Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
The Adrenal Gland
Caricato da
ardevanaDescrizione originale:
Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
The Adrenal Gland
Caricato da
ardevanaCopyright:
Formati disponibili
James F.
Head
The goal of these lectures is to provide you with an understanding of how the adrenal gland functions in producing a range of hormones to regulate a number of homeostatic mechanisms. In the lectures, the major focus is on the nature of the various hormones, how and where they are produced, how their production is regulated, how they are transported in the blood, what they do and where, and how are they degraded. Some aspects of disease states associated with hypo or hyper-secretion and their consequences will be touched on in lecture and considered more in the discussion session. These considerations of pathological conditions, as previously in the course, are not intended to provide a detailed view into disease and treatment, but are primarily intended to emphasize the significance of the underlying control processes and the functional roles of the hormones. N.B. You are not asked to memorize the details of the molecular structures of any of the adrenal hormones or intermediates, only their general structural characteristics.
The Adrenal Gland
Objectives.
The adrenals are a pair of glands weighing a total of about 10g, which are located one near each kidney.
Background
Describe location, structure and organization of adrenal gland Indicate how the general structure of cortical hormones dictates their physical characteristics, and how these dictate many functional characteristics. Describe the synthesis, transportation and mode of action of the cortical hormones Compare the regulation, transportation and effects of glucocorticoids, mineralocorticoids and adrenal androgens. Relate the functions of mineralocorticoids and glucocorticoids to the symptoms and signs of excesses and deficits in these hormones. Describe the synthesis and metabolism of adrenal catecholamines. Describe regulation of secretion, transportation and modes of action of catecholamines.
Fig 1. Diagram of section through adrenal gland showing layers and products.
Adrenal-1
Loss of the adrenal cortex by disease or surgery results in death within 1-2 weeks, unless replacement treatment is instituted. Almost every organ system is affected under these circumstances, although the most likely cause of death is circulatory collapse resulting from sodium depletion. If caloric intake is limited, death may result from hypoglycemia. Based on their ability to protect against these causes of death, the adrenocortical hormones have generally been divided into mineralocorticoids and glucocorticoids. The prime mineralocorticoid is aldosterone, which is required for normal maintenance of sodium and potassium balance. The principal glucocorticoid is cortisol, which is involved in maintaining carbohydrate reserves as well as various other functions. The adrenal androgens have effects similar to (but weaker than) those of the male testicular hormones (T, DHT), and appear to be of importance in both sexes in mediating some of the changes occurring in puberty.
The cortex is itself stratified into three identifiable layers: the zona glomerulosa, which principally produces aldosterone, the zona fasciculata which produces mainly cortisol and the zona reticularis which produces cortisol and the androgens dehydroepiandosterone and androstenedione.
The adrenal is richly vascularized with arterial blood flow to both the cortex and medulla. Blood flowing through the cortex drains into medullary capillary sinusoids, hence part of the blood supply to the medulla is rich in cortical hormones.
Each adrenal is in fact two glands in one, the outer adrenal cortex that produces steroid hormones and the inner adrenal medulla that produces epinephrine and norepinephrine. The cortex (usually about 90% of mass of adrenal) and medulla are morphologically and embryologically distinct, and have distinct products and regulation.
The adrenal medulla arises embryologically from neuroectoderm and is innervated by neurons whose cell bodies reside in the spinal cord. Axons from these cells pass through the paravertebral sympathetic ganglia to form the splanchnic nerves. The medulla stores about 5-6 mgs of the catecholamines epinephrine and norepinephrine in secretory granules within the so-called chromaffin cells. Although the products of the adrenal medulla affect most tissues of the body, humans can survive without it so long as the rest of the sympathetic system is functional.
The fetal adrenal is relatively bigger, for body size, than that of the adult, with a large steroidogenic fetal zone. The role of the fetal adrenal will be discussed briefly below and more fully during the reproductive endocrinology section.
Adrenocorticoids
Overview.
All adrenal cortical hormones derive from cholesterol. The specific product of a given cell depends on the enzymic complement of the cell and the responsiveness to different stimuli. The rate-limiting step in synthesis is the first, from cholesterol to pregnenolone, which is common to all members of this class of hormone (See Fig. 2). It is this step that is regulated by stimulating factors. The stimulatory factors (discussed below) promote the production of steroid acute regulatory protein (StAR) which stimulates the transport of cholesterol from the cytosol into the mitochondria where it Adrenal-2
can be acted on by the first enzyme in the initial common pathway to yield pregnenolone. Although mineralocorticoid activity is predominantly associated with aldosterone and glucocorticoid activity with cortisol, high levels of each will produce the alternate effect. While cortisol has ten times the glucocorticoid effect of aldosterone, it normally has less than 0.25% of the mineralocorticoid effect. (Cortisol can readily bind to and activate the mineralocorticoid receptor protein, see below, but mineralocorticoid sensitive tissues contain an enzyme, type II 11-hydroxysteroid dehydrogenase, that converts cortisol to cortisone, greatly reducing its mineralocorticoid effects).
The major steroid products of the adrenal cortex are shown in capitals. The steps in the pathways, indicated by numbers, are catalyzed by the following enzymes: 1=20,22-desmolase, 2&7=17hydroxylase, 3&8=17,20-desmolase, 4,5&6=3-hydroxysteroid dehydrogenase, 9&10=21-hydroxylase, 11=17-hydroxysteroid dehydrogenase, 12&13=11-hydroxylase, 14=18-hydroxylase, 15=18-hydroxysteroid dehydrogenase. For this course you do not need to memorize these structures nor the enzyme names. Adrenal-3
Figure 2. Steroid hormone production in the adrenal cortex.
The secretion of steroids represents de novo synthesis, since the steroid hormones, unlike the catecholamines of the medulla, cannot be adequately stored. Being lipid soluble, they can simply diffuse across the membranes of the producing cells down their concentration gradient, to enter the circulation. Normally humans secrete about 20mg of cortisol, 0.25mg of aldosterone and (in a young adult) 10-20mg DHEA per day, although this number can be increased many fold under prolonged stimulation.
Free steroid hormones enter cells by passive diffusion and bind to cytoplasmic receptor proteins (figure 3). They subsequently act on their targets mostly through effects at the nucleus. This is distinct from peptide and amine hormones which act via plasma membrane receptors. In the cytosol, mineralocorticoids bind preferentially to socalled type I receptors which are found in greatest amounts in the kidney, colon, sweat and salivary glands. Glucocorticoids bind preferentially to type II receptors found in various tissues of the body (as noted above, cortisol CAN bind to the type I receptor, but is usually intercepted in mineralocorticoid-sensitive tissues and enzymically inactivated).
Once in the circulation, most of the steroids are bound to plasma binding proteins, transcortin or corticosteroid binding globulin (CBG) and plasma albumin. Much of the adrenal androgens are modified by sulfation and travel mostly in that form bound to plasma albumin. Because binding of glucocorticoids is favored over aldosterone, about 95% of the glucocorticoids are bound as opposed to about 60% of the aldosterone. This tighter binding contributes to a longer half-life for corticosteroids in the plasma (about 90 minutes for cortisol versus 30 minutes for aldosterone). The more stable DHEAS has a half life of 10-20hrs and its total plasma concentration therefore often exceeds that of the other adrenal steroids (in young adults). Clearance of the hormones is largely by renal and hepatic mechanisms.
Figure 3. Generalized mechanism of action of steroid hormones. Adrenal-4
The production of ACTH is under the control of corticotrophin releasing hormone (CRH) from the hypothalamus. The secretion of CRH by the hypothalamus, and hence ACTH by the pituitary, follows a circadian rhythm, giving a peak rate of ACTH secretion in the early morning, before waking, followed by a steady decline through to the evening hours. Cortisol secretion parallels the changes in ACTH. Although both CRH and ACTH secretion is subject to feedback inhibition by cortisol, the circadian rhythm appears to be the result of changes in the sensitivity of the CRH secreting cells to cortisol. Low sensitivity to cortisol in the morning hours results in less negative feedback, permitting higher rates of CRH secretion, resulting in a higher basal ACTH production and hence a rise in cortisol secretion. Increasing sensitivity during the course of the day leads to a decline in CRH, ACTH and hence cortisol levels. Stressful stimuli lead to increases in CRH secretion, by neural pathways, over-riding the diurnal rhythm. Chronic stress will lead to the establishment of a new steady state production of ACTH and cortisol. Although many of the hormones of the adrenal gland are associated with stresses of one kind or another, cortisol has become popularly known as the "stress" hormone. In general terms, it is necessary to maintain vital functions during periods of prolonged stress (usually physiological stress, but psychological stress can also be included) and to contain the inflammatory response. The functions of cortisol are diverse including many that are permissive. i.e. the hormone does not itself initiate changes but is required for the changes to be expressed fully. In particular, cortisol is required for catecholamines to be effective and it is the increased responsiveness to catecholamines which is responsible for a number of the effects of cortisol. With cortisol deficit, a decrease in cardiovascular responsiveness to catecholamines and reduced mobilization of fuel stores may account for observed increased vulnerability to "stress" and, conversely, an increase in cortisol, normally stimulated by "stress", is protective. Cortisol function
ACTH is derived in the pituitary from a prohormone, pro-opiomelanocortin POMC. It is required for normal function of the zona fasciculata and reticularis. In the absence of ACTH, these regions atrophy. ACTH can bind to specific receptors on the cells of all three layers of the adrenal cortex. In the zona glomerulosa it may have a minor role in influencing the production of aldosterone, although the prime regulator in this layer is angiotensin II (see below). For cortisol and androgen synthesis, the circulating level of ACTH is the controlling factor, although the necessary enzymes for DHEA production are primarily located in the zona reticularis. On binding of ACTH to the receptor there is a rise in intracellular c-AMP level that leads to the production of StAR protein, entry of cholesterol into the mitochondria and there the conversion of cholesterol to pregnenolone. Stimulation by ACTH leads to a rise in steroid hormone secretion within 1-2 minutes, peaking by about 15 minutes.
Regulation of cortisol and adrenal androgen production by adrenocorticotropic hormone, ACTH.
Table 1 summarizes some of the effects of INCREASING levels of cortisol. This is followed by more detailed considerations of some of these effects.
Adrenal-5
Promotes catabolism. Facilitates the breakdown of protein from muscle and connective tissue to liberate amino acids used in gluconeogenesis. Stimulates production of gluconeogenic enzymes in liver. Decreases glucose utilization. Inhibits glucose transport into cells (but not in the brain).
As implicit in the term "glucocorticoid", increased cortisol leads to elevations of plasma glucose, largely by mechanisms which oppose insulin. These are summarized below and in Figure 4.
Elevation of cortisol levels leads to: Increased blood glucose.
Decreases protein synthesis. Reduces rates of protein synthesis everywhere except in liver. Reduced rates of synthesis are at least in part because cortisol reduces the uptake of amino acids into muscle cells. With increased protein breakdown and reduced amino acid uptake, plasma amino acid levels rise.
Alters fat metabolism. Increases rate of breakdown of peripheral fat to liberate fatty acids and glycerol for gluconeogenesis. Adrenal-6
HOWEVER, increases central fat deposition i.e. on trunk and face. Hence in cortisol excess the limbs lose fat and muscle mass while the trunk and face become fatter. The basis for these redistributions of fat appear to be related to different receptor responsivities to cortisol in different adipose tissues.
Cortisol is required to maintain sensitivity to epinephrine and norepinephrine in the vasculature. In the absence of cortisol there is widespread vasodilation and fall in blood pressure. Adrenal-7
Feedback inhibition of CRH and ACTH production. May also affect perception. In cortisol deficiency taste hearing and smell are frequently accentuated. Cortisol excess is associated with an initial euphoria followed by depression. Cardiovascular effects: Maintenance of circulatory system.
Figure 4. Effects of increased cortisol leading to elevated plasma glucose. Other functions of cortisol include: CNS effects.
Permissive role in maturation of many fetal organ systems, intestinal enzymes and pulmonary surfactant. (Administration of corticosteroids during late pregnancy, if there is an anticipated premature birth, can be protective against respiratory distress syndrome). Renal effects: Free water clearance.
Developmental effects.
In cortisol excess, osteoclastic activity is enhanced, hence promoting the development of osteoporosis. Immune function: Containing inflammatory response.
In the absence of cortisol a patient is unable to produce a hypotonic urine and is therefore unable to eliminate free water. This can result in water intoxication. In addition to a fall in glomerular filtration, subsequent to falling blood pressure, ADH secretion is not depressed by hypo-osmolarity when cortisol is very low. There appears to be a functional relationship between ADH and CRH, such that ADH also appears to be able to promote ACTH production. The interplay between ADH and cortisol levels may therefore be complex. Bone effects: Promotes bone breakdown.
In high doses, cortisol suppresses many of the bodys inflammatory responses and this function is harnessed pharmacologically with the use of synthetic glucocorticoids. The effects may be beneficial, such as in the reduction of swelling or tissue damage caused by inflammation (such as in rheumatoid arthritis), but may be harmful, such as allowing resurgence of a latent tubercular infection. Cortisol achieves its anti-inflammatory effects through many mechanisms, including: stabilizing lysosomal membranes (preventing release of degradative enzymes), decrease in capillary permeability (reducing entry of fluid and white cells into the inflamed area), depressing activity of phagocytes, suppression of synthesis of interleukin-1, inhibition of production of eicosanoids. By depressing antigen/antibody induced release of histamine from mast cells in the lung, it can also act to reduce allergic responses. Other issues: Consequences of pharmacological administration of glucocorticoids.
Prolonged administration of pharmacological glucocorticoids leads to feedback inhibition of ACTH production, atrophying of dependent adrenal cells (zona fasciculata and reticularis) and hence reduced ability to generate cortisol. Withdrawal from long term glucocorticoid treatment must therefore be gradual to permit recovery of the patient's own cortisol generating system. Free cortisol in the plasma can be filtered in the glomerulus and appears in the urine. Cortisol is also enzymically reduced, mostly in the liver, to tetrahydrocortisol and conjugated with glucuronic acid which is also filtered at the kidney and excreted in the urine as glucuronide metabolites. Adrenal-8 Elimination of cortisol from the body
Adrenal androgens are produced in large quantities in the fetus and are very important during fetal development. This will be discussed more during considerations of reproductive endocrinology. Postnatally, adrenal androgen production is low but rises during the prepubertal period (adrenarche) and appears to contribute to events normally preceding puberty, including the onset of axillary and pubic hair growth and growth and secretion from axillary sebaceous glands. In adult females the adrenals are a major source of androgens and, after conversion into testosterone in the periphery, contribute to the stimulation of axilliary and pubic hair growth. Some androstenedione is also converted in the periphery to estrogen. This appears to be the major source of estrogen in post-menopausal women whose ovaries are no longer active. There is some evidence that DHEA levels may have an influence on libido in women. In adult males, the androgenic contribution of the DHEA is insignificant in comparison to the powerful androgen testosterone, produced in the testes, and dihydrotestosterone produced from testosterone in the testes and peripherally. The levels of DHEA production are close to those of cortisol in a young adult, but decline with age. The functional role of DHEA in adults remains poorly understood, although various studies have attributed a range of functions. The decline with age has been used as the basis for an "industry" of dietary DHEA supplementation aimed at reversing the effects of aging. Consistent scientific evidence on the benefits of such supplementation remains lacking. Free DHEA-S in plasma is filtered in the kidney and excreted in the urine mostly as the sulfated form. Aldosterone function. Adrenal androgen production is mainly under the control of ACTH, although age-dependent changes in androgen production in the face of similar levels of ACTH, suggest other factors also have an influence.
Adrenal androgens
As discussed in Renal Physiology, the primary function of aldosterone is to increase sodium reabsorption in the distal nephron, with important consequences for potassium levels. Its production is principally under the control of angiotensin II and of plasma potassium, and ACTH to a lesser extent. In its absence, the body's fluid and electrolyte status is altered, although if sufficient cortisol is present, the mineralocorticiod effects of this hormone may become sufficient to prevent progressive fluid loss. As the prime regulator, angiotensin II is itself produced in response to rising levels of renin. Renin is a protease produced by the granular cells of the juxtaglomerular apparatus. It cleaves circulating angiotensinogen to produce angiotensin I, which is rapidly converted into angiotensin II by converting enzyme in the lung and elsewhere (including intrarenally). Renin release is increased by a fall in the perfusion pressure at the afferent arterioles or by sympathetic stimulation via the renal nerve.
Adrenal-9
Fig 5. Regulation of aldosterone production
Other factors influencing renin release include the sodium and chloride concentration of the tubular fluid passing through the macula densa, plasma electrolyte levels and, through a feedback loop, angiotensin II. Angiotensin II stimulates production of aldosterone by the cells of the zona glomerulosa via a receptor mediated rise in inositol 1,4,5 trisphosphate, which leads to an increased StaR protein, entry of cholesterol into the mitochondria, conversion of cholesterol to pregnenolone and hence entry into the synthetic pathway. It is notable that aldosterone levels are also increased by elevated estrogen, as during pregnancy. This follows an estrogen-stimulated increase in hepatic synthesis of angiotensinogen. Any renin in the circulation then leads to an enhanced production of AGII and thus aldosterone. The consequence of this is an expanded extracellular fluid volume.
As discussed in Physiology, aldosterone acts to increase the activity of the Na/K ATPase in the basolateral membrane of target cells, in part by increasing the rate of entry of sodium on the luminal surface through added sodium channels. These effects are the result of modifications of expression of proteins at the DNA level. The changes may include increased synthesis of the basolateral ATPase and of the luminal membrane sodium channel, although increase in pump activity is also believed to be associated with increased ATP levels, possibly resulting from increased rates of production of some mitochondrial enzymes. (Other effects of aldosterone in the kidney and elsewhere, including possible non-genomic effects, Adrenal-10
have been reported but remain under study. Since the mechanisms of action leading to some of these effects remain tentative, we will not discuss them at this time).
The contents of the granules are secreted in response to sympathetic cholinergic stimulation. Binding of acetylcholine to the chromaffin cells leads to a rise in intracellular calcium levels which promote exocytosis.
The cells of the medulla are modified postganglionic neurons. Innervation is via the splanchnic nerves. The medulla cells contain numerous secretory granules and stain with chromium; hence they have been given the name chromaffin cells. The chromaffin granules contain stored catecholamines, normally in the ratio of about 5:1 epinephrine to norepinephrine. ATP is also present in the granules in a ratio of about 1 ATP to 4 catecholamine molecules. Some peptide hormones, including enkephalin, -endorphin and precursors are also present in small amounts, although their functional significance is unclear. Biosynthesis of catecholamines in the medulla is from tyrosine leading to the formation of norepinephrine which is mostly converted to epinephrine (Figure 6). The enzyme which catalyzes the conversion of norepinephrine to epinephrine (phenylethanolamine-N-methyltransferase, PNMT) is inducible by cortisol. The arrangement of the medulla within the cortical layer, receiving blood rich in corticosteroids, may therefore have functional significance. In this case, it could be modulating or fine-tuning the ratio of epinephrine:norepinephrine released from the medulla such that elevated cortisol will increase the proportion of epinephrine. Once released into the circulation, about half of the catecholamines are free in solution and about half are loosely associated with albumin. The hormones are rapidly cleared from the circulation, having a half-life in the circulation of only about 10-15 seconds. About 90% is cleared on a single pass through the microvasculature. The rapid response time of the medulla and the fast turn-over of catecholamines is in sharp contrast to the more slowly responding cortex and longer lasting effects of the corticosteroids. However, since the overall role of the adrenal is maintaining homeostasis in the face of internal and external stresses, the two sets of hormones provide for a full and complementary range of responses.
In addition to the distal nephron, mineralocorticoid receptors are also found in other tissues where sodium is excreted, e.g. in the sweat and salivary glands and colon. Hyperaldosteronism results in sweat and saliva which are essentially sodium free.
Adrenal medulla Catecholamines
Adrenal catecholamines may be taken up by both neuronal and non-neuronal tissue. Catecholamines taken up by neurons may be repackaged for reuse or may be degraded by the neuronal cytosolic enzyme monoamine oxidase (MAO). Elsewhere in the body the catecholamines are inactivated enzymically by both MAO and catecholamine-O-methyl transferase. Products of degradation are normally coupled with sulfate or glucuronic acid and excreted in the urine. As with corticosteroids, the measurement of urinary concentrations of degradation products is a non-invasive way of monitoring circulating hormone levels. Adrenal-11
The effects of the catecholamines are widespread with most cells having receptors of some kind. The plasma membrane receptors were originally subdivided into and classes, although these have now been further subdivided. The effects of epinephrine and norepinephrine differ mostly by their effects on and 2 receptors. They are equally effective in stimulating 1 receptors. Norepinephrine is a potent agonist and has little effect on 2 receptors. Epinephrine is an even more powerful agonist in many organs and is a powerful 2 agonist. The roles of the catecholamines have been extensively dealt with in other courses. Some are summarized here in table 2. Further consideration of their effects, especially in energy metabolism will also be given in other lectures in this course. Adrenal-12
Most if not all of the epinephrine in the circulation is adrenal in origin, although much of the circulating norepinephrine arises by diffusion from sympathetic synapses elsewhere in the body. Function of adrenal medulla
Figure 6. Biosynthesis of epinephrine (E) and norepinephrine (N) in the adrenal medulla cell. PNMT = phenylethanolamine-N-methyltransferase
Table 2
Adrenal-13
Potrebbero piacerti anche
- Adrenal Fatigue: Understanding the Symptoms: How Malfunctioning Adrenal Glands Negatively Affect the BodyDa EverandAdrenal Fatigue: Understanding the Symptoms: How Malfunctioning Adrenal Glands Negatively Affect the BodyNessuna valutazione finora
- Corticosteroid PDFDocumento21 pagineCorticosteroid PDFAida YuliaNessuna valutazione finora
- ABS Endocrinology PDFDocumento59 pagineABS Endocrinology PDFMichael Olivier WNessuna valutazione finora
- 4 Endocrine 3Documento23 pagine4 Endocrine 3ShenNessuna valutazione finora
- Adrenal Glands 2018 - 2019Documento32 pagineAdrenal Glands 2018 - 2019Bianca BiaNessuna valutazione finora
- Transport in PlantDocumento21 pagineTransport in PlantIsheba WarrenNessuna valutazione finora
- Adrenocortical HormonesDocumento77 pagineAdrenocortical HormonesMaxamed Faarax XaashiNessuna valutazione finora
- The Endocrine SystemDocumento13 pagineThe Endocrine SystemThenmozhi SivajiNessuna valutazione finora
- Endocrine SystemDocumento41 pagineEndocrine SystemMerrin EncarnacionNessuna valutazione finora
- Introduction To Endocrinology: Dr. Jehad Al-ShuneigatDocumento21 pagineIntroduction To Endocrinology: Dr. Jehad Al-Shuneigatyousef sarairehNessuna valutazione finora
- Fkumj Physiology of Hormone 2012Documento84 pagineFkumj Physiology of Hormone 2012sakelengelNessuna valutazione finora
- Adrenal GlandDocumento50 pagineAdrenal GlandAnita YadavNessuna valutazione finora
- Physiology of The Endocrine and Nervous Systems 2017Documento169 paginePhysiology of The Endocrine and Nervous Systems 2017Criss CristinaNessuna valutazione finora
- 337 Pdfsam NMS PhysiologyDocumento166 pagine337 Pdfsam NMS PhysiologydrpnnreddyNessuna valutazione finora
- Dr. Dr. H. Busjra M. Nur, MSC FKK UmjDocumento84 pagineDr. Dr. H. Busjra M. Nur, MSC FKK UmjEza MelindaNessuna valutazione finora
- Endocrine GlandsDocumento13 pagineEndocrine GlandsJoan GalarceNessuna valutazione finora
- Functional Anatomy of The Adrenal GlandDocumento9 pagineFunctional Anatomy of The Adrenal GlandPaula SchaeferNessuna valutazione finora
- Fkumj Physiology of Hormone 2012Documento76 pagineFkumj Physiology of Hormone 2012nudiya azimahNessuna valutazione finora
- Review Chapter 16 EndocrineDocumento11 pagineReview Chapter 16 EndocrineAkul MunjalNessuna valutazione finora
- Endocrinology Notes: Veterinary PhysiologyDocumento16 pagineEndocrinology Notes: Veterinary PhysiologyBrian AllanNessuna valutazione finora
- Endocrine System: University of SurreyDocumento13 pagineEndocrine System: University of SurreyqhqhqNessuna valutazione finora
- Endocrine Metabolism: Adrenal GlandsDocumento2 pagineEndocrine Metabolism: Adrenal GlandsEcaterina Borovic-PavlovschiNessuna valutazione finora
- The Endocrine System Overview/ Introduction: Nur112: Anatomy and Physiology ISU Echague - College of NursingDocumento6 pagineThe Endocrine System Overview/ Introduction: Nur112: Anatomy and Physiology ISU Echague - College of NursingWai KikiNessuna valutazione finora
- Adrenal Gland - GlucocorticoidsDocumento16 pagineAdrenal Gland - GlucocorticoidsDongs McClureNessuna valutazione finora
- Adrenal Gland1Documento27 pagineAdrenal Gland1Rasel IslamNessuna valutazione finora
- Endocrine System (Vertebrate) : HomeostasisDocumento7 pagineEndocrine System (Vertebrate) : HomeostasisKaleem UllahNessuna valutazione finora
- Hormones, ClassificationDocumento10 pagineHormones, ClassificationMenoNessuna valutazione finora
- Endocrine GlandDocumento4 pagineEndocrine Glandakash_zizouNessuna valutazione finora
- CH 45 Guided ReadingDocumento7 pagineCH 45 Guided ReadingmharirajNessuna valutazione finora
- Lecture 3Documento4 pagineLecture 3Ubaid HassanNessuna valutazione finora
- Endocrinology Excerpts: Hypothalamic-Pituitary-Adrenal AxisDocumento9 pagineEndocrinology Excerpts: Hypothalamic-Pituitary-Adrenal AxisKiana TehraniNessuna valutazione finora
- Chapter 13: Endocrine SystemDocumento16 pagineChapter 13: Endocrine SystemKarren Taquiqui PleteNessuna valutazione finora
- Lewis: Medical-Surgical Nursing, 10 Edition: Assessment of Endocrine System Key PointsDocumento3 pagineLewis: Medical-Surgical Nursing, 10 Edition: Assessment of Endocrine System Key PointsDeo FactuarNessuna valutazione finora
- The Endocrine System: An OverviewDocumento12 pagineThe Endocrine System: An Overviewlovelyc95Nessuna valutazione finora
- By Timothy R. Test, SR., Ph.D. National College Everest College of PhoenixDocumento32 pagineBy Timothy R. Test, SR., Ph.D. National College Everest College of PhoenixTimothy Robert TestNessuna valutazione finora
- SteroidsDocumento22 pagineSteroidsLuqman QadirNessuna valutazione finora
- Adrenal Cortex HormonesDocumento21 pagineAdrenal Cortex HormonesAaa JjjjNessuna valutazione finora
- Study of The Endocrine SystemDocumento225 pagineStudy of The Endocrine SystemHuzaifa RashidNessuna valutazione finora
- Glandula SuprarenalisDocumento6 pagineGlandula SuprarenalisYohanes BongNessuna valutazione finora
- The Endocrine System Study Guide 101 PDFDocumento17 pagineThe Endocrine System Study Guide 101 PDFStephanie Kate PelenioNessuna valutazione finora
- Pharmacology Chapter 34Documento5 paginePharmacology Chapter 34languha NgatiNessuna valutazione finora
- EndocrinologyDocumento35 pagineEndocrinologyNasif HussainNessuna valutazione finora
- Adrenal GlandDocumento46 pagineAdrenal GlandhSANNessuna valutazione finora
- Reproductive SystemDocumento114 pagineReproductive SystembookaccountNessuna valutazione finora
- Adrenal GlandDocumento36 pagineAdrenal Glandpranutan739Nessuna valutazione finora
- 1 - Endocrine 1 (Introduction) - MedicineDocumento36 pagine1 - Endocrine 1 (Introduction) - MedicineBHUWAN BASKOTANessuna valutazione finora
- Introduction - CC3: Mechanism of Hormonal ActionDocumento40 pagineIntroduction - CC3: Mechanism of Hormonal ActionAl-hadad AndromacheNessuna valutazione finora
- CUSHINGSDocumento205 pagineCUSHINGSDaroo D.TNessuna valutazione finora
- Endocrine SystemDocumento19 pagineEndocrine Systemethel roseNessuna valutazione finora
- Exam 3 Study Guide (1169)Documento7 pagineExam 3 Study Guide (1169)S. MartinezNessuna valutazione finora
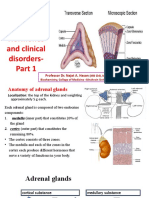
- Adrenal Gland: Structure, Hormones and Clinical Disorders-: Professor Dr. Najat A. HasanDocumento18 pagineAdrenal Gland: Structure, Hormones and Clinical Disorders-: Professor Dr. Najat A. HasanDrFarah Emad Ali100% (1)
- Hormonal Responses To ExerciseDocumento38 pagineHormonal Responses To ExercisesajjadNessuna valutazione finora
- ADRENAL GLAND X.PPTX - 1Documento23 pagineADRENAL GLAND X.PPTX - 1b29jcqnfn9Nessuna valutazione finora
- Learning JournalDocumento82 pagineLearning JournalAnnapurna RavelNessuna valutazione finora
- Endocrine Pathophysiology Nursing NotesDocumento4 pagineEndocrine Pathophysiology Nursing Notesgrad_nurse_2015100% (2)
- Dulutalias - Chapter18-THE ENDOCRINE SYSTEMDocumento10 pagineDulutalias - Chapter18-THE ENDOCRINE SYSTEMGwen Valerie DulutaliasNessuna valutazione finora
- Module 04Documento14 pagineModule 04acrehell8Nessuna valutazione finora
- Care of Clients With Problem On EndocrineDocumento48 pagineCare of Clients With Problem On EndocrineAngel CauilanNessuna valutazione finora
- Chapt 8 AnswersDocumento6 pagineChapt 8 AnswershulsercNessuna valutazione finora
- 45 Lecture PresentationDocumento95 pagine45 Lecture PresentationCourtney TaylorNessuna valutazione finora
- Adrenargic Antiagonist Prepardd by BashirDocumento11 pagineAdrenargic Antiagonist Prepardd by BashirZakarie Abdullahi HusseinNessuna valutazione finora
- Pharma Final ModuleDocumento139 paginePharma Final ModuleQuantum XavierNessuna valutazione finora
- SympatheticDocumento10 pagineSympatheticSharneeshriyaNessuna valutazione finora
- Pharmacology FirecrackerDocumento37 paginePharmacology FirecrackerRehan Usman100% (1)
- Biochemistry of NeurotransmittersDocumento7 pagineBiochemistry of NeurotransmittersHyphophysis 2015Nessuna valutazione finora
- Adrenergic Agonist: Persiapan CBT September 2020 Bimo Kusumo BhirowoDocumento16 pagineAdrenergic Agonist: Persiapan CBT September 2020 Bimo Kusumo BhirowoDendyNessuna valutazione finora
- Test Eng Examen 2017 FRDocumento274 pagineTest Eng Examen 2017 FRRaduNessuna valutazione finora
- Golongan Obat Prekursor Dan OotDocumento2 pagineGolongan Obat Prekursor Dan Ootherfandi ahmadNessuna valutazione finora
- Oxaliplatin For Injection (Preservative-Free) 50 MG Per Vial and 100 MG Per Vial Package Leaflet - Taj PharmaDocumento1 paginaOxaliplatin For Injection (Preservative-Free) 50 MG Per Vial and 100 MG Per Vial Package Leaflet - Taj PharmaTAJ PHARMA — A Health Care ProviderNessuna valutazione finora
- Investigating Factors Affecting The Heart Rate of DaphniaDocumento8 pagineInvestigating Factors Affecting The Heart Rate of DaphniaPinto PintoNessuna valutazione finora
- The Basics of Iridology - Iris Patterns (The Basics of Iridology 1 - Iris Patterns)Documento139 pagineThe Basics of Iridology - Iris Patterns (The Basics of Iridology 1 - Iris Patterns)Silviane Silvério100% (2)
- Catecholamine: Epinephrine (Adrenaline)Documento7 pagineCatecholamine: Epinephrine (Adrenaline)Unggul YudhaNessuna valutazione finora
- Pharmacology Test 1Documento39 paginePharmacology Test 1Niki BolinNessuna valutazione finora
- Furman 2014 MARDDocumento10 pagineFurman 2014 MARDJuan Hernández GarcíaNessuna valutazione finora
- 2020-Biochem-Activity-16 - BIOCHEMISTRY OF HORMONESDocumento32 pagine2020-Biochem-Activity-16 - BIOCHEMISTRY OF HORMONESGabrielle John HernaezNessuna valutazione finora
- Pre Workout Nutrition 2016Documento60 paginePre Workout Nutrition 2016Genaro Alberto Levi TrismegistoNessuna valutazione finora
- Moderator: DR - Rupesh ChaudharyDocumento37 pagineModerator: DR - Rupesh ChaudharyAarti GuptaNessuna valutazione finora
- Pharmacology MnemonicsDocumento17 paginePharmacology MnemonicsKrizzia SuñerNessuna valutazione finora
- Autonomic Nervous System DrugsDocumento128 pagineAutonomic Nervous System DrugsMubashra Habib100% (1)
- Neurotransmitters & NeuromodulatorsDocumento41 pagineNeurotransmitters & Neuromodulatorsafiwahyu100% (1)
- Autonomic Nervous System: Dr. Ahmed Elfatih Ahmed Clinical PharmacologistDocumento25 pagineAutonomic Nervous System: Dr. Ahmed Elfatih Ahmed Clinical Pharmacologistكسلان اكتب اسميNessuna valutazione finora
- Precision Medicine Care in ADHD The Case For Neural Excitation and InhibitionDocumento12 paginePrecision Medicine Care in ADHD The Case For Neural Excitation and InhibitionDaria DanielNessuna valutazione finora
- DM Critical Care Medicine PDFDocumento23 pagineDM Critical Care Medicine PDFArun Sahaya RajaNessuna valutazione finora
- Noradrenaline (Norepinephrine) : 1mg/mLDocumento5 pagineNoradrenaline (Norepinephrine) : 1mg/mLBrian RelsonNessuna valutazione finora
- Orderan Obat Desember 2021Documento25 pagineOrderan Obat Desember 2021Suci IndrianiNessuna valutazione finora
- Chapter 6 Introduction To Autonomic PharmacologyDocumento30 pagineChapter 6 Introduction To Autonomic PharmacologyImrana AamirNessuna valutazione finora
- Brain Facts Book PDFDocumento96 pagineBrain Facts Book PDFjlangseth100% (1)
- Traumatic Stress - Effects On The Brain PDFDocumento17 pagineTraumatic Stress - Effects On The Brain PDFDaniel Londoño GuzmánNessuna valutazione finora
- Biokimia Syaraf Dan Otak: Biochemistry Department Hasanuddin UniversityDocumento52 pagineBiokimia Syaraf Dan Otak: Biochemistry Department Hasanuddin Universityraynhard b. fandresNessuna valutazione finora