Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
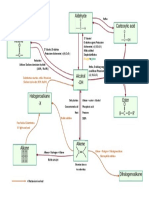
Organic Chem Reactions
Caricato da
Teo Jia Ming NickolasCopyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Organic Chem Reactions
Caricato da
Teo Jia Ming NickolasCopyright:
Formati disponibili
Organic Chem Reactions
1. Alkanes
a. Reactions Combustion Free-radical substitution o Steps Initiation Propagation Termination o Conditions Cl2(g)/Br2(l), UV light b. Formation Hydrogenation of Alkenes o H2(g) with Nickel catalyst, at ~150oC, ~5atm o H2(g) with Pt or Pd catalyst, at room temperature Decarboxylation of sodium salt of carboxylic acid o Heating with sodalime
2. Alkenes
a. Reactions Addition of bromine o Br2(l) / Br2 dissolved in CCl4, room temperature Addition of bromine water o Bromine water at room temperature Addition of HBr o HBr(g)/ HBr dissolved in CCl4, room temperature Direct hydration o Steam, H3PO4 catalyst, 300oC, 65 atm Indirect hydration o Cold concentrated H2SO4, followed by heating in the presence of water Addition of Hydrogen o H2(g) with Nickel catalyst, at ~150oC, ~5atm o H2(g) with Pt or Pd catalyst, at room temperature Oxidation o Partial bond cleavage Cold alkali/ cold, dilute acidified KMnO4 o Total bond cleavage Hot, acidified KMnO4 Combustion b. Formation Dehydration of Alcohols o Excess, concentrated H2SO4, 180oC o Al2O3, 400oC Dehydrohalogenation of halogenoalkane Done by Nickolas Teo Jia Ming, CG 12/11
Alcoholic KOH, relfux
3. Benzene
a. Reactions Nitration o Concentrated HNO3, concentrated H2SO4, reflux at less than 60oC Halogenation o Anhydrous AlCl3 / FeCl3/ Fe power, at room temperature Friedel-Crafts Alkylation o Anhydrous AlCl3 / FeCl3/ Fe power, at room temperature Friedel-Crafts Acylation o Anhydrous AlCl3 / FeCl3/ Fe power, at room temperature
4. Alkylbenzene
a. Reactions (side-chain) Halogenation o Cl2(g)/Br2(l), UV light Oxidation o Alkaline/ acidified KMnO4, reflux o Entire side chain is oxidized into CO2H b. Formation Friedel-Crafts Alkylation o Anhydrous AlCl3 / FeCl3/ Fe power, at room temperature
5. Alcohols
a. Reactions Esterification o Concentrated H2SO4, reflux Acylation Halogenation (hydrogen halide) o NaCl(s), concentrated H2SO4, reflux o HX, reflux Halogenation (phosphorus halide) o Cold PCl5 o Red P and Br2, reflux o Red P and I2, reflux Halogenation (Sulfur dichloride oxide) o SOCl2, dissolved in pyridine Dehydration o Excess, concentrated H2SO4, 180oC o Al2O3, 400oC Formation of alkyl hydrogensulfate o Concentrated H2SO4, reflux at 80oC Combustion Oxidation o For aldehyde Acidified K2Cr2O7, heat to distill o For carboxylic acid/ ketone Done by Nickolas Teo Jia Ming, CG 12/11
Acidified K2Cr2O7/ KMnO4 , reflux
b. Formation Alkaline hydrolysis of halogenoalkane o Aqueous KOH, reflux Direct hydration of alkenes o Steam, H3PO4 catalyst, 300oC, 65 atm Indirect hydration of alkenes o Cold concentrated H2SO4, followed by heating in the presence of water Reduction of carbonyl compounds o H2(g) with Ni catalyst at 140oC o LiAlH4, dissolved in dry ether at room temperature c. Test Tri-iodomethane/ Iodoform test o Alkaline I2(aq), heat o Solid I2, NaOH(aq), heat o NaOI(l), heat
6. Phenol
a. Reactions Esterification o Acid chloride and NaOH(aq) Halogenation o Br2 dissolved in CCl4 o Chlorine/ Bromine water at room temperature Nitration o Dilute HNO3, room temperature b. Test Neutral Iron(III) chloride solution at room temperature o Violet coloration Bromine water at room temperature o Decolourisation and white precipitate
7. Aliphatic carbonyl compounds
a. Reactions Oxidation of aldehydes o Acidified K2Cr2O7/ KMnO4 , heat o Alkaline KMnO4 , heat o Tollens Reagent, heat Ammoniacal silver(I) nitrate o Fehlings Solution, heat Alkaline copper(II) tartrate Reduction o H2(g) with Ni catalyst at 140oC o LiAlH4, dissolved in dry ether at room temperature Addition of HCN o Slightly alkaline HCN (aq) at 10-20oC Done by Nickolas Teo Jia Ming, CG 12/11
o HCN (aq), small amount of KCN, 10-20oC Condensation reaction with Bradys Reagent o Bradys Reagent, room temperature. b. Test Tri-iodomethane test o Alkaline I2(aq), heat o Solid I2, NaOH(aq), heat o NaOI(l), heat c. Formation Oxidation of alcohols o Acidified K2Cr2O7/ KMnO4 , heat (to distill for aldehyde)
8. Aromatic carbonyl compounds
a. Reactions Oxidation of aldehydes o Acidified KMnO4 , heat o Alkaline KMnO4 , heat o Tollens Reagent, heat Ammoniacal silver(I) nitrate o NOT Fehlings solution Reduction o LiAlH4, dissolved in dry ether at room temperature Condensation reaction with Bradys Reagent o Bradys Reagent, room temperature. Electrophilic substitution of benzene ring o Fuming HNO, concentrated H2SO4, reflux at 40oC o Cl2 , anhydrous FeCl3, at room temperature b. Test Tri-iodomethane test o Alkaline I2(aq), heat o Solid I2, NaOH(aq), heat o NaOI(l), heat c. Formation For benzaldehyde o Excess Cl2 gas and UV light, followed by reflux with NaOH (aq) For phenylethanone o CH3COCl with anhydrous AlCl3 catalyst, 40oC
9. Carboxylic acid
a. Reactions Metal, metal carbonate, alkali o Room temperature Formation of acyl chloride by a halogen atom o PCl5(s), cold o PCl3(l), cold o SOCl2, dissolved in pyridine Esterification Done by Nickolas Teo Jia Ming, CG 12/11
o Concentrated H2SO4, reflux Reductions o LiAlH4, dry ether, room temperature b. Formation Oxidation of primary alcohol o Acidified K2Cr2O7/ KMnO4 , reflux Oxidation of aldehydes o Acidified K2Cr2O7/ KMnO4 , reflux Oxidation of methyl benzene o Acidified KMnO4 , heat o Alkaline KMnO4 , heat Acid hydrolysis of nitriles o Dilute HCl, reflux Alkali hydrolysis of nitriles o Dilute NaOH, reflux, followed by acidify
10.Carboxylic acid derivatives
a. Reactions Hydrolysis o Ester Dilute H2SO4, reflux o Acyl chloride Water, room temperature o Amide Dilute HCl, reflux React with alcohol/ phenol o Acyl chloride Room temperature Reaction with NH3 o Ester Concentrated alcoholic NH3 o Acyl chloride Room temperature Reduction o Ester LiAlH4, dissolved in dry ether at room temperature o Acyl chloride LiAlH4, dissolved in dry ether at room temperature o Amide LiAlH4, dissolved in dry ether at room temperature b. Formation Acyl chloride o Formation of acyl chloride by a halogen atom Ester o Esterification Done by Nickolas Teo Jia Ming, CG 12/11
Amide o Acyl chloride, NH3/ RNH2/ R2NH, cold
11. Amine
a. Reactions Alkylation of amine o RX dissolved in ethanol in a sealed tube; Heat Acylation of amine o Acyl chloride; Cold b. Formation Reduction of nitriles o LiAlH4, dry ether, room temperature o H2(g) with Ni catalyst at 140oC o Na and ethanol Reduction of amides o LiAlH4, dry ether, room temperature o H2(g) with Ni catalyst at 140oC o Na and ethanol
12. Phenylamine
a. Reactions Alkylation of amine o RX dissolved in ethanol in a sealed tube; Heat Acylation of amine o Acyl chloride; Cold Halogenation o Br2 dissolved in CCl4 o Chlorine/ Bromine water at room temperature b. Formation Reduction of nitrobenzene o Sn, concentrated HCl, reflux followed by addition of NaOH (aq)
13. Halogenoalkane
a. Reactions Nucleophilic substitution o Alkaline hydrolysis NaOH (aq); Reflux KOH (aq); Reflux o Formation of Nitrile Alcoholic KCN; Reflux o Formation of ether Na in excess alcohol; Reflux o Formation of ester Silver(I) salt of carboxylic acid in alcohol; Reflux o Formation of amine Excess concentrated NH3 in alcohol; Reflux Elimination o Dehalogenation of halogenoalkane Done by Nickolas Teo Jia Ming, CG 12/11
Alcoholic KOH; Reflux Alcoholic CH3O-Na+; Reflux
b. Formation Free radical substitution of alkane Halogenation of alcohols Addition of halogen to alkene
Done by Nickolas Teo Jia Ming, CG 12/11
Potrebbero piacerti anche
- Electron Transfer Reactions of Complex Ions in SolutionDa EverandElectron Transfer Reactions of Complex Ions in SolutionNessuna valutazione finora
- Unit 4 Organic Chemistry ReactionsDocumento6 pagineUnit 4 Organic Chemistry ReactionsRobbing_Hood100% (1)
- Reaction List v002Documento5 pagineReaction List v002cecil3414Nessuna valutazione finora
- Unit 5 Organic Chemistry ReactionsDocumento9 pagineUnit 5 Organic Chemistry ReactionsRobbing_Hood100% (1)
- Chemistry SME Notes (Organic Chemmistry)Documento14 pagineChemistry SME Notes (Organic Chemmistry)Sayeef MahdiNessuna valutazione finora
- HalogensDocumento15 pagineHalogenskmoiz427Nessuna valutazione finora
- 1 3 F Calculations Involving Gas VolumesDocumento45 pagine1 3 F Calculations Involving Gas VolumesForm 4BNessuna valutazione finora
- Chemsheets As 008 (Amount of Substance)Documento36 pagineChemsheets As 008 (Amount of Substance)takashi_leeNessuna valutazione finora
- Chap 9 Thermochemistry-1415 AznitaDocumento84 pagineChap 9 Thermochemistry-1415 Aznita黄麒安Nessuna valutazione finora
- ElectrolysisDocumento3 pagineElectrolysisRaymond ChanNessuna valutazione finora
- HalogensDocumento3 pagineHalogensselvabala_Nessuna valutazione finora
- Hydroxy CompoundsDocumento9 pagineHydroxy Compoundschong56Nessuna valutazione finora
- Covalent Bonding NotesDocumento1 paginaCovalent Bonding Noteschongkee56100% (1)
- Edexcel IAS Bonding 1Documento14 pagineEdexcel IAS Bonding 1mostafa barakatNessuna valutazione finora
- Hydroxyl Compounds Tutorial 6 Key ConceptsDocumento21 pagineHydroxyl Compounds Tutorial 6 Key ConceptsJohnNessuna valutazione finora
- Organic reactions of carbonyl compoundsDocumento3 pagineOrganic reactions of carbonyl compoundsmohdburhantalatNessuna valutazione finora
- Relative Oxidising Powers of Chlorine and Iodine Measured Using an Electrochemical CellDocumento7 pagineRelative Oxidising Powers of Chlorine and Iodine Measured Using an Electrochemical CellkitoniumNessuna valutazione finora
- U3 Oxidation and Reduction PPT WatermarkDocumento45 pagineU3 Oxidation and Reduction PPT Watermarkapi-125934329Nessuna valutazione finora
- Carbonyl Condensation ReactionsDocumento41 pagineCarbonyl Condensation ReactionsVladislav PapperNessuna valutazione finora
- H2 Chem Notes 9729 PDFDocumento78 pagineH2 Chem Notes 9729 PDFBobNessuna valutazione finora
- AS Chemistry Unit 1 Class Test Jan 2015 QuestionsDocumento26 pagineAS Chemistry Unit 1 Class Test Jan 2015 Questionsecs90603Nessuna valutazione finora
- 16 Organ PDFDocumento3 pagine16 Organ PDFAya ZhNessuna valutazione finora
- ScP020 Chemical Equations 2Documento1 paginaScP020 Chemical Equations 2ORBeducationNessuna valutazione finora
- Faraday's Law WorksheetDocumento4 pagineFaraday's Law WorksheetBrianna MalcolmNessuna valutazione finora
- Inorganic Chemistry Practice QuestionsDocumento7 pagineInorganic Chemistry Practice QuestionskitoniumNessuna valutazione finora
- Applications of Solubility Product: (I) Purification of Common SaltDocumento6 pagineApplications of Solubility Product: (I) Purification of Common SaltSiddhartha GautamaNessuna valutazione finora
- Revision Notes Bonding and StructureDocumento4 pagineRevision Notes Bonding and StructureSomeRandomDude - Tutorials - TechNessuna valutazione finora
- 2019 c3.5 Organic ChemistryDocumento197 pagine2019 c3.5 Organic Chemistryhydesh100% (1)
- A2 Test 11 Notes - Transition ElementsDocumento11 pagineA2 Test 11 Notes - Transition Elementswill bellNessuna valutazione finora
- Mini Mock Unit 4 4 To 4 11 A2 Organic Chemistry and Structure DeterminationDocumento15 pagineMini Mock Unit 4 4 To 4 11 A2 Organic Chemistry and Structure DeterminationSahanNivanthaNessuna valutazione finora
- Aakash - Some Basic Concept of Chemistry & BEGINNER'S BOXDocumento10 pagineAakash - Some Basic Concept of Chemistry & BEGINNER'S BOXCartoons World100% (1)
- Equilibria Questions and Answers For A2 ChemistryDocumento303 pagineEquilibria Questions and Answers For A2 ChemistrybloodymerlinNessuna valutazione finora
- 2.1 Molecules To Metabolism WorksheetDocumento3 pagine2.1 Molecules To Metabolism WorksheetSayedMuhammadNessuna valutazione finora
- A2-Chemistry Unit 5 Sample PaperDocumento22 pagineA2-Chemistry Unit 5 Sample PaperDimuthu SandaruwanNessuna valutazione finora
- Chemical Formulae and Equations Part 2Documento18 pagineChemical Formulae and Equations Part 2Mohd NorihwanNessuna valutazione finora
- Chap 8 Reaction Kinetics 1415FARRADocumento129 pagineChap 8 Reaction Kinetics 1415FARRA黄麒安Nessuna valutazione finora
- Synthetic Routes (A Level) - Reaction Pathways Aliphatic CompoundsDocumento6 pagineSynthetic Routes (A Level) - Reaction Pathways Aliphatic CompoundsJunior GonzalesNessuna valutazione finora
- 2015 H2 Carbonyl Cpds Tutorial Answer Updated PDFDocumento25 pagine2015 H2 Carbonyl Cpds Tutorial Answer Updated PDFJohnNessuna valutazione finora
- 1 Matter & The Atomic Structure ModulDocumento43 pagine1 Matter & The Atomic Structure Modulryder1man6433100% (1)
- Chemistry Paper 3 SampleDocumento3 pagineChemistry Paper 3 Samplerihdus2100% (2)
- CBSE Class 12 Chemistry Worksheet - ElectrochemistryDocumento4 pagineCBSE Class 12 Chemistry Worksheet - ElectrochemistryArya VermaNessuna valutazione finora
- Chapter 1 - Introduction To Organic ChemistryDocumento102 pagineChapter 1 - Introduction To Organic ChemistryMELVINDO JACOBNessuna valutazione finora
- Empirical Versus Molecular FormulasDocumento5 pagineEmpirical Versus Molecular FormulasJaz SantosNessuna valutazione finora
- H2 Chem Summary of Transition ElementDocumento7 pagineH2 Chem Summary of Transition Elementonnoez100% (2)
- The S-Block ElementsDocumento51 pagineThe S-Block ElementsDiksha TNessuna valutazione finora
- Chemical Kinetics Part - IDocumento43 pagineChemical Kinetics Part - ISanskar BhattacharyaNessuna valutazione finora
- Chemistry Form 5 Chapter 1 - Rate of ReactionDocumento63 pagineChemistry Form 5 Chapter 1 - Rate of ReactionSiti Nursyafiqah100% (7)
- Chem-Study - Transition A Level WorkDocumento47 pagineChem-Study - Transition A Level WorkAdeeba AbdullahNessuna valutazione finora
- IGCSE Chemistry Oxygen Hydrogen and Carbon DioxideDocumento15 pagineIGCSE Chemistry Oxygen Hydrogen and Carbon DioxideS M AkashNessuna valutazione finora
- As Chemistry Organic MindmapDocumento1 paginaAs Chemistry Organic MindmapDương Thị Ngọc HiềnNessuna valutazione finora
- Section A: Mcqs Halogen DerivativesDocumento11 pagineSection A: Mcqs Halogen DerivativesBint A. Qadir100% (1)
- Electron Affinity and Ionization EnergiesDocumento251 pagineElectron Affinity and Ionization Energiesgkawsar22Nessuna valutazione finora
- Alkanes Lecture Notes PDFDocumento23 pagineAlkanes Lecture Notes PDFPrivate AccountNessuna valutazione finora
- Periodic TrendsDocumento31 paginePeriodic TrendsAndrew Bondad100% (1)
- Organic Chemistry Final Exam BreakdownDocumento74 pagineOrganic Chemistry Final Exam BreakdownkaleijaNessuna valutazione finora
- Catholic Junior College H2 Chemistry 9729 2019 Practical Handbook - Part 6Documento13 pagineCatholic Junior College H2 Chemistry 9729 2019 Practical Handbook - Part 6Timothy HandokoNessuna valutazione finora
- Thermochemistry (Important Notes)Documento2 pagineThermochemistry (Important Notes)HenrySeowNessuna valutazione finora
- Organometallic Transition Metal Catalysis: A Holistic Approach to Understanding and Predicting their MechanismsDa EverandOrganometallic Transition Metal Catalysis: A Holistic Approach to Understanding and Predicting their MechanismsNessuna valutazione finora
- CM1401 NotesDocumento33 pagineCM1401 NotesTeo Jia Ming NickolasNessuna valutazione finora
- Maths Notes (Definite Integrals and Applications)Documento1 paginaMaths Notes (Definite Integrals and Applications)Teo Jia Ming Nickolas100% (1)
- Summary of Research PaperDocumento5 pagineSummary of Research PaperTeo Jia Ming NickolasNessuna valutazione finora
- LSM1101 NotesDocumento43 pagineLSM1101 NotesTeo Jia Ming NickolasNessuna valutazione finora
- Maths Notes (Applications of Differentiation)Documento1 paginaMaths Notes (Applications of Differentiation)Teo Jia Ming NickolasNessuna valutazione finora
- Subjects For A LevelsDocumento4 pagineSubjects For A LevelsTeo Jia Ming NickolasNessuna valutazione finora
- Econs SkillsDocumento45 pagineEcons SkillsTeo Jia Ming Nickolas100% (2)
- LSM1102 NotesDocumento34 pagineLSM1102 NotesTeo Jia Ming Nickolas100% (1)
- LSM1102 CA Reference Paper SummaryDocumento4 pagineLSM1102 CA Reference Paper SummaryTeo Jia Ming NickolasNessuna valutazione finora
- Organic Chem Reactions MindmapDocumento10 pagineOrganic Chem Reactions MindmapTeo Jia Ming NickolasNessuna valutazione finora
- GP SkillsDocumento38 pagineGP SkillsTeo Jia Ming Nickolas67% (3)
- Maths Notes (Differentiation Techniques)Documento3 pagineMaths Notes (Differentiation Techniques)Teo Jia Ming NickolasNessuna valutazione finora
- Chemistry SkillsDocumento11 pagineChemistry SkillsTeo Jia Ming Nickolas50% (2)
- Maths SkillsDocumento19 pagineMaths SkillsTeo Jia Ming Nickolas100% (1)
- Organic Chem Identification ReactionsDocumento3 pagineOrganic Chem Identification ReactionsTeo Jia Ming NickolasNessuna valutazione finora
- BCME 2011 Exam TimetableDocumento2 pagineBCME 2011 Exam TimetableTeo Jia Ming NickolasNessuna valutazione finora
- Biology Notes (Proteins)Documento9 pagineBiology Notes (Proteins)Teo Jia Ming Nickolas100% (1)
- Maths Notes (Mathematical Induction)Documento1 paginaMaths Notes (Mathematical Induction)Teo Jia Ming NickolasNessuna valutazione finora
- Maths Formular SheetDocumento3 pagineMaths Formular SheetTeo Jia Ming NickolasNessuna valutazione finora
- Biology Notes (Lipids)Documento5 pagineBiology Notes (Lipids)Teo Jia Ming Nickolas100% (1)
- Maths Notes (Techniques of Integration)Documento2 pagineMaths Notes (Techniques of Integration)Teo Jia Ming NickolasNessuna valutazione finora
- Maths Notes (Maclaurin's Series)Documento1 paginaMaths Notes (Maclaurin's Series)Teo Jia Ming NickolasNessuna valutazione finora
- Biology Notes (Carbohydrates)Documento4 pagineBiology Notes (Carbohydrates)Teo Jia Ming NickolasNessuna valutazione finora
- Maths Notes (Recurrence Relations)Documento1 paginaMaths Notes (Recurrence Relations)Teo Jia Ming NickolasNessuna valutazione finora
- Maths Notes (Inequalities)Documento1 paginaMaths Notes (Inequalities)Teo Jia Ming NickolasNessuna valutazione finora
- Maths Notes (Equations)Documento1 paginaMaths Notes (Equations)Teo Jia Ming NickolasNessuna valutazione finora
- Maths Notes (Binomial Expansion)Documento1 paginaMaths Notes (Binomial Expansion)Teo Jia Ming NickolasNessuna valutazione finora
- Maths Notes (Functions)Documento2 pagineMaths Notes (Functions)Teo Jia Ming NickolasNessuna valutazione finora
- Maths Notes (APGP)Documento1 paginaMaths Notes (APGP)Teo Jia Ming NickolasNessuna valutazione finora
- Coordination Trends in Alkali Metal Crown Ether Uranyl Halide Complexes: The Series (A (Crown) ) (UO X) Where A) Li, Na, K and X) CL, BRDocumento6 pagineCoordination Trends in Alkali Metal Crown Ether Uranyl Halide Complexes: The Series (A (Crown) ) (UO X) Where A) Li, Na, K and X) CL, BRGrace Ann SamsonNessuna valutazione finora
- Terpenoids and SteroidsDocumento370 pagineTerpenoids and SteroidsJessica PizzoNessuna valutazione finora
- OQR (Oragnic Quick Revision) (ALIPHATIC) : Mcpba Conc. H SO DDocumento2 pagineOQR (Oragnic Quick Revision) (ALIPHATIC) : Mcpba Conc. H SO Dmanya9b32100% (1)
- Oxidation: Increase in Oxidation Number. in Organic Chemistry, These Definitions, While StillDocumento17 pagineOxidation: Increase in Oxidation Number. in Organic Chemistry, These Definitions, While StillSohail NadeemNessuna valutazione finora
- 05 Chapter2Documento115 pagine05 Chapter2Aaron AsneNessuna valutazione finora
- (Alan R. Katritzky, Otto Meth-Cohn, and Charles W.Documento1.287 pagine(Alan R. Katritzky, Otto Meth-Cohn, and Charles W.Ganesh KashinathNessuna valutazione finora
- Halogen Containing CompoundsDocumento67 pagineHalogen Containing Compoundsonline_mktg100% (6)
- Aldehyde Ketone NotesDocumento46 pagineAldehyde Ketone Noteshareharanbt22Nessuna valutazione finora
- KFUPM Organic Chemistry Chapter 18 QuestionsDocumento2 pagineKFUPM Organic Chemistry Chapter 18 Questionsعبدالله الغامديNessuna valutazione finora
- By Dr. Rashid HassanDocumento43 pagineBy Dr. Rashid HassanDRPRIYA007Nessuna valutazione finora
- Success Achiever Chmeistry Organic Chemistry PDFDocumento44 pagineSuccess Achiever Chmeistry Organic Chemistry PDFmadheshNessuna valutazione finora
- Preparation and Properties of Alcohols, Phenols, Ethers and EpoxidesDocumento14 paginePreparation and Properties of Alcohols, Phenols, Ethers and Epoxidesitsleochandu21Nessuna valutazione finora
- CBSE Class 12 Chemistry Chapter 11 - Alcohols, Phenols and Ethers Important Questions 2023-24Documento37 pagineCBSE Class 12 Chemistry Chapter 11 - Alcohols, Phenols and Ethers Important Questions 2023-24Subramanian VasanthiNessuna valutazione finora
- Infrared Spectroscopy Absorption Table - Chemistry LibreTexts PDFDocumento7 pagineInfrared Spectroscopy Absorption Table - Chemistry LibreTexts PDFNurhayati HasanahNessuna valutazione finora
- Oxidation Reactions of Organic ChemistryDocumento28 pagineOxidation Reactions of Organic ChemistryviejayNessuna valutazione finora
- Essential Guide for Exporting to BrazilDocumento44 pagineEssential Guide for Exporting to Brazilshailendra singhNessuna valutazione finora
- Chapter 1 - Nomenclature - Reaction Classification 2Documento55 pagineChapter 1 - Nomenclature - Reaction Classification 2Duong Hoang Thoai ChauNessuna valutazione finora
- Transparent Soap Formulation and Production ProcessDocumento6 pagineTransparent Soap Formulation and Production Processacit marocitNessuna valutazione finora
- PO via H2O2Documento12 paginePO via H2O2Bruno BelloNessuna valutazione finora
- Modified Starches - Substituted, Cross-Linked and Their UsesDocumento12 pagineModified Starches - Substituted, Cross-Linked and Their UsesTeong JawaNessuna valutazione finora
- Notes Organic ReactionsDocumento44 pagineNotes Organic ReactionsTrecy Jane RicabordaNessuna valutazione finora
- Djokic JCS Perkin Trans.1 1986 Pp1881Documento10 pagineDjokic JCS Perkin Trans.1 1986 Pp1881Mario Micciarelli100% (1)
- Reasons for acidity and reactivity of alcohols and phenolsDocumento3 pagineReasons for acidity and reactivity of alcohols and phenolsSushantNessuna valutazione finora
- Phenols: Classification, Properties and ReactionsDocumento58 paginePhenols: Classification, Properties and ReactionsNova sounds - No copyright musicNessuna valutazione finora
- Carbohydrates Protecing GroupsDocumento11 pagineCarbohydrates Protecing GroupsApparao ThoomuNessuna valutazione finora
- 1 s2.0 S0223523410004836 MainDocumento12 pagine1 s2.0 S0223523410004836 MainWACHIRACHAI PABUPRAPAPNessuna valutazione finora
- AcetyleneDocumento52 pagineAcetyleneJessie Z100% (2)
- Aldehydes and Ketones FinalDocumento67 pagineAldehydes and Ketones FinalAnil Kumar VermaNessuna valutazione finora
- Decker 1998 Polymer InternationalDocumento9 pagineDecker 1998 Polymer InternationalreneNessuna valutazione finora
- Exp't 13: Phase-Transfer-Catalyzed Alkylation of Diethyl MalonateDocumento5 pagineExp't 13: Phase-Transfer-Catalyzed Alkylation of Diethyl MalonatelovehopeNessuna valutazione finora