26.11.2018 PDFSantosh KalkutkiNessuna valutazione finora
- Modern RF and Microwave Measurement Techniques PDFDocumento476 pagineModern RF and Microwave Measurement Techniques PDFYuliyan TopalovNessuna valutazione finora
- Chemical - Mechanical PolishingDocumento7 pagineChemical - Mechanical PolishingMorcos Nashaat Daneil100% (1)
- Airy Wave TheoryDocumento14 pagineAiry Wave TheoryAnirban Guha50% (2)
- Long Life Corrosion Protection of Steel by Zinc-Aluminium Coating Formed by Thermal Spray ProcessDocumento8 pagineLong Life Corrosion Protection of Steel by Zinc-Aluminium Coating Formed by Thermal Spray ProcessmotokaliNessuna valutazione finora
- Daniel J. Gaspar, Evgueni Polikarpov - OLED Fundamentals - Materials, Devices, and Processing of Organic Light-Emitting Diodes-CRC Press (2015)Documento492 pagineDaniel J. Gaspar, Evgueni Polikarpov - OLED Fundamentals - Materials, Devices, and Processing of Organic Light-Emitting Diodes-CRC Press (2015)jeren1228Nessuna valutazione finora
- Daniel J. Gaspar, Evgueni Polikarpov - OLED Fundamentals - Materials, Devices, and Processing of Organic Light-Emitting Diodes-CRC Press (2015)Documento492 pagineDaniel J. Gaspar, Evgueni Polikarpov - OLED Fundamentals - Materials, Devices, and Processing of Organic Light-Emitting Diodes-CRC Press (2015)jeren1228Nessuna valutazione finora
- Daniel J. Gaspar, Evgueni Polikarpov - OLED Fundamentals - Materials, Devices, and Processing of Organic Light-Emitting Diodes-CRC Press (2015)Documento492 pagineDaniel J. Gaspar, Evgueni Polikarpov - OLED Fundamentals - Materials, Devices, and Processing of Organic Light-Emitting Diodes-CRC Press (2015)jeren1228Nessuna valutazione finora
- Daniel J. Gaspar, Evgueni Polikarpov - OLED Fundamentals - Materials, Devices, and Processing of Organic Light-Emitting Diodes-CRC Press (2015)Documento492 pagineDaniel J. Gaspar, Evgueni Polikarpov - OLED Fundamentals - Materials, Devices, and Processing of Organic Light-Emitting Diodes-CRC Press (2015)jeren1228Nessuna valutazione finora
- Damascene ProcessDocumento25 pagineDamascene ProcessfudgemonsterNessuna valutazione finora
- 1.1 General BackgroundDocumento14 pagine1.1 General BackgroundChris TanNessuna valutazione finora
- The Introduction of The Ceramic PCB Manufacturing ProcessDocumento8 pagineThe Introduction of The Ceramic PCB Manufacturing ProcessjackNessuna valutazione finora
- Dual DamasceneDocumento21 pagineDual Damascenebisma_waseeqNessuna valutazione finora
- 08 MetallizationDocumento25 pagine08 Metallizationrojas mdangaNessuna valutazione finora
- Whisker Free Tin Jan Feb White Paper DownloadDocumento6 pagineWhisker Free Tin Jan Feb White Paper DownloadRajashekhar MahantinamathNessuna valutazione finora
- C 12 S 20Documento21 pagineC 12 S 20Varda ShahidNessuna valutazione finora
- Study of Electrochemical Polishing Applications in Some Alloys For High Surface FinishDocumento7 pagineStudy of Electrochemical Polishing Applications in Some Alloys For High Surface Finishieom2012Nessuna valutazione finora
- Applied Surface Science Advances: P.N. Belkin, S.A. Kusmanov, E.V. ParfenovDocumento30 pagineApplied Surface Science Advances: P.N. Belkin, S.A. Kusmanov, E.V. ParfenovKaren AnnNessuna valutazione finora
- The Effects of MG, Cu and Ca On The Rolling and Drawing Performance of 5Xxx Aluminum Alloy WireDocumento6 pagineThe Effects of MG, Cu and Ca On The Rolling and Drawing Performance of 5Xxx Aluminum Alloy Wiresatish_trivediNessuna valutazione finora
- EDM With ECM PDFDocumento8 pagineEDM With ECM PDFlogeshboy007Nessuna valutazione finora
- Microelectronic Engineering: Alexander Vladimirov GrigorovDocumento9 pagineMicroelectronic Engineering: Alexander Vladimirov Grigorov135713571357Nessuna valutazione finora
- Stretchable Silicon EssayDocumento8 pagineStretchable Silicon Essayrachit1986Nessuna valutazione finora
- Plating Processes On Aluminum and Application To Novel Solar Cell ConceptsDocumento9 paginePlating Processes On Aluminum and Application To Novel Solar Cell ConceptsĐức PhạmNessuna valutazione finora
- GN - 8-04-Thermally Sprayed Metal CoatingsDocumento4 pagineGN - 8-04-Thermally Sprayed Metal CoatingsGeorge AlexiadisNessuna valutazione finora
- Corrosion Behavior of AA5038 Nanostructured Aluminum Alloy Produced by Accumulative Roll-BondingDocumento7 pagineCorrosion Behavior of AA5038 Nanostructured Aluminum Alloy Produced by Accumulative Roll-Bondinglaura arciniegasNessuna valutazione finora
- CH 15Documento12 pagineCH 15afnene1Nessuna valutazione finora
- About Die CastingDocumento3 pagineAbout Die CastingJoelNessuna valutazione finora
- MechanicalPlating60136 146Documento11 pagineMechanicalPlating60136 146jim clarkNessuna valutazione finora
- Chromium Electroplating On PlasticsDocumento4 pagineChromium Electroplating On PlasticsMateus SpinelliNessuna valutazione finora
- Guo - 2012 - Charact. of Cr+3 Process Coating On AA2024T3 (Surf.& Coatings Techn., 2012)Documento8 pagineGuo - 2012 - Charact. of Cr+3 Process Coating On AA2024T3 (Surf.& Coatings Techn., 2012)Luis Gustavo PachecoNessuna valutazione finora
- Surface & Coatings Technology: J. Zechner, G. Mohanty, C. Frantz, H. Cebeci, L. Philippe, J. MichlerDocumento5 pagineSurface & Coatings Technology: J. Zechner, G. Mohanty, C. Frantz, H. Cebeci, L. Philippe, J. MichlerCahyo ArdoyoNessuna valutazione finora
- Process Development For Solder Joints On Power ChipsDocumento44 pagineProcess Development For Solder Joints On Power ChipsYeong-Tsuen PanNessuna valutazione finora
- Rare MetalsDocumento10 pagineRare MetalssrijroxNessuna valutazione finora
- Electrodeposition of MetalDocumento4 pagineElectrodeposition of MetalvkmsNessuna valutazione finora
- Roll Bonding Properties of Al/Cu Bimetallic Laminates Fabricated by The Roll Bonding TechniqueDocumento10 pagineRoll Bonding Properties of Al/Cu Bimetallic Laminates Fabricated by The Roll Bonding Techniqueamalendu_biswas_1Nessuna valutazione finora
- Ceramics: 1.0 OverviewDocumento9 pagineCeramics: 1.0 OverviewMehmet Can HatibogluNessuna valutazione finora
- Electrical Conductivity of Chromate Conversion Coating On Electrodeposited ZincDocumento6 pagineElectrical Conductivity of Chromate Conversion Coating On Electrodeposited ZincMoeen Iqbal ShahNessuna valutazione finora
- Rautomead CC PDFDocumento13 pagineRautomead CC PDFDayanand SharmaNessuna valutazione finora
- Structural and Spectroscopic Characterisations of The Surface Oxide Scales and Inclusions Present On Edge-Burst Hot-Rolled Steel CoilsDocumento8 pagineStructural and Spectroscopic Characterisations of The Surface Oxide Scales and Inclusions Present On Edge-Burst Hot-Rolled Steel CoilsmusonlyNessuna valutazione finora
- Electric Feedthroughs and Insulating Parts: Helmut Mayer, FRIATEC AG, MannheimDocumento10 pagineElectric Feedthroughs and Insulating Parts: Helmut Mayer, FRIATEC AG, MannheimRonakPatelNessuna valutazione finora
- Magnetic Pulse Welding (MPW) For Joining Similar and Dissimilar MatalsDocumento18 pagineMagnetic Pulse Welding (MPW) For Joining Similar and Dissimilar MatalsBANOTH SHIVAKUMARNessuna valutazione finora
- Consideration On Zinc Content On The Microstructure, Mechanical, and Corrosion Evolution of Aluminum/zinc Composites Fabricated by CARB ProcessDocumento16 pagineConsideration On Zinc Content On The Microstructure, Mechanical, and Corrosion Evolution of Aluminum/zinc Composites Fabricated by CARB ProcessADRIANONessuna valutazione finora
- 8.. - Study of Tin .FullDocumento8 pagine8.. - Study of Tin .FullTJPRC PublicationsNessuna valutazione finora
- Detailed Report of EMSRDocumento40 pagineDetailed Report of EMSROfficialRF WRNessuna valutazione finora
- Texturing Methods PDFDocumento4 pagineTexturing Methods PDFAnoop KizhakathNessuna valutazione finora
- Texturing of Rollers For The Production of Auto-Industry SheetDocumento4 pagineTexturing of Rollers For The Production of Auto-Industry SheetAnoop KizhakathNessuna valutazione finora
- Galvanizing AHSSDocumento3 pagineGalvanizing AHSSTrial_TNessuna valutazione finora
- Copper Foils For High Frequency Circuit Materials PDFDocumento8 pagineCopper Foils For High Frequency Circuit Materials PDFrahul05singhaNessuna valutazione finora
- 2 ND Proposal With RamiDocumento9 pagine2 ND Proposal With Ramiyunus_mohammed1641Nessuna valutazione finora
- Application of Electroslag Strip CladdingDocumento12 pagineApplication of Electroslag Strip CladdingSANKET SINGHNessuna valutazione finora
- Increase in Wear Resistance of 5083 Al-Alloy Using Micro Arc Oxidation"Documento14 pagineIncrease in Wear Resistance of 5083 Al-Alloy Using Micro Arc Oxidation"ashwani kaushikNessuna valutazione finora
- Laser Technology: Applications in Adhesion and Related AreasDa EverandLaser Technology: Applications in Adhesion and Related AreasNessuna valutazione finora
- Compendium of Atomic Alkali Resistant Optical Thin Films, Diffusion and Electrical Mobility in Diode Pumped Alkali Lasers (DPALs)Da EverandCompendium of Atomic Alkali Resistant Optical Thin Films, Diffusion and Electrical Mobility in Diode Pumped Alkali Lasers (DPALs)Nessuna valutazione finora
- Atomic Layer Etch ReviewDocumento15 pagineAtomic Layer Etch Reviewjeren1228Nessuna valutazione finora
- Error Control For Network-on-Chip LinksDocumento158 pagineError Control For Network-on-Chip Linksjeren1228Nessuna valutazione finora
- 2 Sanghun Jeon - SeminarDocumento26 pagine2 Sanghun Jeon - Seminarjeren1228Nessuna valutazione finora
- Cost Effective Reconfigurable LogicDocumento102 pagineCost Effective Reconfigurable Logicjeren1228Nessuna valutazione finora
- Keysight RF MeasurementDocumento14 pagineKeysight RF Measurementjeren1228Nessuna valutazione finora
- Introduction To FPGA: Guan-Lin WuDocumento37 pagineIntroduction To FPGA: Guan-Lin Wujeren1228Nessuna valutazione finora
- Building Blocks For Digital Design: Adders, Registers, and MultiplexersDocumento33 pagineBuilding Blocks For Digital Design: Adders, Registers, and Multiplexerspkn.11ee56Nessuna valutazione finora
- Magnum OpusDocumento4 pagineMagnum OpusPharaohgodking100% (1)
- Assignment#2, Potential, Capacitors, Magnetic Force and FieldDocumento5 pagineAssignment#2, Potential, Capacitors, Magnetic Force and FieldBilal KhalidNessuna valutazione finora
- ThermAsset 2 Low ResDocumento2 pagineThermAsset 2 Low ResThonny Barrera QuispeNessuna valutazione finora
- Effects of Joint Geometries On Welding of Mild Steel by Shielded Metal Arc Welding (Smaw)Documento6 pagineEffects of Joint Geometries On Welding of Mild Steel by Shielded Metal Arc Welding (Smaw)Sudeep Kumar SinghNessuna valutazione finora
- Level Sensor AnimationDocumento2 pagineLevel Sensor AnimationInstrumentation ToolsNessuna valutazione finora
- Interfacing of Flame Sensor With ArduinoDocumento14 pagineInterfacing of Flame Sensor With Arduinotech agentNessuna valutazione finora
- Peugeot Elystar Diagnostic AidDocumento13 paginePeugeot Elystar Diagnostic Aidpiter4012Nessuna valutazione finora
- Multistage and Multilevel Power Electronic Converter-Based Power Supply For Plasma DBD DevicesDocumento4 pagineMultistage and Multilevel Power Electronic Converter-Based Power Supply For Plasma DBD DevicesBrightworld ProjectsNessuna valutazione finora
- CSB-F: Three-Phase Power Capacitor With Fuse ProtectionDocumento2 pagineCSB-F: Three-Phase Power Capacitor With Fuse ProtectionAbdul RafaeNessuna valutazione finora
- SAPPHIRE® PLUS 70-BarDocumento2 pagineSAPPHIRE® PLUS 70-BarAyhan ÖZKALNessuna valutazione finora
- Rockfall Embankment - in Cretaz (Cogne - Aosta) : Case HistoryDocumento2 pagineRockfall Embankment - in Cretaz (Cogne - Aosta) : Case HistoryHammer SondirNessuna valutazione finora
- Mantenedor de Bateria LBC1206Documento6 pagineMantenedor de Bateria LBC1206YERKO OVIEDONessuna valutazione finora
- Catalog - Regent RSV Ed 3Documento14 pagineCatalog - Regent RSV Ed 3Nyu123456Nessuna valutazione finora
- Pneumatically Operated Valve P-12Documento4 paginePneumatically Operated Valve P-12Weldmap DrawingNessuna valutazione finora
- Overview of Fluid Catalytic Cracking Unit (FCC, Fccu) : Catalyst AluminumDocumento4 pagineOverview of Fluid Catalytic Cracking Unit (FCC, Fccu) : Catalyst AluminumAdemola RabiuNessuna valutazione finora
- Prepared By: Mr. Harsh PanchalDocumento46 paginePrepared By: Mr. Harsh PanchalKáûshàl PãtëlNessuna valutazione finora
- UBL3&4 E 0 G0 ES AA0 002P - R3 - Color Schedule For Power PlantDocumento12 pagineUBL3&4 E 0 G0 ES AA0 002P - R3 - Color Schedule For Power PlantAdvent ManurungNessuna valutazione finora
- Brushless DC MotorDocumento3 pagineBrushless DC MotorSourav KumarNessuna valutazione finora
- ENDA-5000 HRE2406B- Gas Analyzer- Thiết Bị Phân Tích KhíDocumento6 pagineENDA-5000 HRE2406B- Gas Analyzer- Thiết Bị Phân Tích KhíminhNessuna valutazione finora
- "Enron Energy Scandal": Assignment ONDocumento11 pagine"Enron Energy Scandal": Assignment ONDarshan GohilNessuna valutazione finora
- Air Blast Circuit Breaker 1Documento19 pagineAir Blast Circuit Breaker 1Bidya Bhusan MajiNessuna valutazione finora
- Design and Fabrication of Oil Skimmer RobotDocumento46 pagineDesign and Fabrication of Oil Skimmer RobotSathiya Udumalpet100% (2)
- Training ReportDocumento21 pagineTraining ReportPiyush Khandait0% (1)