Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
The New Flavone Ester Apigenin 7-O-P-hydroxybenzoate and
Caricato da
Anym de CièloDescrizione originale:
Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
The New Flavone Ester Apigenin 7-O-P-hydroxybenzoate and
Caricato da
Anym de CièloCopyright:
Formati disponibili
The New Flavone Ester Apigenin 7-O-p-hydroxybenzoate and Three Di-C-Glycosylflavones from Pteris vittata
Author(s) :Filippo Imperato Source: American Fern Journal, 96(2):62-65. 2006. Published By: The American Fern Society DOI: http://dx.doi.org/10.1640/0002-8444(2006)96[62:SN]2.0.CO;2 URL: http://www.bioone.org/doi/ full/10.1640/0002-8444%282006%2996%5B62%3ASN%5D2.0.CO%3B2
BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOnes Terms of Use, available at www.bioone.org/ page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and noncommercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder.
BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research.
American Fern Journal 96(2):6265 (2006)
SHORTER NOTES
The New Flavone Ester Apigenin 7-O-p-hydroxybenzoate and Three Di-CGlycosylavones from Pteris vittata.It has been shown by Ma et al. (Nature 409:579580, 2001) that Pteris vittata L. is efcient in extracting arsenic from soils; these workers have shown that in fronds of this fern (growing in soil spiked with 1,500 ppm arsenic) arsenic concentrations increased from 29.4 to 15,861 ppm in two weeks whereas arsenic concentration in roots was less than 303 ppm. This is the rst report of arsenic hyperaccumulation by an unmanipulated plant. Hence, Pteris vittata may be of great interest for phytoremediation of arsenic-contaminated soils that are among the major arsenic sources for drinking water. In addition, Ma et al. suggested that these data might be of interest in research dealing with arsenic speciation and distribution in plants. Previous work on the avonoids of Pteris vittata has led to the identication of luteolidin 5-O-glucoside by Harborne (Phytochemistry 5:589600, 1966). In addition, acid hydrolysis of extracts of this fern has led to the identication of kaempferol, quercetin, leucocyanidin and leucodelphinidin by Voirin (Ph. D. thesis, University of Lyon, p. 151, 1970). More recently, 3-C-(60-acetyl-b-cellobiosyl)-apigenin (Amer. Fern J. 89:217220, 1999) and 6C-b-cellobiosyl-isoscutellarein-8-methyl ether together with quercetin 3-Oglucuronide and rutin (Amer. Fern J. 90:4245, 2000) have been identied by Imperato and Telesca. In addition, three kaempferol glycosides (3-O-glucoside, 3-O-glucuronide and 3-O-(X0-X0-di-protocatechuoyl)-glucuronide), two di-Cglycosylavones (3,8-di-C-arabinosyllutelin and 6-C-arabinosyl-8-C-glucosylluteolin), three avonol glycosides acylated with hydroxycinnamic acids (kaempferol and quercetin 3-O-(20,30-di-O-p-coumaroyl)-glucosides together with kaempferol 3-O-(X0-O-p-coumaroyl-X0-O-feruloyl)-glucoside), vitexin 7O-rhamnoside and kaempferol 3-O-rutinoside have been found by Imperato (Amer. Fern J. 90:141144, 2000; Amer. Fern J. 92:244246, 2002; Amer. Fern J. 93:157160, 2003; Amer. Fern J. 94:159162, 2004). In the present paper four avonoids (IIV) have been isolated from aerial parts of Pteris vittata collected in the Botanic Garden of the University of Naples. The fern has been identied by Dr. R. Nazzaro (University of Naples); a voucher specimen (149.001.001.01) has been deposited in the Herbarium Neapolitanum (NAP) of the University of Naples. Flavonoids IIV were isolated from an ethanolic extract of aerial parts of Pteris vittata by preparative paper chromatography in BAW (n-butanol-acetic acid-water, 4:1:5, upper phase), 15% HOAc (acetic acid) and BEW (n-butanolethanol-water, 4:1:2.). Further purication was carried out by Sephadex LH-20 column chromatography eluting with methanol. Color reactions (brown to yellow in UVNH3), chromatographic behaviour (Rf values on Watman No 1 paper: 0.74 in BAW; 0.43 in 15% HOAc) and ultraviolet spectral analysis in the presence of usual shift reagents (kmax (nm)
SHORTER NOTES
63
FIG. 1. Structure of Flavonoid I.
(MeOH) 272, 334; AlCl3 276, 385; AlCl3/HCl 277, 384; NaOAc 272, 382; NaOMe 273, 383) suggested that avonoid I may be a avone with free hydroxyl groups at positions 5 and 49. Electrospray mass spectrum showed a pseudomolecular ion at m/z 413 [MNa] suggesting that avonoid I may be a avone aglycone esteried with benzoic acid or a hydroxybenzoic acid. Acid hydrolysis (2N HCl; 2h under reux) gave p-hydroxybenzoic acid and apigenin. These data show that avonoid I is apigenin 7-O-p-hydroxybenzoate (Fig. 1), a new natural product. The structure of this compound was conrmed by 1H NMR spectrum (300 MHz; DMSO-d6) which showed signals of apigenin at d 6.03 (1H, doublet, J 2 Hz; H-6), d 6.24 (1H, doublet, J 2 Hz; H-8), d 6.71 (1H, singlet; H-3), d 6.94 (2H, doublet, J 8.6 Hz; H-39 and H-59), d 7.98 (2H, doublet, J 8.6 Hz; H-29 and H69); signals of p-hydroxybenzoyl group appeared at d 6.82 (2H, doublet, J 8.3 Hz; H-40 and H-60) and d 7.61 (2H, doublet, J 8.3 Hz; H-30 and H-70). This is the rst report of the occurrence of a avone aglycone acylated with an aromatic acid in ferns. A single ester of a avonoid aglycone with an aromatic acid has previously been reported from plants; this compound was isolated from leaf resin of Baccaris bigelovii A. Gray (Asteraceae) and has been identied as chrysin 7-O-benzoate by Arriaga-Giner et al. (Z. Naturforsch 41c:946948. 1986). Thirteen methylated avonol aglycones, acylated with aliphatic acids (acetic and butyric) have previously been found in ferns belonging to the genus Notholaena as shown in the review of Wollenweber (pp. 259335, in J. B. Harborne, ed., The Flavonoids, Advances in Research Since 1986, Chapman and Hall, London, 1994). In avonoid glycosides p-hydroxybenzoic has been found as acyl substituent in kaempferol 3-(phydroxybenzoylglucoside)-7-glucoside from Narcissus poeticus L. (Amaryllidaceae) by Schoensiegel et al. (Z. Naturforsch 24 b:12131214, 1969) and in quercetin 3-a-(20-p-hydroxybenzoyl-40-p-coumaroylrhamnoside) from Libocedrus bidwillii Hook. f. (Cupressaceae) by Franke and Markham (Phytochemistry 28:35653567, 1989). Rf values on Whatman No 1 paper, color reactions and UV spectral analysis in the presence of usual shift reagents suggested that avonoid II may be a avone glycoside with free hydroxyl groups at positions 5, 7, 39 and 49. This product may be a C-glycosylavone since it was resistant to acid hydrolysis
64
AMERICAN FERN JOURNAL: VOLUME 96 NUMBER 2 (2006)
(2N HCl; 2 hr under reux ). Electrospray mass spectrum showed a pseudomolecular ion at m/z 603 [MNa], an ion at m/z 1183 [(Mx2)Na] (dimer), an ion at m/z 1809 [(Mx3)3 Na] (trimer) and a fragment ion at m/z 441 [CpentosylluteolinNa]; these data show that Flavonoid II is a C-exosyl-Cpentosylluteolin. Since Wessely-Moser isomerization (3N HCl; 3h under reux) gave 6-C-arabinosyl-8-C-glucosylluteolin (previously found in Pteris vittata by Imperato (Amer. Fern J. 92:244246, 2002)), Flavonoid II is identied as 6-C-glucosyl-8-C-arabinosylluteolin (carlinoside) which is a new fern constituent. Identication was conrmed by 1H NMR spectrum and 13C NMR spectrum. Flavonoid III was identied as schaftoside (6-C-glucosyl-8-C-arabinosylapigenin) by UV spectral analysis in the presence of usual shift reagents, Rf values, color reactions, electrospray mass spectrum (which showed a pseudomolecular ion at m/z 587 [MNa] (C-exosyl-C-pentosylapigenin) and an ion at m/z 1151 [Mx2Na] (dimer)), 1H NMR spectrum and 13C NMR spectrum. Schaftoside has previously been found in Angiopteris lygodiifolia Ros. (Mariattaceae) by Wallace et al. (Phytochemistry 20:27012703, 1981), in Polypodium vulgare L. (Polypodiaceae) by Karl et al. (Z. Naturforsh 37c:148 153, 1982) and in Archangiopteris henryi Christ & Gies. (Angiopteridaceae) by Wallace (Biochem. Syst. Ecol. 15:529530, 1987). Flavonoid IV was present in trace amounts and was resistant to acid hydrolysis (2N HCl; 2h under reux). It was partially characterized as di-Cpentosylapigenin by UV spectral analysis with usual shift reagents and electrospray mass spectrum which showed a pseudomolecular ion at m/z 557 [MNa] (di-C-pentosylapigenin) and an ion at m/z 1091 [M32Na] (dimer). The presence of three dimers and one trimer in electrospray mass spectra of di-C-glycosylavones IIIV may be due to hydrogen bonding in gas phase. Hydrogen bonding in crystal structures of avonoids has been shown by a number of authors, e.g. by Cantrell (pp. 391394, in V. Cody, E. Middleton, Jr. and J. B. Harborne, eds., Plant Flavonoids in Biology and Medicine, Alan R. Liss, New York, 1986). The presence of C-glycosylavones IIIV in Pteris vittata and the four Cglycosylavones previously reported from this fern show that Pteris vittata has an impressive range of C-glycosylavones; a similar situation has been observed in Angiopteris evecta (G. Forst.) Hoffm. (Mariattaceae) as shown in the review of Markham (pp. 427468, in J. B. Harborne, ed., The Flavonoids: Advances in Research since 1980; Chapman and Hall, London, New York, 1988) but this fern lacks avonoid O-glycosides which are present in Pteris vittata. Polyphenols of ferns have been investigated by Imperato and co-workers since 1978. It is well known that avonoids are of interest in chemotaxonomic and phylogenetic studies but avonoid data are scanty for many fern families. Ninety avonoids including sixty-three new fern constituents have been isolated from ferns in this laboratory as shown in the review by Markham (1988), in the review by Imperato (pp. 3975 in Current Topics in Phytochemistry, Research Trends, Trivandrum, 2000) and in the introduction of this paper; these avonoids include fty new natural products. Among the
SHORTER NOTES
65
new compounds, kaempferol 3-O-(X0-p-coumaroyl-X0-heptaglycylapioside) isolated by Imperato (Amer. Fern J. 86:127128, 1996) from Pteridium aquilinum is the only known avonoid occurring in such bound form in plants. The isolation of bracteatin by Imperato (La Chimica & LIndustria 71:8687, 1989) from Asplenium kaulfussii Schltdl. represents the only known report of an aurone from ferns. 3-C-Xylosylapigenin 7-methyl ether and 3-Crhamnosylluteolin isolated by Imperato (Phytochemistry (Life Science Advances) 11:225230, 1992) from Asplenium viviparum belong to a new type of mono-C-glycosylavones; in addition the isolation by Imperato (Phytochemistry 33:729730, 1993) of 3,6,8-tri-C-xylosylapigenin from Asplenium viviparum (L.f.) Pr. represents the rst report of a tri-C-glycosylavonoid from plants. It is well known that Smith and Levin (Am. J. Bot. 50:952958, 1963) provided a clear example of additive inheritance of chemical characters in Asplenium hybrids; subsequently Richardson and Lorenz-Liburnau showed (Amer. Fern J. 72:103106, 1982) that the chemistry of European Asplenium adiantum-nigrum L. complex is analogous to that of Appalachian Asplenium complex. Thirty-two avonoid glycosides from Asplenium species have been fully characterized in this laboratory as shown in two reviews by Imperato (Biochem. Syst. Ecol. 17:161166, 1989 and the above review (2000)). In 1961 Harborne and Corner (Biochem. J. 81:242250, 1961) showed that hydroxycinnamic acid-sugar derivatives are of wide occurrence in higher plants; subsequently three papers by Bohm and Tyron (Can. J. Bot. 45:585593, 1967), by Bohm (Phytochemistry 7:18251830, 1968) and by Glass and Bohm (Phytochemistry 8:629632, 1969) showed that hydroxycinnamic acids are widespread in ferns with the exception of sinapic acid. Seventeen hydroxycinnamic acid-sugar derivatives including nine new natural products have been isolated by Imperato from ferns belonging to the genera Asplenium, Ceterach, Adiantum and Cystopteris as shown in the review by Imperato (2000). Most of these compounds are 1-p-coumaroylglucose sulphates and 1caffeoylglucose sulphates having sulphate attached to an hydroxyl group of sugar moiety; in two of these products glucose is replaced by galactose or by a disaccharide (laminaribiose). As shown in the review by Richardson (Biochem Syst. Ecol. 12:16, 1984) xanthones of ferns may conrm or reveal the relationships between diploids and allopolyploids, although these compounds are of little interest in fern taxonomy. Fourteen xanthones, including nine new natural products have been found in ferns in this laboratory; among these products 3,7,8-trihydroxy1-O-b-laminaribioside isolated by Imperato from Asplenium adiantum-nigrum (Phytochemistry 19:20302031,1980) is the rst xanthone glycoside having the carbohydrate moiety attached to a phenolic hydroxyl group. In addition, 1,6-dihydroxy-3,5,7-trimethoxyxanthone isolated by Imperato from Cystopteris fragilis (J. Nat. Prod. 54:603604, 1991) is the rst pentaoxygenated xanthone found in ferns. ` The author thanks Universita della Basilicata for nancial support. Mass spectra were provided by SESMA (CNR, Naples).FILIPPO IMPERATO, Diparti` mento di Chimica, Universita della Basilicata, 85100 Potenza, ITALY.
Potrebbero piacerti anche
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5795)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (400)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (74)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- 365 Days (Blanka Lipińska)Documento218 pagine365 Days (Blanka Lipińska)rjalkiewiczNessuna valutazione finora
- HSN-Lube 2007 PDFDocumento45 pagineHSN-Lube 2007 PDFCecilio Valderrama100% (3)
- This Is No Way To Treat An Aorta.: Edwards EZ Glide Aortic CannulaDocumento5 pagineThis Is No Way To Treat An Aorta.: Edwards EZ Glide Aortic CannulaAhmadNessuna valutazione finora
- Pineapple PDFDocumento7 paginePineapple PDFDestia AyuNessuna valutazione finora
- HDFC Life Insurance (HDFCLIFE) : 2. P/E 58 3. Book Value (RS) 23.57Documento5 pagineHDFC Life Insurance (HDFCLIFE) : 2. P/E 58 3. Book Value (RS) 23.57Srini VasanNessuna valutazione finora
- 15 UrinalysisDocumento9 pagine15 UrinalysisJaney Ceniza تNessuna valutazione finora
- Section IIDocumento8 pagineSection IIapi-471272376Nessuna valutazione finora
- Starbucks Reconciliation Template & Instructions v20231 - tcm137-84960Documento3 pagineStarbucks Reconciliation Template & Instructions v20231 - tcm137-84960spaljeni1411Nessuna valutazione finora
- Intellectual Disability: Definition, Classification, Causes and CharacteristicsDocumento12 pagineIntellectual Disability: Definition, Classification, Causes and CharacteristicsRyan RV ViloriaNessuna valutazione finora
- Case Study LenovoDocumento10 pagineCase Study LenovoGOHAR GHAFFARNessuna valutazione finora
- AluminumPresentationIEEE (CompatibilityMode)Documento31 pagineAluminumPresentationIEEE (CompatibilityMode)A. HassanNessuna valutazione finora
- EE2401 Power System Operation and ControlDocumento93 pagineEE2401 Power System Operation and ControlPrasanth GovindarajNessuna valutazione finora
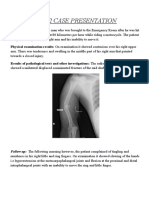
- PBL 2 Case PresentationDocumento12 paginePBL 2 Case PresentationRamish IrfanNessuna valutazione finora
- 2nd Term Study Guide 4th Grade Feb 2024 Cambridge ObjectivesDocumento8 pagine2nd Term Study Guide 4th Grade Feb 2024 Cambridge Objectivessofi.cardenas1968Nessuna valutazione finora
- PMEGP Revised Projects (Mfg. & Service) Vol 1Documento260 paginePMEGP Revised Projects (Mfg. & Service) Vol 1Santosh BasnetNessuna valutazione finora
- Region Iii - Central Luzon Padapada National High SchoolDocumento2 pagineRegion Iii - Central Luzon Padapada National High SchoolRotipNessuna valutazione finora
- Pamela Johnson Arnold and Nancy Fortson On Behalf of A Minor Vs Hamilton Co. Dept. of EducationDocumento27 paginePamela Johnson Arnold and Nancy Fortson On Behalf of A Minor Vs Hamilton Co. Dept. of EducationDan LehrNessuna valutazione finora
- 1 SMDocumento10 pagine1 SMAnindita GaluhNessuna valutazione finora
- 1 A Finalexam FNH330 June 2015 Final Review QuestionsDocumento6 pagine1 A Finalexam FNH330 June 2015 Final Review QuestionsChinley HinacayNessuna valutazione finora
- Docu Ifps Users Manual LatestDocumento488 pagineDocu Ifps Users Manual LatestLazar IvkovicNessuna valutazione finora
- Pre-Operative Check Up of Farm Tools, ImplementsDocumento19 paginePre-Operative Check Up of Farm Tools, ImplementsLaurence Fabiala50% (2)
- An Assignment On "Mycology Laboratory Technique"Documento1 paginaAn Assignment On "Mycology Laboratory Technique"BsksvdndkskNessuna valutazione finora
- Pearls and Pitfalls in Emergency Radiology Variants and Other Difficult Diagnoses 2013Documento389 paginePearls and Pitfalls in Emergency Radiology Variants and Other Difficult Diagnoses 2013mmbire@gmail.comNessuna valutazione finora
- Martins Taylorb Os 10742 Final Opinion 2 11 2022 02898337xd2c78Documento9 pagineMartins Taylorb Os 10742 Final Opinion 2 11 2022 02898337xd2c78Live 5 NewsNessuna valutazione finora
- Course Weekly Schedule Health Science TheoryDocumento6 pagineCourse Weekly Schedule Health Science Theoryapi-466810096Nessuna valutazione finora
- Upper Limb OrthosesDocumento29 pagineUpper Limb OrthosesMaryam KhalidNessuna valutazione finora
- Aquamine 50.01Documento17 pagineAquamine 50.01Armando RelajoNessuna valutazione finora
- Trane Air Cooled Scroll Chillers Installation Operation MaintenanceDocumento276 pagineTrane Air Cooled Scroll Chillers Installation Operation MaintenanceBay Mưa100% (1)
- Marketing ProjectDocumento82 pagineMarketing ProjectSumit GuptaNessuna valutazione finora
- BRS PDFDocumento14 pagineBRS PDFGautam KhanwaniNessuna valutazione finora