Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
A 10-Year Old Girl With Neck Pain: Clinical History
Caricato da
willygopeDescrizione originale:
Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
A 10-Year Old Girl With Neck Pain: Clinical History
Caricato da
willygopeCopyright:
Formati disponibili
doi:10.1111/j.1750-3639.2009.00365.
x
COM DECEMBER 2009 CASE 2 bpa_365 519..522
A 10-YEAR OLD GIRL WITH NECK PAIN
Craig Horbinski, M.D., Ph.D.,† Ian F. Pollack, M.D.,§ Clayton Wiley, M.D., Ph.D.,† and
Geoff Murdoch, M.D., Ph.D.†
Departments of † Pathology and § Pediatric Neurosurgery, University of Pittsburgh, Pittsburgh, PA 15213
Affiliations: CH was supported by a Callie Rohr American Brain Tumor Association Fellowship
CLINICAL HISTORY circumferentially. Gross total resection was achieved, confirmed by
ultrasound guidance during the resection.
The patient was a 10-year-old African-American female with a 1 Postoperatively, the patient had only transient left upper extrem-
year history of intermittent pain when she turned her neck, as well ity paresis and recovered extremely well, regaining full strength in
as pain in her shoulder and back. She also had a persistent right- both upper extremities, with resolution of her preoperative symp-
ward head tilt and progressive right hand weakness with paresthe- toms and neurological deficits. The patient was transferred to an
sias, as well as difficulty with ambulation secondary to progressive inpatient rehabilitation facility on postoperative day 6. Her impres-
leg weakness. A full physical and neurological examination was sive recovery persisted, with no evidence of lesion recurrence on a
significant for profound right hand weakness and sensory impair- follow-up MRI obtained 2 months after surgery.
ment, and bilateral lower extremity weakness and proprioceptive
impairment.
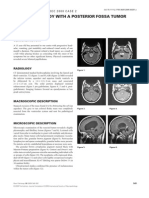
Magnetic resonance imaging showed widening of the cervical
spine with hypointensity on T1 (Fig. 1), increased intradural
PATHOLOGICAL FINDINGS
intramedullary T2 signal (Fig. 2), and contrast enhancement Sections of the excised lesion showed oblique profiles of abnormal
(Fig. 3). The abnormal T2 signal extended from the craniocervical spinal cord elements with hypercellularity in the parenchymal
junction to T5, and the abnormal enhancement extended from the and perivascular regions (Fig. 5, 6, 7). An LFB-PAS stain (Fig. 8)
craniocervical junction to T3. No abnormalities were seen in the showed sharply demarcated zones of myelinated and demyelinated
remainder of the brain. axon fascicles. NF1 immunostain (Fig. 9) confirmed that the long
Corticosteroid therapy with dexamethasone was instituted, processes were axons, and were focally swollen and dystrophic.
although the patient showed no improvement in either her symp- GFAP immunostain showed widespread reactive piloid astrocytic
toms or clinical findings. The spinal cord was thus decompressed processes and occasional gemistocytic reactive astrocytes in
via a C1 laminectomy and C2 through T3 osteoplastic laminotomy the parenchyma. CD68 immunostain highlighted columns of
was then performed. The dura was tense from the underlying mass, foamy macrophages interdigitating between the dystrophic and
which upon exposure was found to be brownish gray and well focally swollen neurofilament immunopositive axons. There were
demarcated from the surrounding spinal cord, splaying the poste- perivascular cuffs composed predominantly of CD3 positive
rior columns laterally on each side (Fig. 4). A distinct plane T-lymphocytes (Fig. 10) with some CD20 positive B-cells
between the mass and the surrounding cord was apparent (Fig. 11). What is the diagnosis?
Figure 1. Figure 2. Figure 3.
Brain Pathology 20 (2010) 519–522 519
© 2010 The Authors; Journal Compilation © 2010 International Society of Neuropathology
Correspondence
Figure 4.
Figure 5.
520 Brain Pathology 20 (2010) 519–522
© 2010 The Authors; Journal Compilation © 2010 International Society of Neuropathology
Correspondence
Figure 6. Figure 7.
Figure 8. Figure 9.
Figure 10. Figure 11.
Brain Pathology 20 (2010) 519–522 521
© 2010 The Authors; Journal Compilation © 2010 International Society of Neuropathology
Correspondence
FINAL DIAGNOSIS:
NEUROMYELITIS OPTICA (NMO)
An extensive work-up was conducted for potential etiologies of
an inflammatory pseudotumor. A full serologic panel was positive
only for anti-aquaporin IgG antibody, consistent with the NMO
spectrum of disorders. On further questioning, the family recalled
that the patient had been evaluated several years ago for a “bad
eye.” Ophthalmological examination confirmed visual impairment
in the right eye.
NMO was once considered an aggressive variant of multiple
sclerosis (MS), but with some differences (4). For example, unlike
MS, NMO is relatively common in sub-Saharan Africa, Asia,
India, and the Caribbean, and interferon-beta is not an effective
therapy (6). Furthermore, detection of anti-aquaporin IgG (a.k.a.
NMO-IgG) has been shown to be >90% specific and 75% sensi-
tive for NMO (1, 3). These findings led to the modified NMO
diagnostic criteria, which included optic neuritis and acute myeli-
tis as major criteria plus either a spinal MRI lesion longer than 3 Figure 12.
vertebral segments or the presence of anti-aquaporin-4 (AQP4)
antibody (7). The mean age of NMO is around 40 years, but it is
known to occur in children. Relapses with severe sequelae have 2. Hinson SR, Pittock SJ, Lucchinetti CF, Roemer SF, Fryer JP, Kryzer
been reported, especially if the patient is NMO-IgG positive at TJ, Lennon VA (2007) Pathogenic potential of IgG binding to water
initial presentation (1). Other risk factors for a relapsing clinical channel extracellular domain in neuromyelitis optica. Neurology
69(24):2221–31.
course include female gender and severe motor weakness at pre-
3. Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF,
sentation. Each recurrent attack during the first 2 years of disease Fujihara K, Nakashima I, Weinshenker BG (2004) A serum
increases risk of early death (8). autoantibody marker of neuromyelitis optica: distinction from multiple
Long-standing NMO lesions typically show reduced AQP4 sclerosis. Lancet 364(9451):2106–12.
immunostaining as do ischemic lesions and inactive MS plaques 4. Lucchinetti CF, Mandler RN, McGavern D, Bruck W, Gleich G,
(5). Fluorescent microscopy showed increased AQP4 immunoreac- Ransohoff RM, et al. (2002) A role for humoral mechanisms in the
tivity in our case (Fig. 12, red). This increased staining may be due pathogenesis of Devic’s neuromyelitis optica. Brain 125(Pt
to the acute phase of the disease. Aquaporin channels increase 7):1450–61.
plasma membrane osmotic permeability by facilitating the bidirec- 5. Misu T, Fujihara K, Kakita A, Konno H, Nakamura M, Watanabe S,
tional movement of water. AQP4 is the main aquaphorin subtype et al (2007) Loss of aquaporin 4 in lesions of neuromyelitis optica:
distinction from multiple sclerosis. Brain 130(Pt 5):1224–34.
found in the brain and is primarily expressed at key parenchymal-
6. Papeix C, Vidal JS, de Seze J, Pierrot-Deseilligny C, Tourbah A,
fluid interfaces (2) Proposed pathologic mechanisms of action Stankoff B, et al. (2007) Immuno-suppressive therapy is more effective
by the anti-AQP4 antibody include endocytosis and degradation than interferon in neuromyelitis optica. Mult Scler 13(2):256–9.
of membrane-bound AQP4, disrupting the blood brain barrier, 7. Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker
destroying AQP4-producing astrocytes via complement fixation, BG (2006) Revised diagnostic criteria for neuromyelitis optica.
and/or by indirect killing of nearby oligodendroglial cells via Neurology 66(10):1485–9.
glutamate excitotoxicity (go to http://path.upmc.edu/divisions/ 8. Wingerchuk DM, Weinshenker BG (2003) Neuromyelitis optica:
neuropath/bpath/cases/case201/dx.html for references). clinical predictors of a relapsing course and survival. Neurology
60(5):848–53.
POSTSCRIPT
The patient was treated with low-dose oral corticosteroids and ABSTRACT
azathioprine but she had relapses at 4, 7, and 11 months, requiring Neuromyelitis optica (NMO), (Devic’s disease), is a relatively
high-dose IV Solu-Medrol each time. Plasmapheresis during the uncommon autoimmune disease predominantly involving the
second relapse produced temporary remission. A fourth minor spinal cord and optic nerves. We present a 10 year-old female with
relapse 15 months after diagnosis prompted replacement of aza- intermittent neck pain, progressive right upper and bilateral lower
thioprine with mycophenolate. While the patient has not yet dem- extremity weakness. MR imaging and intraoperative findings were
onstrated additional deficits, she has developed side effects to the strongly suggestive of a neoplastic process. However, pathologic
steroid therapy, including Cushingoid features and diabetes. examination showed an inflammatory demyelinating lesion and
serological studies were positive for NMO-IgG. The patient
REFERENCES improved dramatically following resection of the compressive
1. Banwell B, Tenembaum S, Lennon VA, Ursell E, Kennedy J, Bar-Or A, “pseudotumor”, with resolution of her preoperative deficits. This
Weinshenker BG, Lucchinetti CF, Pittock SJ (2008) Neuromyelitis case underscores the diverse clinical presentation of neuromyelitis
optica-IgG in childhood inflammatory demyelinating CNS disorders. optica and the importance of maintaining a broad differential diag-
Neurology 70(5):344–52. nosis in pediatric lesions resembling neoplasms.
522 Brain Pathology 20 (2010) 519–522
© 2010 The Authors; Journal Compilation © 2010 International Society of Neuropathology
Potrebbero piacerti anche
- 31-Year-Old Man With Balint'S Syndrome and Visual Problems: Clinical History and NeuroimagingDocumento4 pagine31-Year-Old Man With Balint'S Syndrome and Visual Problems: Clinical History and NeuroimagingwillygopeNessuna valutazione finora
- 1109 Case 2Documento4 pagine1109 Case 2willygopeNessuna valutazione finora
- Acute Disseminated Encephalomyelitis Following Varicella InfectionDocumento4 pagineAcute Disseminated Encephalomyelitis Following Varicella InfectionIJAR JOURNALNessuna valutazione finora
- Syncope: How The EEG Helps in Understanding Clinical FindingsDocumento3 pagineSyncope: How The EEG Helps in Understanding Clinical FindingsCarlos AcostaNessuna valutazione finora
- A 45-Year Old Male With Left-Sided Hemihypesthesia: Clinical HistoryDocumento4 pagineA 45-Year Old Male With Left-Sided Hemihypesthesia: Clinical HistorywillygopeNessuna valutazione finora
- Syringomyelia in Neuromyelitis Optica Seropositive For Aquaporin-4 Antibody: A Case ReportDocumento4 pagineSyringomyelia in Neuromyelitis Optica Seropositive For Aquaporin-4 Antibody: A Case ReportIJAR JOURNALNessuna valutazione finora
- Articulometodos 1Documento19 pagineArticulometodos 1Sadness NeededNessuna valutazione finora
- 1009 Case 1Documento4 pagine1009 Case 1willygopeNessuna valutazione finora
- 0909 Case 2Documento2 pagine0909 Case 2willygopeNessuna valutazione finora
- 0709 Case 2Documento4 pagine0709 Case 2willygopeNessuna valutazione finora
- Pathology and Clinical Research: ClinmedDocumento4 paginePathology and Clinical Research: ClinmedSpt 03Nessuna valutazione finora
- TJN 44370 Images in Clinical Neurology Aggarwal (A)Documento3 pagineTJN 44370 Images in Clinical Neurology Aggarwal (A)VisshwajeetNessuna valutazione finora
- Genes 12 01513 v2Documento10 pagineGenes 12 01513 v2jacopo pruccoliNessuna valutazione finora
- Cureus 0012 00000008855 PDFDocumento11 pagineCureus 0012 00000008855 PDFAlberto BrahmNessuna valutazione finora
- E RefdDocumento13 pagineE RefdGabriella TjondroNessuna valutazione finora
- Magnetic Resonance Imaging - 2010 - Kumar - Eccentric Target Sign in Cerebral Toxoplasmosis Neuropathological Correlate ToDocumento4 pagineMagnetic Resonance Imaging - 2010 - Kumar - Eccentric Target Sign in Cerebral Toxoplasmosis Neuropathological Correlate ToMartha OktaviaNessuna valutazione finora
- Abnormalities of Neuromuscular Transmission in Patients With Miller-Fisher SyndromeDocumento3 pagineAbnormalities of Neuromuscular Transmission in Patients With Miller-Fisher SyndromearaliNessuna valutazione finora
- Handbook of Clinical Electrophysiology of VisionDa EverandHandbook of Clinical Electrophysiology of VisionMinzhong YuNessuna valutazione finora
- Neurohistiocytosis: Two Cases of A Rare DiseaseDocumento8 pagineNeurohistiocytosis: Two Cases of A Rare DiseaseIJAR JOURNALNessuna valutazione finora
- Toronto Study in TBIDocumento10 pagineToronto Study in TBICarlos Esteban Lopez GocheNessuna valutazione finora
- Change in Psychiatric Symptoms in A Bipolar Patient Revealing Endocranial Extension of A Frontal MucoceleDocumento3 pagineChange in Psychiatric Symptoms in A Bipolar Patient Revealing Endocranial Extension of A Frontal MucoceleIJAR JOURNALNessuna valutazione finora
- Isolated Optic Perineuritis As The Presenting Sign of SarcoidosisDocumento3 pagineIsolated Optic Perineuritis As The Presenting Sign of Sarcoidosiswawan 88Nessuna valutazione finora
- Art Acta - Neurol.belgicaDocumento6 pagineArt Acta - Neurol.belgicaAna GabrielaNessuna valutazione finora
- Electrical Stimulation - A Therapeutic Strategy For Retinal and Optic Nerve Disease?Documento3 pagineElectrical Stimulation - A Therapeutic Strategy For Retinal and Optic Nerve Disease?Noura RoseNessuna valutazione finora
- Para Lab Case Study 2Documento6 paginePara Lab Case Study 2jmezzmdc.mdNessuna valutazione finora
- 1208 Case 2Documento4 pagine1208 Case 2willygopeNessuna valutazione finora
- Proof of Progression Over Time Finally Fulminant Brain Muscle An 2009 SeiDocumento3 pagineProof of Progression Over Time Finally Fulminant Brain Muscle An 2009 Seibilal hadiNessuna valutazione finora
- Pocd 2Documento21 paginePocd 2fulgentius juliNessuna valutazione finora
- Post-Hypoxic Myoclonus After COVID-19 Infection RecoveryDocumento2 paginePost-Hypoxic Myoclonus After COVID-19 Infection RecoverykkkljlkjNessuna valutazione finora
- Clinical Neuroimmunology: Multiple Sclerosis and Related DisordersDa EverandClinical Neuroimmunology: Multiple Sclerosis and Related DisordersNessuna valutazione finora
- Botulinum Toxin A For Upper Limb SpasticityDocumento3 pagineBotulinum Toxin A For Upper Limb SpasticityTerrence ChanNessuna valutazione finora
- Use of Electrophysiologic TestingDocumento5 pagineUse of Electrophysiologic Testinggstam69Nessuna valutazione finora
- Neuropathology Simplified: A Guide for Clinicians and NeuroscientistsDa EverandNeuropathology Simplified: A Guide for Clinicians and NeuroscientistsNessuna valutazione finora
- Epilepsy: New Advances: Journal ReadingDocumento29 pagineEpilepsy: New Advances: Journal Readingastra yudhaTagamawanNessuna valutazione finora
- Epilepsy & Behavior Case ReportsDocumento4 pagineEpilepsy & Behavior Case ReportsPutu Filla JfNessuna valutazione finora
- A Case Report On Potts Spine Spinal TubeDocumento3 pagineA Case Report On Potts Spine Spinal TubeyaneemayNessuna valutazione finora
- An Autopsy Case of Group A Streptococcus MeningoencephalitisDocumento5 pagineAn Autopsy Case of Group A Streptococcus MeningoencephalitisFadilla NurrahmaNessuna valutazione finora
- A 7-Year-Old Boy Dying of Acute Encephalopathy: Case History Autopsy FindingsDocumento4 pagineA 7-Year-Old Boy Dying of Acute Encephalopathy: Case History Autopsy FindingswillygopeNessuna valutazione finora
- Algoritmo Diagnostico de La Ceguera Monocular en Una Paciente Con NeurosarcoidosisDocumento8 pagineAlgoritmo Diagnostico de La Ceguera Monocular en Una Paciente Con NeurosarcoidosisFarid Santiago Abedrabbo LombeydaNessuna valutazione finora
- Clin Nuc Med 2015. EncefalitisDocumento3 pagineClin Nuc Med 2015. EncefalitisPuig CozarNessuna valutazione finora
- TMP 82 D3Documento5 pagineTMP 82 D3FrontiersNessuna valutazione finora
- B-Lymphocyte-Mediated Delayed Cognitive Impairment Following StrokeDocumento13 pagineB-Lymphocyte-Mediated Delayed Cognitive Impairment Following Strokeasoly giovanoNessuna valutazione finora
- Posterior Reversible Encephalopathy Syndrome After Lumbar PunctureDocumento2 paginePosterior Reversible Encephalopathy Syndrome After Lumbar PunctureMedtext PublicationsNessuna valutazione finora
- Multiple Sclerosis and Related DisordersDocumento7 pagineMultiple Sclerosis and Related DisordersScerbatiuc CristinaNessuna valutazione finora
- 0209 Case 2Documento4 pagine0209 Case 2willygopeNessuna valutazione finora
- Head Injury For NeurologistDocumento10 pagineHead Injury For NeurologistAmsal Darmawan DakhiNessuna valutazione finora
- MCQs NeuroDocumento2 pagineMCQs NeuroBagadi Suneel100% (2)
- Inflammatory Cortical Demyelination in Early Multiple SclerosisDocumento10 pagineInflammatory Cortical Demyelination in Early Multiple SclerosisWulan AzmiNessuna valutazione finora
- Secondary Syphilis Presenting As Diffused Folliculitis: ReferencesDocumento3 pagineSecondary Syphilis Presenting As Diffused Folliculitis: ReferencesNazihan Safitri AlkatiriNessuna valutazione finora
- Case Report: Contrast-Induced Encephalopathy Following Cerebral Angiography in A Hemodialysis PatientDocumento4 pagineCase Report: Contrast-Induced Encephalopathy Following Cerebral Angiography in A Hemodialysis PatientAnaNessuna valutazione finora
- Y Yyy Y Yyy Y YY Y Yy Yyy Y ! YY"Y#Y$Y# Y Y % Y &Y'Y (Y) +#Y"Y Y Y, Yyyy-Yyy Y.Y Y Y/Y "Y.Y Y Y YDocumento15 pagineY Yyy Y Yyy Y YY Y Yy Yyy Y ! YY"Y#Y$Y# Y Y % Y &Y'Y (Y) +#Y"Y Y Y, Yyyy-Yyy Y.Y Y Y/Y "Y.Y Y Y Ydoni007Nessuna valutazione finora
- 439 2007 Article 362Documento6 pagine439 2007 Article 362Zinik EşanuNessuna valutazione finora
- Ophthalmoplegia Diagnosis: Fig 2 Org Fig 2Documento4 pagineOphthalmoplegia Diagnosis: Fig 2 Org Fig 2QarmeyNessuna valutazione finora
- Case Report: Characteristics and Treatment Results of 5 Patients With Fibrous Dysplasia and Review of The LiteratureDocumento8 pagineCase Report: Characteristics and Treatment Results of 5 Patients With Fibrous Dysplasia and Review of The Literaturesolikin ikinNessuna valutazione finora
- A Homozygous MED11 C Terminal Variant Causes A Lethal Ne - 2022 - Genetics in MeDocumento10 pagineA Homozygous MED11 C Terminal Variant Causes A Lethal Ne - 2022 - Genetics in Meronaldquezada038Nessuna valutazione finora
- Emb Oj 2009327 ADocumento14 pagineEmb Oj 2009327 Athemoonwalker2014Nessuna valutazione finora
- Neurology Multiple Choice Questions With Explanations: Volume IDa EverandNeurology Multiple Choice Questions With Explanations: Volume IValutazione: 4 su 5 stelle4/5 (7)
- 人工智能在神经病理学中的应用:基于深度学习的tau蛋白病变评估Documento23 pagine人工智能在神经病理学中的应用:基于深度学习的tau蛋白病变评估meiwanlanjunNessuna valutazione finora
- Adrenal Neuroblastoma With Bone Marrow Metastasis in Anadult: A Case Report and Review of The LiteratureDocumento5 pagineAdrenal Neuroblastoma With Bone Marrow Metastasis in Anadult: A Case Report and Review of The LiteratureIJAR JOURNALNessuna valutazione finora
- 0309 Case 2Documento4 pagine0309 Case 2willygopeNessuna valutazione finora
- A Tiling of The Plane With TrianglesDocumento6 pagineA Tiling of The Plane With TriangleswillygopeNessuna valutazione finora
- Chords RegionsDocumento3 pagineChords RegionswillygopeNessuna valutazione finora
- Geometry BasicsDocumento2 pagineGeometry BasicswillygopeNessuna valutazione finora
- 1208 Case 1Documento2 pagine1208 Case 1willygopeNessuna valutazione finora
- 1208 Case 2Documento4 pagine1208 Case 2willygopeNessuna valutazione finora
- Trig SubstitutionDocumento13 pagineTrig SubstitutionwillygopeNessuna valutazione finora
- A 7-Year-Old Boy Dying of Acute Encephalopathy: Case History Autopsy FindingsDocumento4 pagineA 7-Year-Old Boy Dying of Acute Encephalopathy: Case History Autopsy FindingswillygopeNessuna valutazione finora
- 0909 Case 2Documento2 pagine0909 Case 2willygopeNessuna valutazione finora
- 1009 Case 1Documento4 pagine1009 Case 1willygopeNessuna valutazione finora
- 1009 Case 2Documento4 pagine1009 Case 2willygopeNessuna valutazione finora
- 0709 Case 2Documento4 pagine0709 Case 2willygopeNessuna valutazione finora
- 0610case1 Metastatic MelanomaDocumento4 pagine0610case1 Metastatic MelanomawillygopeNessuna valutazione finora
- 0209 Case 2Documento4 pagine0209 Case 2willygopeNessuna valutazione finora
- References: American Society For Clinical Pathology 2006 Resident In-Service ExaminationDocumento9 pagineReferences: American Society For Clinical Pathology 2006 Resident In-Service ExaminationwillygopeNessuna valutazione finora
- 0309 Case 2Documento4 pagine0309 Case 2willygopeNessuna valutazione finora
- 2006FallRISE ContentOutlineDocumento1 pagina2006FallRISE ContentOutlinewillygopeNessuna valutazione finora
- Item Descriptors Anatomical Pathology: American Society For Clinical Pathology 2006 Resident In-Service ExaminationDocumento6 pagineItem Descriptors Anatomical Pathology: American Society For Clinical Pathology 2006 Resident In-Service ExaminationwillygopeNessuna valutazione finora
- 3200AMMe - Part 4Documento207 pagine3200AMMe - Part 4Tanja Kesic100% (1)
- The FOA Reference For Fiber Optics - Fiber Optic TestingDocumento19 pagineThe FOA Reference For Fiber Optics - Fiber Optic TestingvsalaiselvamNessuna valutazione finora
- Dual Op Amp and Voltage Reference Ap4310/ADocumento12 pagineDual Op Amp and Voltage Reference Ap4310/AМихаил ЯненкоNessuna valutazione finora
- Napoleonic WargamingDocumento13 pagineNapoleonic WargamingandyNessuna valutazione finora
- Data Bulletin Group Motor Installations:: Understanding National Electrical Code (NEC) 430.53 RequirementsDocumento8 pagineData Bulletin Group Motor Installations:: Understanding National Electrical Code (NEC) 430.53 RequirementsshoaibNessuna valutazione finora
- The Light Fantastic by Sarah CombsDocumento34 pagineThe Light Fantastic by Sarah CombsCandlewick PressNessuna valutazione finora
- Kindergarten Math Problem of The Day December ActivityDocumento5 pagineKindergarten Math Problem of The Day December ActivityiammikemillsNessuna valutazione finora
- LG250CDocumento2 pagineLG250CCarlosNessuna valutazione finora
- Harmonics PatternsDocumento4 pagineHarmonics PatternsIzzadAfif1990Nessuna valutazione finora
- Syllabus Unit Iv Unit Commitment and Economic DispatchDocumento23 pagineSyllabus Unit Iv Unit Commitment and Economic DispatchBALAKRISHNANNessuna valutazione finora
- Mockery Breed Murder Birds PDFDocumento12 pagineMockery Breed Murder Birds PDFLautaro BojanichNessuna valutazione finora
- Goliath 90 v129 eDocumento129 pagineGoliath 90 v129 eerkanNessuna valutazione finora
- Curriculum Vitae - RadikaDocumento3 pagineCurriculum Vitae - RadikaradikahendryNessuna valutazione finora
- Chapter 3.c (Centroid by Intergration)Documento15 pagineChapter 3.c (Centroid by Intergration)Ariff AziziNessuna valutazione finora
- 300 PSI CTS (MP-1115) Operation Manual Rev1.3Documento18 pagine300 PSI CTS (MP-1115) Operation Manual Rev1.3Juan Manuel VizosoNessuna valutazione finora
- Lecture Planner - Inorganic Chemistry (Legend) - Yakeen NEET 2.0 2024Documento1 paginaLecture Planner - Inorganic Chemistry (Legend) - Yakeen NEET 2.0 2024Dipendra KumarNessuna valutazione finora
- De Vault 1996Documento22 pagineDe Vault 1996Harumi OONessuna valutazione finora
- Food Taste Panel Evaluation Form 2Documento17 pagineFood Taste Panel Evaluation Form 2Akshat JainNessuna valutazione finora
- GCSE AstronomyDocumento30 pagineGCSE Astronomyharris123mc100% (1)
- EXP4 The Diels Alder ReactionsDocumento3 pagineEXP4 The Diels Alder ReactionsLaura GuidoNessuna valutazione finora
- TreesDocumento69 pagineTreesADITYA GEHLAWATNessuna valutazione finora
- Bulacan Agricultural State College: Lesson Plan in Science 4 Life Cycle of Humans, Animals and PlantsDocumento6 pagineBulacan Agricultural State College: Lesson Plan in Science 4 Life Cycle of Humans, Animals and PlantsHarmonica PellazarNessuna valutazione finora
- DMDWLab Book AnswersDocumento44 pagineDMDWLab Book AnswersNarpat Makwana Pune100% (1)
- Tugas Dikumpulkan Pada Hari Sabtu, 11 April 2020. Apabila Email Bermasalah Dapat Mengirimkan Via WA PribadiDocumento4 pagineTugas Dikumpulkan Pada Hari Sabtu, 11 April 2020. Apabila Email Bermasalah Dapat Mengirimkan Via WA PribadiFebry SugiantaraNessuna valutazione finora
- Cathodic Protection Catalog - New 8Documento1 paginaCathodic Protection Catalog - New 8dhineshNessuna valutazione finora
- PPT DIARHEA IN CHILDRENDocumento31 paginePPT DIARHEA IN CHILDRENRifka AnisaNessuna valutazione finora
- VBAC MCQsDocumento3 pagineVBAC MCQsHanaNessuna valutazione finora
- 4 Force & ExtensionDocumento13 pagine4 Force & ExtensionSelwah Hj AkipNessuna valutazione finora
- Module 1 Notes The White Bird Reading The Image Painting Analysis PDFDocumento4 pagineModule 1 Notes The White Bird Reading The Image Painting Analysis PDFMelbely Rose Apigo BaduaNessuna valutazione finora
- EN Manual Lenovo Ideapad S130-14igm S130-11igmDocumento33 pagineEN Manual Lenovo Ideapad S130-14igm S130-11igmDolgoffNessuna valutazione finora