Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Assignment 8 Solution
Caricato da
Brishen HawkinsDescrizione originale:
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Assignment 8 Solution
Caricato da
Brishen HawkinsCopyright:
Formati disponibili
MSE 280B Fall 2011
Assignment 8
Solutions
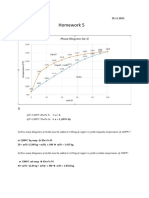
1. (10 Points) Is it possible to have a coppersilver alloy that, at equilibrium, consists of a
phase of composition 92 wt% Ag8 wt% Cu, and also a liquid phase of composition 77
wt% Ag23 wt% Cu? If so, what will be the approximate temperature of the alloy? If
this is not possible, explain why.
It is possible to have a Cu-Ag alloy, which at equilibrium consists of a | phase of
composition 92 wt% Ag-8 wt% Cu and a liquid phase of composition 77 wt% Ag-23
wt% Cu. From Figure 10.7 a horizontal tie line can be constructed across the | + L phase
region at about 800C which intersects the L(| + L) phase boundary at 76 wt% Ag, and
also the (| + L)| phase boundary at 92 wt% Ag.
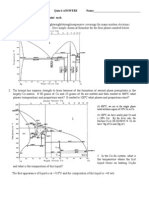
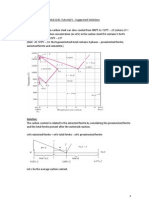
2. (15 Points) A leadtin alloy of composition 30 wt% Sn70 wt% Pb is slowly heated
from a temperature of 150C (300F).
(a) At what temperature does the first liquid phase form?
(b) What is the composition of this liquid phase?
(c) At what temperature does complete melting of the alloy occur?
(d) What is the composition of the last solid remaining prior to complete
melting?
Upon heating a lead-tin alloy of composition 30 wt% Sn-70 wt% Pb from 150C and
utilizing Figure 10.8 as shown below:
(a) The first liquid forms at the temperature at which a vertical line at this composition
intersects the eutectic isotherm--i.e., at 183C.
(b) The composition of this liquid phase corresponds to the intersection with the (o + L)
L phase boundary, of a tie line constructed across the o + L phase region just above this
eutectic isotherm--i.e., C
L
= 61.9 wt% Sn.
(c) Complete melting of the alloy occurs at the intersection of this same vertical line at
30 wt% Sn with the (o + L)L phase boundary--i.e., at about 260C.
(d) The composition of the last solid remaining prior to complete melting corresponds to
the intersection with o(o + L) phase boundary, of the tie line constructed across the o +
L phase region at 260C--i.e., C
o
is about 13 wt% Sn.
3. (20 Points) A 2.0-kg specimen of an 85 wt% Pb15 wt% Sn alloy is heated to 200C
(390F); at this temperature it is entirely an -phase solid solution (Figure 10.8). The
alloy is to be melted to the extent that 50% of the specimen is liquid, the remainder being
the phase. This may be accomplished by heating the alloy or changing its composition
while holding the temperature constant.
(a) To what temperature must the specimen be heated?
(b) How much tin must be added to the 2.0-kg specimen at 200C to achieve this state?
(a) This part of the problem calls for us to cite the temperature to which a 85 wt% Pb-15
wt% Sn alloy must be heated in order to have 50% liquid. Probably the easiest way to
solve this problem is by trial and error--that is, on the Pb-Sn phase diagram (Figure 10.8),
moving vertically at the given composition, through the o + L region until the tie-line
lengths on both sides of the given composition are the same. This occurs at
approximately 280C (535F).
(b) We can also produce a 50% liquid solution at 200C, by adding Sn to the alloy. At
200C and within the o + L phase region
C
o
= 17 wt% Sn-83 wt% Pb
C
L
= 57 wt% Sn-43 wt% Pb
Let C
0
be the new alloy composition to give W
o
= W
L
= 0.5. Then,
W
o
= 0.5 =
C
L
C
0
C
L
C
o
=
57 C
0
57 17
And solving for C
0
gives 37 wt% Sn. Now, let m
Sn
be the mass of Sn added to the alloy
to achieve this new composition. The amount of Sn in the original alloy is
(0.15)(2.0 kg) = 0.30 kg
Then, using a modified form of Equation 5.6
0.30 kg + m
Sn
2.0 kg + m
Sn
(
(
(
100 = 37
And, solving for m
Sn
(the mass of tin to be added), yields m
Sn
= 0.698 kg.
4. (30 Points) Consider 6.0 kg of austenite containing 0.45 wt% C, cooled to below 727C
(1341F).
(a) What is the proeutectoid phase?
(b) How many kilograms each of total ferrite and cementite form?
(c) How many kilograms each of pearlite and the proeutectoid phase form?
(d) Schematically sketch and label the resulting microstructure.
(a) Ferrite is the proeutectoid phase since 0.45 wt% C is less than 0.76 wt% C.
(b) For this portion of the problem, we are asked to determine how much total ferrite and
cementite form. For ferrite, application of the appropriate lever rule expression yields
W
o
=
C
Fe
3
C
C
0
C
Fe
3
C
C
o
=
6.70 0.45
6.70 0.022
= 0.94
which corresponds to (0.94)(6.0 kg) = 5.64 kg of total ferrite.
Similarly, for total cementite,
W
Fe
3
C
=
C
0
C
o
C
Fe
3
C
C
o
=
0.45 0.022
6.70 0.022
= 0.06
Or (0.06)(6.0 kg) = 0.36 kg of total cementite form.
(c) Now consider the amounts of pearlite and proeutectoid ferrite. Using Equation 10.20
W
p
=
C
0
'
0.022
0.74
=
0.45 0.022
0.74
= 0.58
This corresponds to (0.58)(6.0 kg) = 3.48 kg of pearlite.
Also, from Equation 10.21,
W
o'
=
0.76 0.45
0.74
= 0.42
Or, there are (0.42)(6.0 kg) = 2.52 kg of proeutectoid ferrite.
(d) Schematically, the microstructure would appear as:
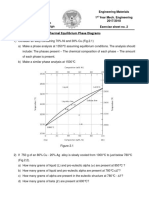
5. (25 Points) Below is a portion of the copperaluminum phase diagram for which only
single-phase regions are labeled. Specify temperaturecomposition points at which all
eutectics, eutectoids, peritectics, and congruent phase transformations occur. Also, for
each, write the reaction upon cooling.
There is one eutectic on this phase diagram, which exists at 8.3 wt% Al-91.7 wt% Cu and
1036C. Its reaction upon cooling is
L o + |
There are four eutectoids for this system. One exists at 11.8 wt% Al-88.2 wt% Cu and
565C. This reaction upon cooling is
| o +
2
Another eutectoid exists at 15.4 wt% Al-84.6 wt% Cu and 964C. For cooling the reaction is
_ | +
1
A third eutectoid exists at 15.5 wt% Al-84.5 wt% Cu and 786C. For cooling the reaction is
1
| +
2
The other eutectoid exists at 23.5 wt% Al-76.5 wt% Cu and 560C. For cooling the reaction is
c
2
o + ,
1
There are four peritectics on this phase diagram. One exists at 15.3 wt% Al-84.7 wt% Cu
and 1037C. The reaction upon cooling is
| + L _
Another peritectic exists at 17 wt% Al-83 wt% Cu and 1021C. It's cooling reaction is
_ + L
1
Another peritectic exists at 20.5 wt% Al-79.5 wt% Cu and 961C. The reaction upon cooling is
1
+ L c
1
Another peritectic exists at 28.4 wt% Al-71.6 wt% Cu and 626C. The reaction upon cooling is
c
2
+ L q
1
There is a single congruent melting point that exists at 12.5 wt% Al-87.5 wt% Cu and
1049C. The reaction upon cooling is
L |
Potrebbero piacerti anche
- Week 11Documento12 pagineWeek 11lduran_63Nessuna valutazione finora
- Assignment 7 SolutionsDocumento11 pagineAssignment 7 SolutionsIsaiah Qe LiewNessuna valutazione finora
- CH 10Documento9 pagineCH 1000gp1200r100% (1)
- CH 09Documento8 pagineCH 09Antoinette ChuaNessuna valutazione finora
- HW 9 2011 SolutionsDocumento15 pagineHW 9 2011 SolutionsParamesh Anugu100% (1)
- Mse230 E2 Practice SolDocumento20 pagineMse230 E2 Practice SolAkshit PandeyNessuna valutazione finora
- HW SolutionsDocumento16 pagineHW SolutionsoerbilNessuna valutazione finora
- Closed-Book Practice-Ch 09 (2017!08!07)Documento9 pagineClosed-Book Practice-Ch 09 (2017!08!07)Juan100% (1)
- 9 SolutionsDocumento31 pagine9 SolutionsLaurertan TavaresNessuna valutazione finora
- Phase Diagram - : Dr. Aneela WakeelDocumento21 paginePhase Diagram - : Dr. Aneela WakeelHassan KhanNessuna valutazione finora
- HW 8 TrustedDocumento20 pagineHW 8 TrustedEldeniz AliyevNessuna valutazione finora
- Binary Alloy Phase DiagramsDocumento12 pagineBinary Alloy Phase Diagramseeng.ali651550% (2)
- HW9 PracticexDocumento7 pagineHW9 PracticexJod JDNessuna valutazione finora
- Phase Diagram Analysis of the Al-Cu Binary SystemDocumento6 paginePhase Diagram Analysis of the Al-Cu Binary Systemnilanga123Nessuna valutazione finora
- 4Documento63 pagine4ARJUN PESARU 19BME0161Nessuna valutazione finora
- Eng Mat Chapter 4Documento126 pagineEng Mat Chapter 4VC Chua Yee LeongNessuna valutazione finora
- Phase DiagramDocumento47 paginePhase DiagramMuhammed MansNessuna valutazione finora
- Tutorial 2 HelperDocumento3 pagineTutorial 2 HelperDedy SaputraNessuna valutazione finora
- Chapter 8 - Phase Diagram PART2Documento26 pagineChapter 8 - Phase Diagram PART2Mohd IqbalNessuna valutazione finora
- Cu-Ni Phase DiagramDocumento5 pagineCu-Ni Phase DiagramFahri Ali ImranNessuna valutazione finora
- Composition Solidus Temperature Liquidus Temperature: (WT% Si) (°C) (°C)Documento7 pagineComposition Solidus Temperature Liquidus Temperature: (WT% Si) (°C) (°C)Muhammad Ibkar YusranNessuna valutazione finora
- Phase Diagram: Type III group with Eutectic ReactionDocumento20 paginePhase Diagram: Type III group with Eutectic ReactionAhmad NawazNessuna valutazione finora
- Phase Diagram Exercises - Worked Answers - CorrectedDocumento14 paginePhase Diagram Exercises - Worked Answers - CorrectedHayden Reeves75% (4)
- Phase Diagram ExDocumento23 paginePhase Diagram ExTey KaijingNessuna valutazione finora
- Solving Phase Equilibrium ProblemsDocumento5 pagineSolving Phase Equilibrium ProblemsJulian GarcíaNessuna valutazione finora
- Problems Phase FeDocumento9 pagineProblems Phase FeAshutoshKumarNessuna valutazione finora
- Phase Diagram AssignmentDocumento10 paginePhase Diagram AssignmentSyafiqah RusdiNessuna valutazione finora
- Chapter 9: Phase Diagrams: Issues To Address..Documento41 pagineChapter 9: Phase Diagrams: Issues To Address..Faiz AkhtarNessuna valutazione finora
- Fe-C Phase Diagram MCQ & Essay QuestionsDocumento5 pagineFe-C Phase Diagram MCQ & Essay QuestionsĐỗ ĐăngNessuna valutazione finora
- Tugas LiburDocumento5 pagineTugas LiburAnonymous AjrDxGNessuna valutazione finora
- Materials Science and Engineering Concept Check Part2 PDFDocumento27 pagineMaterials Science and Engineering Concept Check Part2 PDF李宛芸Nessuna valutazione finora
- Phase Diagrams and Microstructures in AlloysDocumento4 paginePhase Diagrams and Microstructures in AlloysTia AgustinNessuna valutazione finora
- Phase Diagrams ExplainedDocumento222 paginePhase Diagrams ExplainedFAIQNessuna valutazione finora
- ENSC 3313, Fall 2012 Quiz 6 Answers Name - Answer Any 4 Questions Only, 1 Point EachDocumento2 pagineENSC 3313, Fall 2012 Quiz 6 Answers Name - Answer Any 4 Questions Only, 1 Point EachterratempestNessuna valutazione finora
- 8 - Phase Diagram 0 PDFDocumento28 pagine8 - Phase Diagram 0 PDFAtif IrshadNessuna valutazione finora
- Chapter 9: Phase Diagrams: Issues To Address..Documento38 pagineChapter 9: Phase Diagrams: Issues To Address..yunlu0705Nessuna valutazione finora
- Che 3330 - Spring 2012 HW 5Documento5 pagineChe 3330 - Spring 2012 HW 5Brett CasserlyNessuna valutazione finora
- Chapt 08Documento21 pagineChapt 08Jesse McClure100% (5)
- 98materials Phase DiagramsDocumento27 pagine98materials Phase DiagramsHari PrasathNessuna valutazione finora
- Recitation 2 QuestionsDocumento14 pagineRecitation 2 QuestionsfzfwsbyxrhNessuna valutazione finora
- Ceramic Calculations Sample 3Documento24 pagineCeramic Calculations Sample 3AkonSayagyiNessuna valutazione finora
- CH 09Documento99 pagineCH 09AbdoNessuna valutazione finora
- Eutectics: Lecture 3 Manufacturing TechnologyDocumento24 pagineEutectics: Lecture 3 Manufacturing TechnologyAyush BhadauriaNessuna valutazione finora
- MLE1101 Tutorial 4 - Suggested Solutions AnalysisDocumento7 pagineMLE1101 Tutorial 4 - Suggested Solutions AnalysisYin HauNessuna valutazione finora
- Phase Diagrams & Heat Treatment of Carbon SteelDocumento84 paginePhase Diagrams & Heat Treatment of Carbon SteelTanmay DuttaNessuna valutazione finora
- Introduction to Phase Diagrams and Their Application to Mantle MeltingDocumento4 pagineIntroduction to Phase Diagrams and Their Application to Mantle MeltingJOSE MIGUELNessuna valutazione finora
- Engineering Materials Phase DiagramsDocumento6 pagineEngineering Materials Phase DiagramsOmar AssalNessuna valutazione finora
- Ch10 Phase DiagramsDocumento79 pagineCh10 Phase DiagramsDhileepan KumarasamyNessuna valutazione finora
- Phase DiagramsDocumento79 paginePhase DiagramsArun V NairNessuna valutazione finora
- Calculate phase amounts in Cu-Ni and Pb-Sn alloysDocumento8 pagineCalculate phase amounts in Cu-Ni and Pb-Sn alloysBukti NegalNessuna valutazione finora
- Assignment 4 Phase DiagramDocumento4 pagineAssignment 4 Phase DiagramAhmedAhmedNessuna valutazione finora
- Homework 5Documento4 pagineHomework 5Ferhat PeynirciNessuna valutazione finora
- Exam 3 Material Science MATS 2001 UMN Fall 2012Documento7 pagineExam 3 Material Science MATS 2001 UMN Fall 2012Zaki Smn100% (1)
- Phases PDFDocumento13 paginePhases PDFc1a5c7Nessuna valutazione finora
- W, H, F L T: ORK EAT AND THE Irst AW OF HermodynamicsDocumento7 pagineW, H, F L T: ORK EAT AND THE Irst AW OF HermodynamicsonetimeuploadNessuna valutazione finora
- Chapter 8 (Heat Treatment)Documento12 pagineChapter 8 (Heat Treatment)Shekinah KanindaNessuna valutazione finora
- MLE1101 - Tutorial 5 - Suggested SolutionsDocumento5 pagineMLE1101 - Tutorial 5 - Suggested SolutionsYin HauNessuna valutazione finora
- Solution Manual Callister 8th EditionDocumento15 pagineSolution Manual Callister 8th EditionAbdur Rozaq25% (8)
- A Modern Course in Statistical PhysicsDa EverandA Modern Course in Statistical PhysicsValutazione: 3.5 su 5 stelle3.5/5 (2)
- GMAT R2016a User GuideDocumento921 pagineGMAT R2016a User GuideBrishen HawkinsNessuna valutazione finora
- SPIE AstroPreprint 02Documento9 pagineSPIE AstroPreprint 02Brishen HawkinsNessuna valutazione finora
- Optimal Control and Applications To Aerospace Some Results and ChallengesDocumento46 pagineOptimal Control and Applications To Aerospace Some Results and ChallengesBrishen HawkinsNessuna valutazione finora
- Exoplanet SatDocumento14 pagineExoplanet SatBrishen HawkinsNessuna valutazione finora
- 4gb Gddr5 Sgram BriefDocumento7 pagine4gb Gddr5 Sgram BriefBrishen HawkinsNessuna valutazione finora
- Stability of Triangular Lagrangian PointsDocumento7 pagineStability of Triangular Lagrangian PointsBrishen HawkinsNessuna valutazione finora
- Ryu Et Al. - 2014 - Seismo-Ionospheric Coupling Appearing As Equatorial Electron Density Enhancements Observed Via DEMETER Electron DensityDocumento19 pagineRyu Et Al. - 2014 - Seismo-Ionospheric Coupling Appearing As Equatorial Electron Density Enhancements Observed Via DEMETER Electron DensityBrishen HawkinsNessuna valutazione finora
- The Origin of The Kirkwood Gaps A Mapping Technique For Asteroidal Motion The The 3-1 CommensurabilityDocumento17 pagineThe Origin of The Kirkwood Gaps A Mapping Technique For Asteroidal Motion The The 3-1 CommensurabilityBrishen HawkinsNessuna valutazione finora
- Seattle Minimum Wage Study Finds Modest Impact on Wages and JobsDocumento57 pagineSeattle Minimum Wage Study Finds Modest Impact on Wages and JobsBrishen HawkinsNessuna valutazione finora
- A Statistical Comparison of Vertical Total Electron Content (TEC) From Three Ionospheric ModelsDocumento23 pagineA Statistical Comparison of Vertical Total Electron Content (TEC) From Three Ionospheric ModelsBrishen HawkinsNessuna valutazione finora
- Lenovo x200 x201 Technical Manual 43y6632 05Documento322 pagineLenovo x200 x201 Technical Manual 43y6632 05sindu77166Nessuna valutazione finora
- The Resonance Overlap Criterion and The Onset of Stochastic Behavior in The Restricted Three-Body ProblemDocumento12 pagineThe Resonance Overlap Criterion and The Onset of Stochastic Behavior in The Restricted Three-Body ProblemBrishen HawkinsNessuna valutazione finora
- 5394GMA2Cheat SheetDocumento4 pagine5394GMA2Cheat SheetBrishen HawkinsNessuna valutazione finora
- A Universal Instability of Many-Dimensional Oscillator SystemsDocumento117 pagineA Universal Instability of Many-Dimensional Oscillator SystemsBrishen Hawkins100% (1)
- 2013jgra.50413 Mukhtarov EtalDocumento15 pagine2013jgra.50413 Mukhtarov EtalBrishen HawkinsNessuna valutazione finora
- Avionic Data For Business JetsDocumento20 pagineAvionic Data For Business JetsBrishen HawkinsNessuna valutazione finora
- Chaotic Behavior and The Origin of The 31 Kirkwood GapDocumento24 pagineChaotic Behavior and The Origin of The 31 Kirkwood GapBrishen HawkinsNessuna valutazione finora
- Oth 1Documento8 pagineOth 1Brishen HawkinsNessuna valutazione finora
- Nav20090100002 32837248Documento7 pagineNav20090100002 32837248Brishen HawkinsNessuna valutazione finora
- 61 0500eDocumento3 pagine61 0500eBrishen HawkinsNessuna valutazione finora
- PhysRevB 48 14009Documento4 paginePhysRevB 48 14009Brishen HawkinsNessuna valutazione finora
- 000 0 Tsop1838Documento7 pagine000 0 Tsop1838Andre PazmiñoNessuna valutazione finora
- AE202 Homework 5: 1 ConstantsDocumento4 pagineAE202 Homework 5: 1 ConstantsBrishen HawkinsNessuna valutazione finora
- A Placebo-Controlled Evaluation of Adjunctive Modafinil in The Treatment of Bipolar DepressionDocumento8 pagineA Placebo-Controlled Evaluation of Adjunctive Modafinil in The Treatment of Bipolar DepressionBrishen HawkinsNessuna valutazione finora
- WLesson 1 Intro and Atomic StructureDocumento29 pagineWLesson 1 Intro and Atomic StructureBrishen HawkinsNessuna valutazione finora
- MSE 280B Assignment 7 SolutionsDocumento6 pagineMSE 280B Assignment 7 SolutionsBrishen Hawkins100% (2)
- Week 1Documento7 pagineWeek 1Brishen HawkinsNessuna valutazione finora
- Understanding Blow Molding: Norman C. LeeDocumento11 pagineUnderstanding Blow Molding: Norman C. LeeKiran ModakNessuna valutazione finora
- Super Duplex Stainless SteelDocumento1 paginaSuper Duplex Stainless SteelUma Shankar100% (1)
- Disriuton..: StaunannouncedDocumento44 pagineDisriuton..: StaunannouncedChiao YunNessuna valutazione finora
- ESCO Crushing BrochureDocumento20 pagineESCO Crushing BrochureEdwardNessuna valutazione finora
- Chromium-Nickel Stainless SteelsDocumento6 pagineChromium-Nickel Stainless SteelsrajkmuarNessuna valutazione finora
- Assignment of Electrochemical Grinding MachiningDocumento5 pagineAssignment of Electrochemical Grinding MachiningRajesh PuniaNessuna valutazione finora
- Us Eutronic Arc Spray Wires Brochure SinglesDocumento4 pagineUs Eutronic Arc Spray Wires Brochure SinglesRusli MubarokNessuna valutazione finora
- Stainless Steel Socket, Square Head, and Slotted Headless-Set ScrewsDocumento6 pagineStainless Steel Socket, Square Head, and Slotted Headless-Set ScrewsDanZel Dan100% (1)
- Farsi Steel PunchesDocumento10 pagineFarsi Steel PunchessdrabaNessuna valutazione finora
- SS304 Hardening BehaviourDocumento4 pagineSS304 Hardening BehaviourrbagriNessuna valutazione finora
- ER-5861 SSDA Slotted Web Beam-To-Column Steel Moment Frame ConnectionDocumento6 pagineER-5861 SSDA Slotted Web Beam-To-Column Steel Moment Frame Connectioncancery0707Nessuna valutazione finora
- K2 Materials ReqDocumento71 pagineK2 Materials ReqPoetra PangestuNessuna valutazione finora
- SpekrometerDocumento16 pagineSpekrometerPutra AjaNessuna valutazione finora
- Materiales Solucionario (271 406)Documento136 pagineMateriales Solucionario (271 406)Yhonatan Gotea Zambrano100% (1)
- Technical - Details Ecf ActuatorDocumento7 pagineTechnical - Details Ecf ActuatorDheeraj MohanNessuna valutazione finora
- Trade Fitter 1 ST Semester Multiple Choice Questions: About The Institute & SafetyDocumento42 pagineTrade Fitter 1 ST Semester Multiple Choice Questions: About The Institute & SafetyAnonymous n7jQFvW7rNessuna valutazione finora
- Selective Leaching Type CorrosionDocumento14 pagineSelective Leaching Type CorrosionRifqi MumtazNessuna valutazione finora
- Physical Metallurgy Vol. 1 2 y 3Documento55 paginePhysical Metallurgy Vol. 1 2 y 3veronicaNessuna valutazione finora
- Astm A-350Documento9 pagineAstm A-350jair botelloNessuna valutazione finora
- BLRF 150 H No. 191201Documento2 pagineBLRF 150 H No. 191201suria qaqcNessuna valutazione finora
- AW 887 - EnuDocumento410 pagineAW 887 - EnuGerardo Grijalva AvilaNessuna valutazione finora
- CLOSED AND OPEN DIE HOT FORGING TECHNIQUESDocumento13 pagineCLOSED AND OPEN DIE HOT FORGING TECHNIQUESNaveen PradeepanNessuna valutazione finora
- BK - TNG 001 TS PI SP 001 Piping Material Specification Rev.1Documento45 pagineBK - TNG 001 TS PI SP 001 Piping Material Specification Rev.1Kev Tra100% (1)
- EN16 Carbon Steel - 605M36T - Malaysia Carbon Steel SupplierDocumento5 pagineEN16 Carbon Steel - 605M36T - Malaysia Carbon Steel SupplierSAMNessuna valutazione finora
- A340 SRM Ata 51-43Documento674 pagineA340 SRM Ata 51-43L1049A100% (6)
- Domex 650 MC Hot Rolled, Extra High Strength, Cold Forming SteelDocumento2 pagineDomex 650 MC Hot Rolled, Extra High Strength, Cold Forming SteelBo WangNessuna valutazione finora
- PIpe Class and StandardDocumento3 paginePIpe Class and StandardSamim RahmanNessuna valutazione finora
- Estimating Welding CostsDocumento41 pagineEstimating Welding CostsAnonymous 7yN43wjl100% (1)
- Mobilgrind Series: Honing and Grinding Fluids For Precision MachiningDocumento2 pagineMobilgrind Series: Honing and Grinding Fluids For Precision MachiningJahmia CoralieNessuna valutazione finora
- Analysis of Ultrasonic Indications in Lack of FusionDocumento8 pagineAnalysis of Ultrasonic Indications in Lack of Fusionantant3052Nessuna valutazione finora