Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
2000 AL Chemistry Paper II Marking Scheme
Caricato da
api-37388410 valutazioniIl 0% ha trovato utile questo documento (0 voti)
1K visualizzazioni15 pagineCopyright
© Attribution Non-Commercial (BY-NC)
Formati disponibili
PDF o leggi online da Scribd
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Attribution Non-Commercial (BY-NC)
Formati disponibili
Scarica in formato PDF o leggi online su Scribd
0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
1K visualizzazioni15 pagine2000 AL Chemistry Paper II Marking Scheme
Caricato da
api-3738841Copyright:
Attribution Non-Commercial (BY-NC)
Formati disponibili
Scarica in formato PDF o leggi online su Scribd
Sei sulla pagina 1di 15
7S PR SX DS Fe
Section A
FOR TEACHERS’ USE ONLY
(@) (Chlorine has two isotopes °Cl and "C1 1
Bombardment of e° on chlorine produces atomic ion Cl"
The five peaks correspond to the species: CI", "CI
roe
i (1) relative atomic mass of Cl, =
La
1) Abundance of A ty 1
a) eof Ace txt
Abundance of Bc 2
1
4
Abundance of Ca
ana
3
4
Macks
‘and molecular jon {CI-Cl]" i
PCCP, PCC and 1
BEET 55 5¢emu) 2
(mark for method: | mark for answer; deduct | mark forgiving units other than ama.)
4
(sy
ratio of relative abundance abundance of =
© © & x: Se /pparicte
ay r: Be
(UD 2: {He sa-particle
(i) 10h, = 4x hatttife
aetiviy = 12000%(2)* = 750 counts minut
16 16 16
26:9
(1 mark fo the method; 1 mark forthe answer. Deduct % marks for wrong/no unit )
(Gi) Expose food to ionizing radiation suchas fast electrons /y-rays
Radiation
2000-AL-CHEM 2-17
RRA ME
but will not affect the texture of the 1,1
wa
FOR TEACHERS’ USE ONLY
7 .
CABRANEHBY FOR TEACHERS’ USE ONLY
Marks
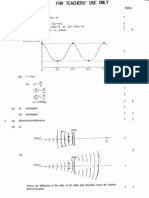
(©) (i) Each line represents the transition of an electron from a higher energy level toa lower energy t
level.
co)
an4
“3 y
> 4
&
5 n=2
“The energy difference between energy levels decreases with increase in principal u
quantum number.
of, The relative increase in energy ofthe photon emitted forthe transition from n=nton=1 (I)
becomes progressively smaller as n increases.
(iii) Assuming that the frequency of the convergence limit of the Lyman series is v» 1
Energy of photon =h Yo -
“This amount of energy equals to the energy required to ionize 1 atom of hydrogen.
LE=Lx Avo 1
where L is the Avogadro Constant.
(iv) To show the presence the element /to identify an element / to determine the concentration 1
ofan element ina sample _
O)
2000-AL-CHEM 2-18,
ARAMEE FOR TEACHERS’ USE ONLY
2 @)_
(b)
)
@
ARRAS FOR TEACHERS’ USE ONLY
Marks
(i) "The enthalpy change when ] male of "is removed from gascous atoms of the element under Yeh \
‘standard conditions, % \
or, the molar enthalpy change involved in the process under standard conditions. (4%)
Mig) > M(e) + (4)
«w Ne 2
= Ar
5 f
Me si
ar
+ atomic number
(A marks for indicating Ne and Ar as maxima with LE. of Ne > Ar;
‘A marks for showing Na as minimum;
Y marks for showing LE. : Mg> Al> Na;
°% marks for showing LE. : P> $> Si
"A marks for labeling the axes.)
(if the curve carries zero marks; the axes will not be marked. )
o
0@) +e + OW 4%
When ane” is added tothe outermost shell of O, the attraction between she nucleus andtheelection %
leads to a decrease in energy of the system. 7
First EA. is negative
O@) +e 3 O'~
‘There is repulsive force between the anion O and the negatively charged electron, %
Second E.A. is positive.
Both Ca and Zn are Period 4 elements / have the same no, of electron shells %
From Ca to Zn, the nuclear charge increases (by 10 units) / 10 electrons are added to an inner ‘4
‘electron shell/d-subshell
Due to the poor screening effect of the d clectrons/ the d-orbitals are more diffused, the effect of 7
increase in nuclear charge outweighs the effect of screening effect / the effective nuclear charge
' an c
*. size of Ca > size of Zn .
Q)
CH, CO,
B.D structure 4
weMH 1d
hi
Ihybridization state sp sp th
- 7 @
2000-AL-CHEM 2-19
RARAMEH FOR TEACHERS’ USE ONLY
Potrebbero piacerti anche
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- 1994 AL Chemistry Paper I Marking SchemeDocumento10 pagine1994 AL Chemistry Paper I Marking Schemeapi-3738841100% (1)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (587)
- 1999 AL Chemistry Marking SchemeDocumento34 pagine1999 AL Chemistry Marking Schemeapi-373884125% (4)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (890)
- 1990 AL Chemistry Paper I Marking SchemeDocumento11 pagine1990 AL Chemistry Paper I Marking Schemeapi-373884160% (5)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- 1993 AL Chemistry Paper I Marking SchemeDocumento7 pagine1993 AL Chemistry Paper I Marking Schemeapi-373884150% (2)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (399)
- 1997 AL Chemistry Paper 2 Marking SchemeDocumento12 pagine1997 AL Chemistry Paper 2 Marking Schemeapi-37388410% (2)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (73)
- 2000 AL Chemistry Paper I Marking SchemeDocumento20 pagine2000 AL Chemistry Paper I Marking Schemeapi-3738841Nessuna valutazione finora
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- 1997 AL Chemistry Paper I Marking SchemeDocumento22 pagine1997 AL Chemistry Paper I Marking Schemeapi-3738841Nessuna valutazione finora
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- 1995 AL Chemistry Paper 1 Marking SchemeDocumento21 pagine1995 AL Chemistry Paper 1 Marking Schemeapi-3738841100% (1)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- 1996 AL Chemistry Marking SchemeDocumento34 pagine1996 AL Chemistry Marking Schemeapi-373884175% (4)
- 2001 AL Chemistry Paper II Marking SchemeDocumento16 pagine2001 AL Chemistry Paper II Marking Schemeapi-373884163% (8)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- 1998 AL Chemistry Paper 1+2 Marking SchemeDocumento36 pagine1998 AL Chemistry Paper 1+2 Marking Schemeapi-3738841100% (1)
- AL 1990 Physics Marking Scheme IIADocumento13 pagineAL 1990 Physics Marking Scheme IIAapi-373884167% (3)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- 1997 AL Chemistry Paper 2 Marking SchemeDocumento12 pagine1997 AL Chemistry Paper 2 Marking Schemeapi-37388410% (2)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- 1999 AL Chemistry Marking SchemeDocumento34 pagine1999 AL Chemistry Marking Schemeapi-373884125% (4)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2219)
- 2003 AL Chemistry Paper 1 Marking SchemeDocumento16 pagine2003 AL Chemistry Paper 1 Marking Schemeapi-3738841100% (1)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- 2002 AL Chemistry Paper 1+2 Marking SchemeDocumento36 pagine2002 AL Chemistry Paper 1+2 Marking Schemeapi-373884138% (8)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (344)
- AL 1993 Physics Marking SchemeDocumento16 pagineAL 1993 Physics Marking Schemeapi-3738841100% (3)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (265)
- AL 1991 Physics Marking Scheme IIADocumento12 pagineAL 1991 Physics Marking Scheme IIAapi-37388410% (3)
- AL 1992 Physics Marking SchemeDocumento22 pagineAL 1992 Physics Marking Schemeapi-373884186% (7)
- AL 1994 Physics Marking Scheme IADocumento14 pagineAL 1994 Physics Marking Scheme IAapi-3738841100% (2)
- 1998 AL Chemistry Paper 1+2 Marking SchemeDocumento36 pagine1998 AL Chemistry Paper 1+2 Marking Schemeapi-3738841100% (1)
- AL 1991 Physics Marking SchemeDocumento22 pagineAL 1991 Physics Marking Schemeapi-373884150% (2)
- AL 1994 Physics Marking Scheme IIBDocumento14 pagineAL 1994 Physics Marking Scheme IIBapi-3738841Nessuna valutazione finora
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- AL 1995 Physics Marking Scheme IIBDocumento7 pagineAL 1995 Physics Marking Scheme IIBapi-3738841100% (1)
- AL 1996 Physics Marking SchemeDocumento14 pagineAL 1996 Physics Marking Schemeapi-3738841100% (4)
- AL 1997 Physics Marking SchemeDocumento15 pagineAL 1997 Physics Marking Schemeapi-373884167% (3)
- AL 1998 Physics Marking SchemeDocumento15 pagineAL 1998 Physics Marking Schemeapi-373884175% (8)
- AL 2000 Physics Marking SchemeDocumento40 pagineAL 2000 Physics Marking Schemeapi-373884180% (10)
- AL 1999 Physics Marking SchemeDocumento15 pagineAL 1999 Physics Marking Schemeapi-373884150% (2)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (119)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)