Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
0909 Case 2
Caricato da
willygopeDescrizione originale:
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
0909 Case 2
Caricato da
willygopeCopyright:
Formati disponibili
doi:10.1111/j.1750-3639.2009.00349.
x
COM SEPT 2009 CASE 2
A 61-YEAR-OLD MAN WITH INSTABILITY OF GAIT AND RIGHT
HAND CLUMSINESS bpa_349 273..274
Anne Vital, MD, PhD ; Emmanuel Ellie, MD2; Hugues Loiseau, MD3
1
1
CNRS UMR 5227, Victor Segalen-Bordeaux 2 University, Bordeaux, France
2
Neurology Department, Côte Basque General Hospital, Bayonne, France
3
Neurosurgery Department, Bordeaux University Hospital, Bordeaux, France
patient was able to resume his daily hobby of golfing. Total body
CLINICAL HISTORY PET scan using fluorodeoxyglucose was normal, as were motor
A 60 year-old man, without relevant medical history, noted a slight and sensory nerve conduction studies and echocardiography.
and progressive instability of gait for one month, and right hand Serum beta2 microglobulin was within normal limits and bone
clumsiness two weeks before admission. marrow biopsy showed no abnormality. No change in the patient
Initial examination showed wide-based gait, mild dysarthria and condition was noted over a follow-up period of more than one year.
right arm dysmetria. Strength and sensation were normal as was
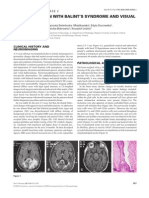
body temperature. Brain MRI showed a unique cerebellar lesion,
posterior to the middle cerebellar peduncle, near the right dentate
MICROSCOPIC PATHOLOGY
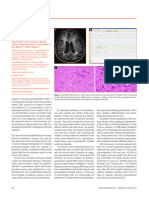
nucleus. The lesion was heterogeneous and hyperintense on FLAIR Microscopic examination on haematoxylin and eosin stained sec-
sequences, isointense on T1-weighted images, and showed gado- tions from three specimens revealed amorphous eosinophilic mate-
linium enhancement (Figure 1). rial, either extra-vascular or thickening vessel walls (Figure 2a). In
Full blood count, erythrocyte sedimentation rate, C-reactive close proximity to the deposited material, the cerebellar paren-
protein level, liver function tests, serum electrolytes, and serum chyma was infiltrated by predominant mature plasma-cells (CD
protein electrophoresis were normal. Plasma and urine immuno- 138 immunopositive; Figure 2b) associated with a few mature T
electrophoresis showed no abnormality. Serologic tests for HIV, lymphocytes (CD3 immunopositive; Figure 2c) and macrophages
Listeria, Legionella, and Lyme disease were negative. CT scans of of the foreign body type (CD68 immunopositive; Figure 2d). The
the abdomen, chest and pelvis were normal as was bone scintigra- weak congophilia of the deposits failed to show any birefringence
phy. Cervical echography was unremarkable and thyroid was in polarized light microscopy. There was a strong immunostaining
normal. CSF analysis showed slightly elevated protein content of the deposited material as well as the plasma-cells with anti-k
(0.72 mg/dl), 5 white cells/mm3, and no oligoclonal banding on light chain (Figure 2e), while anti-l antibodies failed to label them.
CSF protein electrophoresis. The deposits were also immunonegative for transthyretin amyloid,
At surgery, a yellowish and firm lesion associated with abnormal Ab amyloid and AA amyloid. Under UV light, the deposits were
vessels was resected. slightly fluorescent after thioflavine staining, strongly immuno-
The patient’s recovery was good and two months later an iso- fluorescent for k light chain (figure 2f) and negative for l light
lated mild clumsiness of the right hand was noted. However the chain. Electron microscopy revealed the non-fibrillar ultrastructure
of the deposits which presented as finely granular aggregates
(Figure 3).
What is the diagnosis?
Figure 1.
Figure 3. Figure 2.
Brain Pathology 20 (2010) 273–274 273
© 2010 The Authors; Journal Compilation © 2010 International Society of Neuropathology
Correspondence
3. Fischer L, Korfel A, Stoltenburg-Didinger G, Ransco C, Thiel E
DIAGNOSIS (2006) A 19-year-old male with generalized seizures,
Tumor-like deposits of k light chain surrounded by mature kappa unconsciousness and a deviation of gaze. Brain Pathol 16:185–186.
chain-expressing plasma cells. 4. Grassi MP, Clerici F, Perin C, Borella M, Gendarini A, Quattrini A,
Nemni R, Mangoni A (1998) Light chain deposition disease
neuropathy resembling amyloid neuropathy in a multiple myeloma
patient. Ital J Neurol Sci 19:229–233.
DISCUSSION
5. Laeng RH, Altermatt HJ, Scheithauer BW, Zimmermann DR (1998)
Light chains are well known for their amyloidogenic properties, but Amyloidomas of the nervous system. A monoclonal B-cell disorder
in a few cases, they are non amyloidogenic and may cause tissue with monotypic amyloid light chain l amyloid production. Cancer
deposits histologically similar to amyloid but Congo red-negative 82:362–374.
and non fibrillary at ultrastructural examination. Non amyloid light 6. McMillion L, Melton DM, Erickson JC (2008) Teaching neuroimage:
Primary cerebral amyloidoma mimicking CNS neoplasm. Neurology
chain deposits have been reported as a complication of plasma cell
71:e68.
dyscrasia and particularly of multiple myeloma and the condition 7. Popovic M, Tavcar R, Glavac D, Volavsek M, Pirtosek Z, Vizjack A
has been designated light chain deposition (LCD) disease (9). The (2007) Light chain deposition disease restricted to the brain: the first
kidney is usually the first and most severely affected organ but case report. Hum Pathol 38:179–184.
others may be involved, including the peripheral nervous system 8. Ragel BT, Blumenthal DT, Browd SR, Salzman KL, Jensen RL
(4) and ocular globes (1). However, the blood-brain barrier likely (2006) Intracerebral amyloidoma can mimic high-grade glioma on
prevents entrance of protein macromolecules into the central magnetic resonance imaging and spectroscopy. Arch Neurol
nervous system and the brain is not usually affected by systemic 63:906–907.
light chain disorders, excepted in sites missing a tight blood-brain 9. Randall RE, Williamson WC, Mullinax F, Tung MY, Still WJS
barrier such as the choroids plexus and the periventricular areas (1976) Manifestations of systemic light chain deposition. Am J Med
60:293–299.
where they have no effect on the brain (10).
10. Schröder R, Linke RP (1999). Cerebrovascular involvement in
Occurrence of light chain deposits in the brain is rare, with only
systemic AA and AL amyloidosis: a clear hematogenic pattern.
two cases previously reported in the literature (3, 7). In one of these Virchows Arch 434:551–560.
cases, it was suggested that the light chain deposition originated
from a low-grade cerebral lymphoma diagnosed 3 months earlier ABSTRACT
(3), while in the other case a monoclonal B-cell proliferation of
“neoplastic or other undefined nature” was found in close proxim- A 60 year-old man, without relevant medical history, noted a slight
ity to the deposits (7). The cell infiltrate observed in the present and progressive instability of gait for one month and right hand
case was composed of monotypic k producing plasma cells clumsiness. Brain MRI showed a cerebellar lesion, posterior to the
showing no cytologic atypia, associated with a few mature T lym- middle cerebellar peduncle. This lesion was heterogeneous and
phocytes and macrophages of the foreign body type. There was hyperintense on FLAIR sequences, isointense on T1-weighted
no evidence of an aggressive or systemic lymphoplasmacytic images, and showed gadolinium enhancement. Hematological and
neoplasm. biological serum analyses were normal as were plasma and urine
The tumor-like MRI presentation of the light chain disease in immunoelectrophoresis. CSF analysis including protein electro-
this case is reminiscent of primary intracerebral amyloidoma pre- phoresis was unremarkable. CT scans of the abdomen, chest and
senting as a mass lesion (6, 8) and likely produced by B-cell clone. pelvis were normal as were cervical echography and bone scintig-
Nevertheless, most of such cases lack clinical evidence of an raphy. A yellowish and firm lesion was surgically resected. The
aggressive or systemic lymphoplasmacytic neoplasm and are char- patient’s recovery was good, with normal total body PET scan and
acterized by a relatively indolent course (2, 5). Intracerebral light bone marrow biopsy.
chain amyloidomas are almost invariably of l type, as for the two Pathological study evidenced k light chain deposits and
previously reported cases of LCD (3, 7). We report the first case of k-immunopositive mature plasma-cells in the vicinity. The deposits
intracerebral k LCD presumably derived from local synthesis by failed to show any birefringence in polarized light microscopy
mature plasma cells and mimicking CNS neoplasm on MRI. after Congo red staining, and electron microscopy revealed their
granular ultrastructure. Light chains are well known for their
amyloidogenic properties, but in a few cases, they are non amy-
REFERENCES loidogenic and may cause tissue deposits histologically similar to
amyloid but Congo red-negative and non fibrillary at ultrastructural
1. Daicker BC, Mihatsch MJ, Strøm EH, Fogazzi GB (1995). Ocular
pathology in light chain deposition disease. Eur J Ophtalmol 5:75–81.
examination. Occurrence of light chain deposits in the brain is rare
2. Fischer B, Palkovic S, Rickert C, Weckesser M, Wassmann H (2007) and the tumor-like MRI presentation is reminiscent of primary
Cerebral AL l-amyloidoma: clinical and pathomorphological intracerebral amyloidoma presenting as a mass lesion. This is the
characteristics. Review of the literature and of a patient. Amyloid first report of intracerebral k light chain deposits which presumably
14:11–19. derived from local synthesis by mature plasma cells.
274 Brain Pathology 20 (2010) 273–274
© 2010 The Authors; Journal Compilation © 2010 International Society of Neuropathology
Potrebbero piacerti anche
- 31-Year-Old Man With Balint'S Syndrome and Visual Problems: Clinical History and NeuroimagingDocumento4 pagine31-Year-Old Man With Balint'S Syndrome and Visual Problems: Clinical History and NeuroimagingwillygopeNessuna valutazione finora
- 0209 Case 2Documento4 pagine0209 Case 2willygopeNessuna valutazione finora
- A 45-Year Old Male With Left-Sided Hemihypesthesia: Clinical HistoryDocumento4 pagineA 45-Year Old Male With Left-Sided Hemihypesthesia: Clinical HistorywillygopeNessuna valutazione finora
- A 10-Year Old Girl With Neck Pain: Clinical HistoryDocumento4 pagineA 10-Year Old Girl With Neck Pain: Clinical HistorywillygopeNessuna valutazione finora
- Isolated Optic Perineuritis As The Presenting Sign of SarcoidosisDocumento3 pagineIsolated Optic Perineuritis As The Presenting Sign of Sarcoidosiswawan 88Nessuna valutazione finora
- Art Acta - Neurol.belgicaDocumento6 pagineArt Acta - Neurol.belgicaAna GabrielaNessuna valutazione finora
- A Rare Case of Space Occupying Lesion of Brainstem in An Elderly Male PatientDocumento3 pagineA Rare Case of Space Occupying Lesion of Brainstem in An Elderly Male PatientInternational Journal of Clinical and Biomedical Research (IJCBR)Nessuna valutazione finora
- 1009 Case 2Documento4 pagine1009 Case 2willygopeNessuna valutazione finora
- 0709 Case 2Documento4 pagine0709 Case 2willygopeNessuna valutazione finora
- 1208 Case 2Documento4 pagine1208 Case 2willygopeNessuna valutazione finora
- 1109 Case 2Documento4 pagine1109 Case 2willygopeNessuna valutazione finora
- Secondary Syphilis Presenting As Diffused Folliculitis: ReferencesDocumento3 pagineSecondary Syphilis Presenting As Diffused Folliculitis: ReferencesNazihan Safitri AlkatiriNessuna valutazione finora
- Neurocytoma/rhabdomyoma (Myoneurocytoma) of The CerebellumDocumento6 pagineNeurocytoma/rhabdomyoma (Myoneurocytoma) of The CerebellumRafael Mujica OreNessuna valutazione finora
- Magnetic Resonance Spectroscopy A Noninvasive Diagno - 2006 - Magnetic ResonancDocumento3 pagineMagnetic Resonance Spectroscopy A Noninvasive Diagno - 2006 - Magnetic Resonanctejas1578Nessuna valutazione finora
- 104.full AdemDocumento6 pagine104.full AdemayubahriNessuna valutazione finora
- Proof of Progression Over Time Finally Fulminant Brain Muscle An 2009 SeiDocumento3 pagineProof of Progression Over Time Finally Fulminant Brain Muscle An 2009 Seibilal hadiNessuna valutazione finora
- Calcified Pseudoneoplasm of The Neuraxis Capnon A Lesson Learnt From A Rare Entity PDFDocumento3 pagineCalcified Pseudoneoplasm of The Neuraxis Capnon A Lesson Learnt From A Rare Entity PDFKisspetre PetreNessuna valutazione finora
- A New Familial Adult-Onset Leukodystrophy Manifesting As Cerebellar Ataxia and DementiaDocumento9 pagineA New Familial Adult-Onset Leukodystrophy Manifesting As Cerebellar Ataxia and DementiaKristineNessuna valutazione finora
- Hon 2005Documento2 pagineHon 2005Andana TrisaviNessuna valutazione finora
- Neurohistiocytosis: Two Cases of A Rare DiseaseDocumento8 pagineNeurohistiocytosis: Two Cases of A Rare DiseaseIJAR JOURNALNessuna valutazione finora
- Tum DemDocumento2 pagineTum DemKota AnuroopNessuna valutazione finora
- Foster Kennedy Synd 2Documento3 pagineFoster Kennedy Synd 2Anonymous k4F8sAs25Nessuna valutazione finora
- Bifascicular Block Revealing Steinerts Myotonic DystrophyDocumento8 pagineBifascicular Block Revealing Steinerts Myotonic DystrophyIJAR JOURNALNessuna valutazione finora
- Congenitalmyopathies Andmusculardystrophies: Heather R. Gilbreath,, Diana Castro,, Susan T. IannacconeDocumento15 pagineCongenitalmyopathies Andmusculardystrophies: Heather R. Gilbreath,, Diana Castro,, Susan T. IannacconeRizki Nandasari SulbahriNessuna valutazione finora
- Primary Intracranial Leiomyoma: A Case Report and Literature ReviewDocumento3 paginePrimary Intracranial Leiomyoma: A Case Report and Literature ReviewcandiddreamsNessuna valutazione finora
- Lhermitte Duclos Disease - A Case Report: Ramya C, Renuka IV, Durga Prasad B, Pratyusha NDocumento4 pagineLhermitte Duclos Disease - A Case Report: Ramya C, Renuka IV, Durga Prasad B, Pratyusha NajmrdNessuna valutazione finora
- Effect Ofl Dopa On Oculogyric Crises inDocumento2 pagineEffect Ofl Dopa On Oculogyric Crises inJoséNessuna valutazione finora
- Prion BrainDocumento2 paginePrion BrainD SNessuna valutazione finora
- Visual Anosognosia (Anton-Babinski Syndrome) : Report of Two Cases Associated With Ischemic Cerebrovascular DiseaseDocumento5 pagineVisual Anosognosia (Anton-Babinski Syndrome) : Report of Two Cases Associated With Ischemic Cerebrovascular DiseaseHanna EnitaNessuna valutazione finora
- Epithelioid Hemangioendothelioma of The Infundibular-Hypothalamic Region: Case Report and Literature ReviewDocumento6 pagineEpithelioid Hemangioendothelioma of The Infundibular-Hypothalamic Region: Case Report and Literature ReviewcarlosNessuna valutazione finora
- January 1994, Volume 34, Number 1: Neurosurgery 1992-98 163 Intracranial Suprasellar AngiolipomaDocumento8 pagineJanuary 1994, Volume 34, Number 1: Neurosurgery 1992-98 163 Intracranial Suprasellar AngiolipomaZdravko HeinrichNessuna valutazione finora
- 5 5 5 Pontine Atypical Neurocytoma Case Report 副本Documento8 pagine5 5 5 Pontine Atypical Neurocytoma Case Report 副本singhNessuna valutazione finora
- Annals of Neurology - 2022 - Gollion - Unilateral Leukoencephalopathy Revealing Cerebral Autosomal Dominant ArteriopathyDocumento2 pagineAnnals of Neurology - 2022 - Gollion - Unilateral Leukoencephalopathy Revealing Cerebral Autosomal Dominant Arteriopathyalex mondomobeNessuna valutazione finora
- Wallerian of Brain:MRI and CT Findings: DegenerationDocumento3 pagineWallerian of Brain:MRI and CT Findings: Degenerationneela kantaNessuna valutazione finora
- CT and MRI Imaging On Tuberous SclerosisDocumento13 pagineCT and MRI Imaging On Tuberous Sclerosiselmon patadunganNessuna valutazione finora
- Abnormalities of Neuromuscular Transmission in Patients With Miller-Fisher SyndromeDocumento3 pagineAbnormalities of Neuromuscular Transmission in Patients With Miller-Fisher SyndromearaliNessuna valutazione finora
- Unusual Brain StoneDocumento5 pagineUnusual Brain StonekucingNessuna valutazione finora
- Follicular Carcinoma of Thyroid Presenting As Brain MetastasisDocumento3 pagineFollicular Carcinoma of Thyroid Presenting As Brain MetastasisArif Susilo RahadiNessuna valutazione finora
- Apathy and Hypersomnia Are Common Features of Myotonic DystrophyDocumento7 pagineApathy and Hypersomnia Are Common Features of Myotonic DystrophyAnnisa HusainNessuna valutazione finora
- Radio Logical Finding PCNSLDocumento12 pagineRadio Logical Finding PCNSLKhushalchand KanjiNessuna valutazione finora
- 1208 Case 1Documento2 pagine1208 Case 1willygopeNessuna valutazione finora
- Rapid Review: BackgroundDocumento9 pagineRapid Review: BackgroundyusufNessuna valutazione finora
- Intracranial Epidermoid CystDocumento3 pagineIntracranial Epidermoid CystWildaHanimNessuna valutazione finora
- Genes 12 01513 v2Documento10 pagineGenes 12 01513 v2jacopo pruccoliNessuna valutazione finora
- BMJ Case Reports-2014-Yi-bcr-2013-202851Documento2 pagineBMJ Case Reports-2014-Yi-bcr-2013-202851mkfireNessuna valutazione finora
- Granulocytic SarcomaDocumento6 pagineGranulocytic Sarcomafriiday.qNessuna valutazione finora
- Subacute Sclerosing Panencephalitis A Clinical and Pathological Study - 101 108Documento7 pagineSubacute Sclerosing Panencephalitis A Clinical and Pathological Study - 101 108Bhanu PratapNessuna valutazione finora
- E194 FullDocumento3 pagineE194 FullAlex BabyNessuna valutazione finora
- Diag DifDocumento3 pagineDiag DifJulia LemboNessuna valutazione finora
- TJN 44370 Images in Clinical Neurology Aggarwal (A)Documento3 pagineTJN 44370 Images in Clinical Neurology Aggarwal (A)VisshwajeetNessuna valutazione finora
- Brain Imaging in Colloid CystDocumento5 pagineBrain Imaging in Colloid CystMohamed Farouk El-FaresyNessuna valutazione finora
- Magnetic Resonance Imaging - 2010 - Kumar - Eccentric Target Sign in Cerebral Toxoplasmosis Neuropathological Correlate ToDocumento4 pagineMagnetic Resonance Imaging - 2010 - Kumar - Eccentric Target Sign in Cerebral Toxoplasmosis Neuropathological Correlate ToMartha OktaviaNessuna valutazione finora
- ATROFIA MUSCULAR ESPINAL Lancet 2008Documento14 pagineATROFIA MUSCULAR ESPINAL Lancet 2008Sebastián Silva SotoNessuna valutazione finora
- Diagnosis of Brown Sequard SyndromeDocumento3 pagineDiagnosis of Brown Sequard SyndromeOktavianus PrayitnoNessuna valutazione finora
- Heteroplasmy: A New Mitochondrial Disease Associated With Mitochondrial DNADocumento6 pagineHeteroplasmy: A New Mitochondrial Disease Associated With Mitochondrial DNAThienanNessuna valutazione finora
- Cadasil Syndrome: A Case Report With A Literature ReviewDocumento4 pagineCadasil Syndrome: A Case Report With A Literature ReviewAna Carolina RibasNessuna valutazione finora
- Atypical Atlanto Axial Subluxation - Yjocn - 4Documento1 paginaAtypical Atlanto Axial Subluxation - Yjocn - 4KaitlynHynes34Nessuna valutazione finora
- BH 010060 ENGDocumento2 pagineBH 010060 ENGCarlosNessuna valutazione finora
- ParasiteDocumento7 pagineParasiteJOAN REPOLLONessuna valutazione finora
- Neurology Multiple Choice Questions With Explanations: Volume IDa EverandNeurology Multiple Choice Questions With Explanations: Volume IValutazione: 4 su 5 stelle4/5 (7)
- Geometry BasicsDocumento2 pagineGeometry BasicswillygopeNessuna valutazione finora
- A Tiling of The Plane With TrianglesDocumento6 pagineA Tiling of The Plane With TriangleswillygopeNessuna valutazione finora
- Trig SubstitutionDocumento13 pagineTrig SubstitutionwillygopeNessuna valutazione finora
- 1108 Case 1Documento2 pagine1108 Case 1willygopeNessuna valutazione finora
- 1208 Case 1Documento2 pagine1208 Case 1willygopeNessuna valutazione finora
- 1109 Case 2Documento4 pagine1109 Case 2willygopeNessuna valutazione finora
- Chords RegionsDocumento3 pagineChords RegionswillygopeNessuna valutazione finora
- Putnam InequalitiesDocumento15 paginePutnam InequalitieswillygopeNessuna valutazione finora
- 1009 Case 2Documento4 pagine1009 Case 2willygopeNessuna valutazione finora
- 1009 Case 1Documento4 pagine1009 Case 1willygopeNessuna valutazione finora
- 1208 Case 2Documento4 pagine1208 Case 2willygopeNessuna valutazione finora
- 1108 Case 2Documento4 pagine1108 Case 2willygopeNessuna valutazione finora
- 1109 Case 1Documento4 pagine1109 Case 1willygopeNessuna valutazione finora
- 1008 Case 1Documento4 pagine1008 Case 1willygopeNessuna valutazione finora
- 0809 Case 2Documento4 pagine0809 Case 2willygopeNessuna valutazione finora
- 1008 Case 2Documento4 pagine1008 Case 2willygopeNessuna valutazione finora
- 0909 Case 1Documento4 pagine0909 Case 1willygopeNessuna valutazione finora
- 0610case1 Metastatic MelanomaDocumento4 pagine0610case1 Metastatic MelanomawillygopeNessuna valutazione finora
- 0709 Case 2Documento4 pagine0709 Case 2willygopeNessuna valutazione finora
- A 7-Year-Old Boy Dying of Acute Encephalopathy: Case History Autopsy FindingsDocumento4 pagineA 7-Year-Old Boy Dying of Acute Encephalopathy: Case History Autopsy FindingswillygopeNessuna valutazione finora
- 0609 Case 1Documento4 pagine0609 Case 1willygopeNessuna valutazione finora
- 0609 Case 2Documento2 pagine0609 Case 2willygopeNessuna valutazione finora
- 0409 Case 2Documento4 pagine0409 Case 2willygopeNessuna valutazione finora
- 0410case1 GBM With PNETDocumento4 pagine0410case1 GBM With PNETwillygopeNessuna valutazione finora
- 0510 Case 1Documento2 pagine0510 Case 1willygopeNessuna valutazione finora
- 0509 Case 1Documento4 pagine0509 Case 1willygopeNessuna valutazione finora
- A 24-Year-Old Male With Headaches: Clinical HistoryDocumento4 pagineA 24-Year-Old Male With Headaches: Clinical HistorywillygopeNessuna valutazione finora
- Causes of Obesity: Suzanne M. Wright, Louis J. AronneDocumento3 pagineCauses of Obesity: Suzanne M. Wright, Louis J. AronneCarla Fernanda Sanhueza PozoNessuna valutazione finora
- Advanced Article UsageDocumento4 pagineAdvanced Article UsageManuel Dnte JosueNessuna valutazione finora
- Forensic Pathological Investigation of Myocardial Hypoxia-Inducible Factor-1a, Erythropoietin and Vascular Endothelial Growth Factor in Cardiac DeathDocumento9 pagineForensic Pathological Investigation of Myocardial Hypoxia-Inducible Factor-1a, Erythropoietin and Vascular Endothelial Growth Factor in Cardiac DeathMadul15Nessuna valutazione finora
- Molecular Dynamics Simulations and Novel Drug DiscoveryDocumento16 pagineMolecular Dynamics Simulations and Novel Drug DiscoveryRishavNessuna valutazione finora
- Pediatric DehydrationDocumento27 paginePediatric DehydrationAbraham ChiuNessuna valutazione finora
- Paraphilic DisorderDocumento22 pagineParaphilic DisorderVenson CeaNessuna valutazione finora
- Evs Project - Water PollutionDocumento5 pagineEvs Project - Water Pollutionsamiksha3kadamNessuna valutazione finora
- Pathology Restaurant: Menu Pathologic Diagnosis / AppearancesDocumento2 paginePathology Restaurant: Menu Pathologic Diagnosis / AppearancesNirosha ArulNessuna valutazione finora
- Ethics & Theory PDFDocumento21 pagineEthics & Theory PDFDipak ThakurNessuna valutazione finora
- 27th July (Monday), 2015 Daily Global Rice E-Newsletter by Riceplus MagazineDocumento31 pagine27th July (Monday), 2015 Daily Global Rice E-Newsletter by Riceplus MagazineMujahid AliNessuna valutazione finora
- Microbiology of Clostridium Tetani and Wound ClassificationDocumento3 pagineMicrobiology of Clostridium Tetani and Wound ClassificationAZIZAH ARDINALNessuna valutazione finora
- Bites and StingsDocumento23 pagineBites and StingsAngela PabloNessuna valutazione finora
- Chapters 1, 2, & 3Documento29 pagineChapters 1, 2, & 3ndtc100% (13)
- ECG Interpretation: DR S J Bhosale DM, FPCC (Canada) Associate Professor Tata Memorial CentreDocumento90 pagineECG Interpretation: DR S J Bhosale DM, FPCC (Canada) Associate Professor Tata Memorial Centrevaishali TayadeNessuna valutazione finora
- Orthobullet HandDocumento506 pagineOrthobullet HandRicky Wibowo67% (3)
- Pap SmearDocumento16 paginePap SmearLeo_Rabacca_3610Nessuna valutazione finora
- Study Related To PaediatricsDocumento135 pagineStudy Related To PaediatricsSaakshi1211Nessuna valutazione finora
- How Dopamine Works in the Body and BrainDocumento38 pagineHow Dopamine Works in the Body and BrainelisaNessuna valutazione finora
- NCP Submucous MyomaDocumento1 paginaNCP Submucous MyomaRichmon VillaminNessuna valutazione finora
- IncisionsDocumento25 pagineIncisionspriya_edwin100% (1)
- Bipolar DisorderDocumento48 pagineBipolar DisorderRey Jayvee Arcuino Hinunangan50% (2)
- Fundamental Unit of LifeDocumento89 pagineFundamental Unit of LifeKanchan SawantNessuna valutazione finora
- Syringe Services Programs CDC Fact SheetDocumento2 pagineSyringe Services Programs CDC Fact SheetLeslie RubinNessuna valutazione finora
- My Experience With Topical Homoeopathic Application in A Case of Stage Iv Decubitus UlcerDocumento5 pagineMy Experience With Topical Homoeopathic Application in A Case of Stage Iv Decubitus UlcerHomoeopathic PulseNessuna valutazione finora
- Abrus PrecatoriusDocumento39 pagineAbrus PrecatoriusEshaal KhanNessuna valutazione finora
- 15A ArteryDocumento72 pagine15A Arterypavi7muruganathanNessuna valutazione finora
- January 2018 baby monitoring reportDocumento113 pagineJanuary 2018 baby monitoring reportIsran KadonteNessuna valutazione finora
- Department F Health Plans, Programs, Projects NotesDocumento26 pagineDepartment F Health Plans, Programs, Projects NotesEdgar DumagpiNessuna valutazione finora
- Large cardamom review covers crop improvement, management, pests, diseasesDocumento15 pagineLarge cardamom review covers crop improvement, management, pests, diseasesEzagloriaNessuna valutazione finora
- Haematology An Illustrated Colour Text 4th Edition 2Documento132 pagineHaematology An Illustrated Colour Text 4th Edition 2KiTty Kriss100% (3)