Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Macophag Activain Syndrom
Caricato da
Dr-Usama Sayed AboSehlyDescrizione originale:
Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Macophag Activain Syndrom
Caricato da
Dr-Usama Sayed AboSehlyCopyright:
Formati disponibili
Indian Journal of Rheumatology 2009 December Volume 4, Number 4; pp.
162167
Review Article
Macrophage activation syndrome II/II
K Shanmuganandan1, J Kotwal2
ABSTRACT
Macrophage activation syndrome (MAS) is a rare systemic disorder which results from uncontrolled activation and proliferation of T cells and excessive activation of macrophages. Primary haemophagocytic lymphohistiocytosis (HLH) is recognized as having a genetic basis, but the secondary haemophagocytic syndrome (HS), also referred to as MAS, occurs in a number of autoimmune disorders including systemic onset juvenile idiopathic arthritis, systemic lupus erythematosus (SLE), adult onset Stills disease and other disorders. In this second of the two part series, the clinical features and management are described. Keywords: Macrophage activation syndrome, haemophagocytosis, secondary haemophagocytic lymphohistiocytosis, Stills disease.
INTRODUCTION
Macrophage activation syndrome (MAS) results in significant morbidity and mortality. MAS results from uncontrolled activation and proliferation of T cells and excessive activation of macrophages. It usually occurs as a complication of various systemic inflammatory rheumatic diseases, most commonly systemic onset juvenile idiopathic arthritis.1 It has also been reported with systemic lupus erythematosus (SLE), dermatomyositis, Kawasaki disease (KD) and other systemic inflammatory rheumatic diseases.2 In the last issue of the Journal, the pathobiology and molecular mechanisms of causation of MAS was elucidated.3 In this issue, the clinical features and management issues are discussed with particular reference to rheumatic diseases.
CLINICAL AND LABORATORY FEATURES
The initial presentation and the clinical features of MAS are protean and depend upon the primary condition or the background illness.
The hallmark clinical and laboratory features include high fever, hepatosplenomegaly, lymphadenopathy, pancytopaenia, liver dysfunction, disseminated intravascular coagulation (DIC), hypofibrinogenaemia, hyperferritinaemia, and hypertriglyceridaemia. Despite marked systemic inflammation, the erythrocyte sedimentation rate (ESR) is paradoxically depressed, caused by low fibrinogen levels. The low ESR helps to differentiate the disorder from a flare of the underlying rheumatic disorder, in which case the ESR is usually elevated. A bone marrow biopsy or aspirate usually shows haemophagocytosis. Clinical features, according to the organ system involved, are given in Table 1. The clinical presentation is, in many aspects, similar to systemic inflammatory response syndrome (SIRS). Many patients also present with multiple organ failure.5 Rare cases of nephrotic syndrome have also been reported.6 Since MAS is a relatively rare condition occurring over and above a background illness, the diagnosis is often missed unless there is a high index of suspicion.7 It often presents only with non-specific features, thus eluding the diagnosis and delaying the management which often proves fatal. Palazzi et al. has reported that the clinical and laboratory findings of HLH-MAS were present for up to two weeks of
1 Department of Internal Medicine, 2Department of Pathology, AFMC, Pune, Maharashtra, India. Correspondence: Lt Col K Shanmuganandan, email: kshanmu5520@yahoo.com
Macrophage activation syndrome II/II
Review Article
163
Table 1 Clinical and laboratory features of MAS Organ system General Feature Hectic fever, malaise, anorexia with or without weight loss and failure to thrive Anaemia, leucopaenia, thrombocytopaenia, pancytopaenia, echymosis, prolonged aPTT Non-specific maculopapular rash, erythroderma, generalized purpuric macules and papules, morbilliform eruptions Seizures, ataxia, hemiplegia, mental status changes, irritability40 lymphadenopathy hepatosplenomegaly ARDS Myocarditis, capillary leak syndrome
Table 2 Differentiating features between systemic onset juvenile idiopathic arthritis and MAS Feature Fever Joint inflammation Platelets ESR Transamnitis Coagulopathy SoJIA Intermittent Present Thrombocytosis Elevated Absent Absent MAS Continuous May be absent Thrombocytopaenia Decreased Present Present
Hematological system Dermatological4
Neurological Reticuloendothelial Pulmonary Cardiovascular
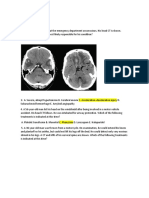
presentation with febrile illness, before diagnosis could be made.8 They may have a mononuclear pleocytosis of the cerebral spinal fluid and evidence of parameningeal infiltrations, subdural effusions, retinal haemorrhages and hypodense or necrotic areas, demonstrated by magnetic resonance imaging of the brain.9 In a report from the Histiocyte Society, children with an abnormal CSF had a higher risk of death and neurologic sequelae.10
and may be detected only by investigations.23 Rarely, MAS may be the presenting manifestation of systemic rheumatic diseases in childhood.24 However, certain subtle clinical and specific laboratory features which could differentiate these two conditions are described in Table 2. A seminal study of 13 cases of MAS from India found that a majority of the patients had an underlying connective tissue disease like SoJIA, SLE, and adult Stills disease. The mortality was 38.4%.25 The accompanying editorial highlights that a high mortality is uncommon and emphasizes that MAS is often missed in clinical practice.26
Diagnosis
MAS should be suspected when there is constellation of following features: high fever, unresponsive to antibiotics, fatigue, falling ESR, maculopapular rash, failure to thrive, central nervous system symptoms, hepatosplenomegaly, lymphadenopathy, cytopaenias, coagulopathy, abnormal liver function tests and elevated ferritin levels. However, in practice the diagnosis is often difficult and therefore delayed due to overlapping clinical and laboratory features. Table 3 lists a few differentiating features between SoJIA and MAS.36
MAS IN RHEUMATIC DISEASES
MAS is most often associated with systemic onset juvenile inflammatory arthritis (SoJIA) and can be the first presenting feature of SoJIA. In adults, MAS is less frequently reported in other rheumatologic disorders such as rheumatoid arthritis and SLE,2,11 MAS erythematosus.11 MAS has also been described in association with Behcets syndrome and ankylosing spondylitis.12 Many factors have been implicated as triggers for MAS: viral agents such as varicella-zoster virus, hepatitis A, Epstein-Barr and coxsackie B, therapies with acetylsalicylic acid and NSAIDs, DMARDs such as gold salts, methotrexate, sulfasalazine and penicillamine and biologic drugs like etanercept.1315 Apart from children with systemic juvenile idiopathic arthritis (SJIA), MAS may also occur less commonly in those with polyarticular juvenile idiopathic arthritis, juvenile SLE, and KD.1622 In a few cases, MAS may be occult
Diagnostic criteria
The initial 1991 diagnostic criteria of HLH (Table 3) has been widely applied for the diagnosis of MAS on the background of the underlying illness1315,27 being idiopathic arthritis. However, additional criteria are increasingly being used for early diagnosis and treatment.2831. This is as in nearly 20% of cases; documenting haemophagocytosis on the first bone marrow specimen is difficult or not possible.32 However, inability to demonstrate haemophagocytosis on the initial specimen should not prevent prompt institution of treatment, provided other clinical criteria are fulfilled.33 However,
164
Indian Journal of Rheumatology 2009 December; Vol. 4, No. 4
Shanmuganandan and Kotwal
Table 3 Diagnostic criteria for HLH27 Major criteria: (1) Fever: Peak temperature > 38.5C for seven or more days (2) Splenomegaly: Spleen palpated > 3 cm below the left costal margin (3) Cytopaenia involving two or more cell lines: Haemoglobin < 9.0 g/dL, or Platelets < 100,000/L, or Absolute neutrophil count < 1000/L (4) Hypertriglyceridaemia or hypofibrinogenaemia: Fasting triglycerides > 2.0 mmol/L, or more than 3 standard deviations (SD) above the normal value for age, or Fibrinogen < 1.5 g/L, or more than 3SD below the normal value for age (5) Hemophagocytosis: Demonstrated in bone marrow, spleen, or lymph node. No evidence for malignancy. Alternative criteria: (a) Low or absent natural killer count (b) Serum ferritin level > 500 g/L (c) Soluble CD25 (sIL-2 receptor) > 2400 U/mL The diagnosis of HLH requires the presence of all five major criteria. Either criterion (a) or a combination of criteria (b) and (c) may substitute for one of the major criteria. Additional criteria for the diagnosis of HLH include: Low or absent NK-cell activity, serum ferritin concentration > 500 mg/L, soluble CD25 (sIL-2 receptor) > 2400 U/mL Either the first of these three additional criteria, or a combination of second and third, may substitute for one of the major clinical criteria listed above.
Table 4 Preliminary diagnostic guidelines for macrophage activation system complicating SoJIA34 Laboratory criteria (1) Decreased platelet count (< 2.6 lakh/L) (2) Elevated levels of aspartate aminotransferase (> 59 IU/L) (3) Decreased white blood cell count (< 4000/L) (4) Hypofibrinogenaemia (< 2.5 g/L) Clinical criteria (1) Central nervous system dysfunction (irritability, disorientation, lethargy, headache, seizures, coma) (2) Haemorrhages (purpura, easy bruising, mucosal bleeding) (3) Hepatomegaly (3 cm below the costal arch) Histopathological criterion Evidence of macrophage hemophagocytosis in the bone marrow aspirate Diagnostic requirement The diagnosis of MAS requires the presence of any two or more laboratory criteria or of any two or three or more clinical or laboratory criteria. A bone marrow aspirate for the demonstration of haemophagocytosis may be required only in doubtful cases.
there are a few caveats with these criteria: since sIL-2 levels vary greatly according to age, one must know the agerelated normal levels to correctly judge these. Regarding NK cell function, patients with the autoimmune lymphoproliferative syndrome (ALPS) have decreased numbers of NK cells and may present with splenomegaly and cytopaenias secondary to the presence of auto antibodies. In MAS occurring in SoJIA, leucocytosis rather than cytopaenias
are common; hence, specific diagnostic guideline for MAS SoJIA has been proposed by Ravelli et al. (Table 4).34
Management
The management of MAS is empirical. Many of the drugs used are similar to HLH protocols.35 The HLH protocol is
Macrophage activation syndrome II/II
Review Article
165
Table 5 HLH 2004 protocol* Initial management 08 weeks Inj Etoposide Inj dexamethasone 02 weeks 150 mg /m2 twice a week 10 mg/m2/day 38 weeks 150 mg once a week 5 mg/m2/day for 2 weeks 2.5 mg/m2/day for 2 weeks 1.25 mg/m2 for 1 week Tapered off in 8th week
Cyclosporine Continuation phase 940 weeks Inj Etoposide Inj dexamaethasone Cyclosporine
6 mg/kg for 8 weeks 150 mg/m2 every fortnightly 10 mg/m2 every fortnightly 6 mg/kg
Additional medications include: (a) Cotrimaxozole prophylaxis week 240, (b) Oral anti-mycotic agents week 19, (c) IVIG q 4 weekly during induction and continuation phase.
the standard of care for primary HLH. The HLH protocol given in Table 5, therefore, merits mention though it is hardly ever used in the management of MAS. Supportive care is important and includes management of underlying disorder precipitating MAS, correction of coagulopathy, treatment of triggering infection with appropriate anti-microbial agents and removal of potential triggering medications like NSAIDs. High-dose steroidparenteral methylprednisolone or dexamethasone is the first line of therapy.36 One schedule is pulse intravenous methylprednisolone 1000 mg/day for three consecutive days, followed by high-dose oral prednisone (60 mg/day, for at least 4 weeks depending on the clinical response and thereafter tapered slowly). More than half of the patients treated with steroids have favourable outcome.14,16 Cyclosporine (35 mg/kg) has been used effectively in the treatment of MAS, especially in MAS associated with JIA.13,37,38 Stephan et al. reported that cyclosporine was effective in 12 patients, five of whom received first line treatment and seven who received second line treatment when steroids failed.39 Ravelli has proposed a switch off effect of cyclosporine wherein there is a rapid resolution of clinical and laboratory features of MAS within 1224 hours of initiation of therapy with this immunosuppressant drug.40 The mechanism of action, in addition to inhibition of 1L-2 production of lymphocytes, is that of a direct inhibition of production of IL-6, IL-1 and TNF- from macrophages and inhibition of expression of inducible nitric oxide synthetase and cyclooxygenase-2, leading to a decreased production of nitric oxide and prostaglandin.41 Thus, many rheumatologists routinely add cyclosporine to steroids in the management of MAS. It has been proposed that cyclosporine be used as a preferred first line of
therapy in MAS due to its rapid onset of action and high efficacy.42 Other drugs used include etopside, IVIG, TNF blockade and IL1ra. Fishman et al. used etoposide in a patient with features of MAS in SoJIA, probably as an extension of its use in primary HLH.43 Though etoposide is an integral part of initial protocol in primary HLH (HLH 2004 protocol), case reports of its use in MAS is sparse. Intravenous immunoglobulin has been used as a second line of treatment in MAS, not responding to corticosteroids. Tristano et al. have reported the successful use of IVIG in MAS.44 IVIG induces expression of the inhibitor Fc[g]RIIB receptor on effector cells, which in turn mediates inhibitory intracellular signalling thereby stopping macrophage activation and TNF blockade. Though intuitively TNF blockers could be considered for MAS, paradoxical use of etarnecept in SoJIA has precipitated MAS.4547 However, efficacy of etarnecept in the treatment of resistant MAS has also been reported.48,49
IL1RA
IL1Ra, anakinra has been successfully used in the treatment of MAS in a child with SoJIA.50,51 One approach is to treat with corticosteroids, starting with pulse methylprednisolone and to introduce second line agents including cyclosporine or etoposide or intravenous immunoglobulin if the response to corticosteroids is poor or if there is worsening or multiorgan involvement. Another approach is to classify patients into high risk and low risk groups. The high-risk groups are those with CNS involvement, severe bleeding diathesis, severe renal impairment,
166
Indian Journal of Rheumatology 2009 December; Vol. 4, No. 4
Shanmuganandan and Kotwal
multiorgan failure or failure to respond to initial therapy. The high-risk groups are treated with corticosteroids, cyclosporine with or without etoposide. The low-risk group is treated with corticosteroids alone.
6.
7.
Prognosis
8.
MAS is a life threatening disorder and fatalities are not uncommon when diagnosed late or if there is presence of multiorgan involvement. The mortality of MAS has varied from 8 to 22%.50 The poor prognostic factors are delayed diagnosis, severe coagulopathy and multisystem involvement.
9.
10.
CONCLUSION
MAS is a multisystem disorder affecting a wide spectrum of rheumatologic and non-rheumatologic illness. Recognizing the condition is difficult due to the absence of characteristic features and the presence of overlapping clinical features. Clinical manifestations include a disseminated intravascular coagulation-like picture with cytopaenias, hyperferritinaemia, coagulopathy and cardiovascular collapse. A high index of suspicion and early directed and specific investigations are essential for (early) diagnosis and treatment. Biomarkers such as sCD25 and sCD163 are important diagnostic tools that are likely to be used in the diagnostic armamentarium. Standardized treatment protocols and immune suppressive regimes go a long way in the mitigation of mortality and morbidity.
11.
12.
13.
14.
15.
16.
REFERENCES
1. Tristano AG. Macrophage activation syndrome: a frequent but under-diagnosed complication associated with rheumatic diseases. Med Sci Monit 2008; 14: RA 2736. Ramanan A, Schneider R. Macrophage activation syndrome: whats in a name! J Rheumatol 2003; 30: 25136. Kotwal J, Shanmuganandan K. Macrophage activation syndrome: I/II. Ind J Rheumatol 2009; 4: 1128. Morrell DS, Pepping MA, Scott JP, Esterly NB, Drolet BA. Cutaneous manifestations of haemophagocytic lymphohistiocytosis. Arch Dermatol 2002; 138: 120812. Nahum E, Ben-Ari J, Stain J, Schonfeld T. Hemophagocytic lymphohistiocytic syndrome: unrecognized cause of multiple organ failure. Pediatr Crit Care Med 2000; 1: 51. 17.
2. 3. 4.
18.
19.
5.
20.
Thaunat O, Delahousse M, Fakhouri F, Martinez F, Stephan JL, Nol LH, et al. Nephrotic syndrome associated with hemophagocytic syndrome. Kidney Int 2006; 69: 1892. Stphan JL, Zeller J, Hubert P, Herbelin C, Dayer JM, Prieur AM. Macrophage activation syndrome and rheumatic disease in childhood: a report of four new cases. Clin Exp Rheumatol 1993; 11: 4516. Palazzi DL, McClain KL, Kaplan SL. Hemophagocytic syndrome in children: an important diagnostic consideration in fever of unknown origin. Clin Infect Dis 2003; 36(3): 30612. Henter JI, Nennesmo I. Neuropathologic findings and neurologic symptoms in twenty-three children with hemophagocytic lymphohistiocytosis. J Pediatr 1997; 130: 35865. Horne A, Trottestam H, Aric M, Egeler RM, Filipovich AH, Gadner H, et al. Frequency and spectrum of central nervous system involvement in 193 children with haemophagocytic lymphohistiocytosis. Br J Haematol 2008; 140: 32735. Kawashiri S, Nakamura H, Kawakami A, Ida H, Izumi Y, Tamai M, et al. Emergence of Epstein-Barr virus-associated haemophagocytic syndrome upon treatment of systemic lupus erythematosus. Lupus 2006; 15: 513. Lee SH, Kim SD, Kim SH, Kim HR, Oh EJ, Yoon CE, et al. EBV-associated haemophagocytic syndrome in a patient with Behcets disease. Scand J Rheumatol 2005; 34: 3203. Ravelli A, Caria MC, Buratti S, Mulattia C, Temporini F, Martini A. Methotrexate as a possible trigger of macrophage activation syndrome in systemic juvenile idiopathic arthritis. J Rheumatol 2001; 28: 8657. Sawhney S, Woo P, Murray KJ. Macrophage activation syndrome: a potentially fatal complication of rheumatic disorders. Arch Dis Child 2001; 85: 4216. Ramanan AV Schneider R. Macrophage activation syndrome , following initiation of etanercept in a child with systemic onset juvenile rheumatoid arthritis. J Rheumatol 2003; 30: 4013. Stephan JL, Kone-Paut I, Galambrun C, Mouy R, BaderMeunier B, Prieur AM. Reactive haemophagocytic syndrome in children with inflammatory disorders: a retrospective study of 24 patients. Rheumatology 2001; 40: 128592. Grom AA, Villanueva J, Lee S, Goldmuntz EA, Passo MH, Filipovich A. Natural killer cell dysfunction in patients with systemic-onset juvenile rheumatoid arthritis and macrophage activation syndrome. J Pediatr 2003; 142: 2926. Muise A, Tallett SE, Silverman ED. Are children with Kawasaki disease and prolonged fever at risk for macrophage activation syndrome? Pediatrics 2003; 112: 4957. Silverman ED, Miller JJ, Bernstein B, Shafai T. Consumption coagulopathy associated with systemic juvenile rheumatoid arthritis. J Pediatr 1983; 103: 8726. Stphan JL, Zeller J, Hubert P, Herbelin C, Dayer JM, Prieur AM. Macrophage activation syndrome and rheumatic disease
Macrophage activation syndrome II/II
Review Article
167
21.
22.
23.
24.
25.
26. 27.
28.
29.
30.
31.
32.
33.
34.
35.
in childhood: a report of four new cases. Clin Exp Rheumatol 1993; 11: 4516. Cuende E, Vesga JC, Perez LB, Ardanaz MT, Guinea J. Macrophage activation syndrome as the initial manifestation of systemic-onset juvenile idiopathic arthritis. Clin Exp Rheumatol 2001; 19: 7645. Kelly A, Ramanan AV Recognition and management of . macrophage activation syndrome in juvenile arthritis. Curr Opin Rheumatol 2007; 19: 47781. Behrens EM, Beukelman T, Paessler M, Cron RQ. Occult macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis. J Rheumatol 2007; 34: 11338. Avcin T, Tse SM, Schneider R, Ngan B, Silverman ED. Macrophage activation syndrome as the presenting manifestation of rheumatic diseases in childhood. J Pediatr 2006; 148: 6836. Pinto L, Kagalwala F, Singh S, Balakrishna C, Prabhu SV , Khodaij S. Macrophages activation syndrome: experience from a tertiary referral center. J Assoc Physicians Ind 2007; 55: 1857. Joshi V Macrophage activation syndrome. J Assoc Physicians . Ind 2007; 55: 1834. Henter JI, Elinder G, Ost A. Diagnostic guidelines for hemophagocytic lymphohistiocytosis. The FHL Study Group of the Histiocyte Society. Semin Oncol 1991; 18: 29. Horne A, Janka G, Maarten Egeler R, Gadner H, Imashuku S, Ladisch S, et al. Haematopoietic stem cell transplantation in haemophagocytic lymphohistiocytosis. Br J Haematol 2005; 129: 622. Sneller MC, Wang J, Dale JK, Strober W, Middelton LA, Choi Y, et al. Clinical, immunologic, and genetic features of an autoimmune lymphoproliferative syndrome associated with abnormal lymphocyte apoptosis. Blood 1997; 89: 13418. Lim MK, Lee CK, Ju YS, Cho YS, Lee MS, Yoo B, Moon HB. Serum ferritin as a serologic marker of activity in systemic lupus erythematosus. Rheumatol Int 2001; 20: 8993. Lambotte O, Cacoub P, Costedoat N, Le Moel G, Amoura Z, Piette JC. High ferritin and low glycosylated ferritin may also be a marker of excessive macrophage activation. J Rheumatol 2003; 30: 10278. Aric M, Janka G, Fischer A, Henter JI, Blanche S, Elinder G, et al. Haemophagocytic lymphohistiocytosis. Report of 122 children from the International Registry. Leukemia 1999. Dhote R, Simon J, Papo T, Detournay B, Sailler L, Andre MH, et al. Reactive hemophagocytic syndrome in adult systemic disease: report of twenty-six cases and literature review. Arthritis Rheum 2003; 49: 6339. Ravelli A, Magni-Manzoni S, Pistorio A, Besana C, Foti T, Ruperto N, et al. Preliminary diagnostic guidelines for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. J Pediatr 2005; 146: 598604. Henter JI, Horne A, Arico M, Egeler RM, Filiporich AH, imashuku S, et al. HLH-2004: diagnostic and therapeutic
36.
37.
38.
39.
40. 41.
42.
43.
44.
45.
46.
47. 48.
49.
50.
guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007; 48: 12431. Kelly A, Ramanan AV Recognition and management of . macrophage activation syndrome in juvenile arthritis. Curr Opin Rheumatol 2007; 19: 47781. Mouy R, Stephan JL, Pillet P, Haddad E, Hubert P, Prieur AM. Efficacy of cyclosporine A in the treatment of macrophage activation syndrome in juvenile arthritis: report of five cases. J Pediatr 1996; 129: 7504. Ravelli A, De Benedetti F, Viola S, Martin A. Macrophage activation syndrome in systemic juvenile rheumatoid arthritis successfully treated with cyclosporine. J Pediatr 1996; 128: 2758. Stphan JL, Kon-Paut I, Galanbrun C, Galambrun C, Mouy R, Bader-Meunier B, et al. Reactive haemophagocytic syndrome in children with inflammatory disorders. A retrospective study of 24 patients. Rheumatology 2001; 40: 128592. Ravelli A. Macrophage activation syndrome. Curr Opin Rheumatol 2002; 14: 54852. Attur MG, Patel R, Thakker G, Vyas P, Levartovsky D, Patel P, et al. Differential anti-inflammatory effects of immunosuppressive drugs: cyclosporin, rapamycin and FK-506 on inducible nitric oxide synthetase, nitric oxide, cyclooxygenase-2 and PGE2 production. Inflamm Res 2000; 49: 2026. Ravelli A, Martini A. Macrophage activation syndrome. In: Cimaz R and Lehman (eds) Pediatrics in systemic autoimmune diseases. 2008; 5565. Fishman D, Rooney M, Woo P. Successful management of reactive haemophagocytic syndrome in systemic-onset juvenile chronic arthritis. Br J Rheumatol 1995; 34: 888. Tristano A, Casanova-Escalona L, Torres A, Rodriguez MA. Macrophage activation syndrome in a patient with Stills disease rescue with intravenous immunoglobulin therapy. J Clin Rheumatol 2003; 9: 2538. Stern A, Riley R, Buckley L. Worsening of macrophage activation syndrome in a patient with adult onset Stills disease after initiation of etanercept therapy. J Clin Rheumatol 2001; 7: 2526. Ramanan AV Scheider R. Macrophage activation syndrome , following initiation of etanercept in a child with systemic onset juvenile rheumatoid arthritis. J Rheumatol 2003; 30: 4013. Makay B. Etarnecept for therapy-resistant macrophage activation syndrome. Pediatr Blood Cancer 2008; 50: 41921. Prahalad S, Bove KE, Dickens D, Lovell DJ, Grom AA. Etanercept in the treatment of macrophage activation syndrome. J Rheumatol 2001; 28: 21204. Behrens EM, Kreiger PA, Cherian S, Cron RQ. Interleukin 1 receptor antagonist to treat cytophagic histiocytic panniculitis with secondary hemophagocytic lymphohistiocytosis. J Rheumatol 2006; 33: 20814.51. Kelly A, Ramanan AV A case of macrophage activation syn. drome successfully treated with anakinra. Nat Clin Pract Rheumatol 2008; 4: 61520.
Potrebbero piacerti anche
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (400)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (345)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (74)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- Preguntas Primer ExamenDocumento5 paginePreguntas Primer ExamenMRVNessuna valutazione finora
- Tramadol, Ketorolac, EterocoxibDocumento4 pagineTramadol, Ketorolac, EterocoxibEric de JulianNessuna valutazione finora
- 2012 EXE 225 Low Vision BookD5-V4Documento39 pagine2012 EXE 225 Low Vision BookD5-V4Brian BowersNessuna valutazione finora
- Caz Clinic PDFDocumento160 pagineCaz Clinic PDFDr.Md.AslamNessuna valutazione finora
- Hirayama's DiseaseDocumento27 pagineHirayama's DiseaseDarshika Vyas MohanNessuna valutazione finora
- Hallucinations: Common Features and CausesDocumento5 pagineHallucinations: Common Features and CausesSissaNessuna valutazione finora
- NCCS Understanding Radiation Therapy (Eng)Documento20 pagineNCCS Understanding Radiation Therapy (Eng)Benjamin NgNessuna valutazione finora
- Pre-Hospital Assessment Sheet: Triage ScoreDocumento2 paginePre-Hospital Assessment Sheet: Triage Scoreratna purwitasariNessuna valutazione finora
- Critical Apprasisal 2Documento5 pagineCritical Apprasisal 2api-678326591Nessuna valutazione finora
- PhysioEx Exercise 7 Activity 1Documento6 paginePhysioEx Exercise 7 Activity 1Jorge CuevaNessuna valutazione finora
- Abnormal Uterine Bleeding in Reproductive-Age Women - Terminology and PALM-COEIN Etiology Classification - UpToDateDocumento46 pagineAbnormal Uterine Bleeding in Reproductive-Age Women - Terminology and PALM-COEIN Etiology Classification - UpToDateCristinaCaprosNessuna valutazione finora
- Pernicious Anaemia and B12 2020Documento42 paginePernicious Anaemia and B12 2020viraaj pawarNessuna valutazione finora
- 1004-First Aid For Exposure To ChemicalDocumento3 pagine1004-First Aid For Exposure To ChemicalderyhermawanNessuna valutazione finora
- Drug Study MetoclopramideDocumento2 pagineDrug Study MetoclopramidePrince Rupee Gonzales100% (2)
- ErythromycinDocumento18 pagineErythromycinjane pletaNessuna valutazione finora
- Daftar PustakaDocumento3 pagineDaftar PustakaNahrijah JahrinaNessuna valutazione finora
- ComplaintDocumento28 pagineComplaintLia TabackmanNessuna valutazione finora
- Evaluation of Antimicrobial Effectiveness of Ophthalmic Drops Sold in Nigeria Pharmacy Stores and Market PlacesDocumento15 pagineEvaluation of Antimicrobial Effectiveness of Ophthalmic Drops Sold in Nigeria Pharmacy Stores and Market Placesvelagapudi surajNessuna valutazione finora
- Silver Book Part A Medication Management ID3104Documento7 pagineSilver Book Part A Medication Management ID3104Anton BalansagNessuna valutazione finora
- Hema - PointersDocumento5 pagineHema - PointersLUALHATI VILLASNessuna valutazione finora
- Murder Mystery LabDocumento19 pagineMurder Mystery Labapi-451038689Nessuna valutazione finora
- BPHM4149 - Chemotherapy-Induced Nausea Vomiting - 22jan2018Documento42 pagineBPHM4149 - Chemotherapy-Induced Nausea Vomiting - 22jan2018kkyyyhNessuna valutazione finora
- NevroblastomprotokollDocumento217 pagineNevroblastomprotokollsfghhhNessuna valutazione finora
- Grand Case Study V7 Final 030310Documento49 pagineGrand Case Study V7 Final 030310Jemimah Ruth Madayag ValenzuelaNessuna valutazione finora
- Discharge Planning PaperDocumento5 pagineDischarge Planning Paperapi-283173905Nessuna valutazione finora
- COH 315: Epidemiology Midterm Exam MAXIMUM 75 Points: Attack Rate 80/90 0.889Documento5 pagineCOH 315: Epidemiology Midterm Exam MAXIMUM 75 Points: Attack Rate 80/90 0.889Farah FarahNessuna valutazione finora
- Republic Act No. 11223 or The Universal Health Care ActDocumento2 pagineRepublic Act No. 11223 or The Universal Health Care ActChristine Joy MolinaNessuna valutazione finora
- Dka in PedsDocumento11 pagineDka in PedsMohamed YasserNessuna valutazione finora
- 05092016ybct 2ND EdDocumento42 pagine05092016ybct 2ND Edpwilkers36100% (4)
- Howtousea Nebulizer in Pediatric ClientsDocumento11 pagineHowtousea Nebulizer in Pediatric ClientsAngelina Nicole G. TungolNessuna valutazione finora