Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
0409 Case 2
Caricato da
willygopeDescrizione originale:
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
0409 Case 2
Caricato da
willygopeCopyright:
Formati disponibili
doi:10.1111/j.1750-3639.2009.00308.
x
COM APRIL 2009 CASE 2
22-YEAR-OLD GIRL WITH STATUS EPILEPTICUS AND
PROGRESSIVE NEUROLOGICAL SYMPTOMS bpa_308 727..730
Pasquale Striano ; Cameron A. Ackerley ; Mariarosaria Cervasio ; Jean-Marie Girard5; Julie Turnbull6;
1 3 4
Maria Laura Del Basso-De Caro4; Salvatore Striano2; Federico Zara1; and Berge A. Minassian5, 6
1
Muscular and Neurodegenerative Disease Unit, Institute G. Gaslini, Genova, Italy; 2 Epilepsy Center, Department of Neurological Sciences, Federico
II University, Napoli, Italy, Italy; 3 Department of Pathology and Laboratory Medicine, The Hospital for Sick Children, Toronto, Canada; 4 Human
Anatomopathology Unit, Federico II University, Napoli, Italy;5 Division of Neurology, Department of Paediatrics, The Hospital for Sick Children,
Toronto, Canada; 6 Program in Genetics and Genome Biology, The Hospital for Sick Children, Toronto, Canada
which was her clinical state prior to her admission for status
CLINICAL HISTORY epilepticus and death.
A 22-year-old girl presented with convulsive status epilepticus and
a previous history of recurrent seizures, myoclonus, ataxia and
impaired cognitive functions. Testing revealed severe metabolic
acidosis, elevated transaminases and creatine kinase, and respira-
MICROSCOPIC AND
tory insufficiency. After intubation and ventilation, thiopental was
ULTRASTRUCTURAL FINDINGS
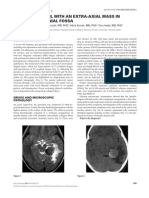
introduced but the patient’s condition worsened dramatically with Histochemical analysis revealed that the CNS was diffusely
death after a few hours. The parents gave permission for autopsy. replete with Intracellular inclusions (Figs 1a, 1b and 2a–c) which
Born of healthy unrelated parents the patient had developed nor- were highlighted by positive periodic acid–Schiff (PAS) staining
mally until the age of 16 years, when she suffered tonic-clonic (figure 2d e). The inclusions were most plentiful in the thalamus,
seizures and visual hallucinations. Some months prior to seizure cerebellum, and brainstem, and in lesser numbers in the frontal
onset, her parents had noted erratic jerks of the upper limbs, per- and occipital cortices. The inclusions were absent in subcortical
sonality changes, and decline in school performance. An electro- white matter but were abundant in the spinal cord gray matter.
encephalographic (EEG) recording had shown spikes over the Two types of inclusions could be distinguished: type I, punctate
occipital regions and photosensitivity. Therapy with valproate had structures present in neuronal processes (Fig 2d;bar = 50 mm, and
decreased the frequency of seizures. However, progressive worsen- type II, large structures found in neuronal cell bodies usually
ing of cognitive and motor functions occurred within a few months. apposed to the nucleus and often so large as to occupy the
In addition, sudden and brief episodes of loss of contact were entire soma (Figure 2e; bar = 10 mm). All sections were
reported. Neurological examination had shown ataxia due to severe ubiquitin-negative.
myoclonus at rest and action-induced myoclonus, pyramidal signs, Electron microscopy showed that the inclusions consisted of
and opposition hypertonia. She could not tandem walk, and deep accumulations of a fibrillar material in the dendrites but not in the
tendon reflexes were barely detectable. EEG showed diffusely slow axon terminals, recognized through their synaptic vesicles (SV)
background activity with superimposed generalized, high- voltage, (Figures 3a, 3b; bar = 500 nm). Additional ultrastructural images
paroxysmal abnormalities. Brain MRI displayed diffuse cerebral show the filamentous nature (Figures 3c, 3d) In addition, the same
and cerebellar atrophy. Neuropsychological testing demonstrated a inclusions were present in clinically unaffected organs too, espe-
full-scale IQ of 70 (verbal 75; performance 60). Adjunctive therapy cially skeletal and cardiac muscle, and the liver. In the apocrine
with clonazepam and zonisamide significantly reduced the inten- sweat gland of skin (Fig 3e; bar = 50 mm) pathological inclusions
sity of myoclonus. In the following years, mental decline continued (round bodies at base of cell) were clearly distinguishable from
unabated resulting in severe dementia, disorientation, akinesia, normal PAS-positive, diastase-resistant secretory material of the
marked rigidity with opposition hypertonia, and inability to feed, gland (granular material on luminal side).
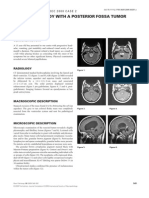
A B
Figure 1.
Brain Pathology 19 (2009) 727–730 727
© 2009 The Authors; Journal Compilation © 2009 International Society of Neuropathology
Correspondence
A B C
D E
Figure 2.
728 Brain Pathology 19 (2009) 727–730
© 2009 The Authors; Journal Compilation © 2009 International Society of Neuropathology
Correspondence
A B
C D
Figure 3.
Brain Pathology 19 (2009) 727–730 729
© 2009 The Authors; Journal Compilation © 2009 International Society of Neuropathology
Correspondence
REFERENCES
DIAGNOSIS
1. Andrade DM, Ackerley CA, Minett TS, Teive HA, Bohlega S, Scherer
Progressive myoclonus epilepsy Lafora-type (LD, OMIM#
SW, Minassian BA (2003) Skin biopsy in Lafora disease:
254780), confirmed also by genetic testing, revealing a homozy- genotype-phenotype correlations and diagnostic pitfalls. Neurology
gous missense mutation (c.205C > G, P69A) in the EPM2B 61:1611–4.
(NHLRC1) gene. 2. Chan EM, Young EJ, Ianzano L, Munteanu I, Zhao X, Christopoulos
CC, Avanzini G, Elia M, Ackerley CA, Jovic NJ, Bohlega S,
DISCUSSION Andermann E, Rouleau GA, Delgado-Escueta AV, Minassian BA,
Scherer SW (2003) Mutations in NHLRC1 cause progressive
LD is the most common and severe form of adolescent-onset myoclonus epilepsy. Nat Genet 35:125–127.
progressive myoclonus epilepsies (PMEs), a group of devastating 3. Ganesh S, Puri R, Singh S, Mittal S, Dubey D (2006) Recent advances
inherited neurodegenerative disorders characterized by progres- in the molecular basis of Lafora’s progressive myoclonus epilepsy. J
sively worsening myoclonus, epilepsy, early dementia and death Hum Genet 51:1–8.
(5). LD, first described by Lafora in 1911, is particularly frequent in 4. Minassian BA, Lee JR, Herbrick JA, Huizenga J, Soder S, Mungall AJ,
Mediterranean countries (Spain, Italy, France), Northern Africa, Dunham I, Gardner R, Fong CY, Carpenter S, Jardim L, Satishchandra
the Middle East, and in some regions of Southern India where a P, Andermann E, Snead OC, 3rd, Lopes-Cendes I, Tsui LC,
high rate of consanguinity is present (3, 5). LD classically starts in Delgado-Escueta AV, Rouleau GA, Scherer SW (1998) Mutations in a
gene encoding a novel protein tyrosine phosphatase cause progressive
adolescence in otherwise neurologically normal individuals,
myoclonus epilepsy. Nat Genet 20:171–174.
usually with action and stimulus-sensitive myoclonus as well as
5. Minassian BA (2001) Lafora’s disease: towards a clinical, pathologic,
tonic–clonic, absence, atonic, and visual seizures. Neuropsychiat- and molecular synthesis. Pediatr Neurol 25:21–29.
ric symptoms such as behavioral changes, depression, apathy, are 6. Van Heycop Ten Ham MW (1975) Lafora disease, a form of
also often present. Initial symptoms are followed by rapidly pro- progressive myoclonus epilepsy. In The Epilepsies. Handbook of
gressive dementia, refractory status epilepticus, psychosis, cer- Clinical Neurology, vol. 15, 382–422 (Eds. Vinken PJ and Bruyn GW),
ebellar ataxia, dysarthria, mutism, and respiratory failure which North-Holland, Amsterdam.
lead to death within about a decade (3, 5).
The main differential diagnosis is with four other forms of
PMEs: Unverricht-Lundborg disease (EPM1), the neuronal ceroid
lipofuscinoses, myoclonic epilepsy with ragged red fibers
ABSTRACT
(MERRF), and sialidosis (3, 5). The age of onset, presenting symp-
toms, occurrence of occipital seizures and the progressive and A 22-year-old girl presented with convulsive status epilepticus and
rapid course, together with the EEG features suggest LD, until the a previous history of recurrent seizures, myoclonus, ataxia and
diagnosis is definitively confirmed by skin biopsy or genetic analy- impaired cognitive functions. Neurological examination revealed
sis. The pathologic hallmark of LD is the presence of the typical rest and action-induced myoclonus, pyramidal signs and opposi-
PAS-positive intraneuronal inclusions (Lafora bodies, LBs). LBs tion hypertonia. Testing revealed severe metabolic acidosis,
are also found in other tissues such as heart, liver, muscle and skin elevated transaminases and creatine kinase, and respiratory insuffi-
composed of an abnormal form of glycogen (5, 6). Importantly, ciency. After intubation and ventilation, thiopental was introduced
neuronal LB exclusively localize in perikarya and dendrites but not but the patient’s condition worsened dramatically with death in a
in axons. LB may be conveniently identified in the eccrine ducts or few hours. Autopsy showed profuse periodic acid–Schiff (PAS)
apocrine myoepithelia of sweat glands obtained from skin biopsy positive intracellular inclusions in the CNS (Lafora bodies), most
(1). LD is caused by mutations in the EPM2A (4) or the EPM2B abundant in thalamus, cerebellum, and brainstem, as well as in
(NHLRC1) (2) genes, encoding the laforin dual specificity protein other organs. Genetic testing revealed a homozygous missense
phosphatase and the malin E3 ubiquitin ligase respectively, both mutation (c.205C > G, P69A) in the EPM2B (NHLRC1) gene,
involved in a complex poorly understood pathway regulating gly- confirming the diagnosis of progressive myoclonic epilepsy
cogen metabolism. At present, LD treatment remains palliative. Lafora-type.
730 Brain Pathology 19 (2009) 727–730
© 2009 The Authors; Journal Compilation © 2009 International Society of Neuropathology
Potrebbero piacerti anche
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (344)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (74)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- Pharmacom Labs CatalogDocumento32 paginePharmacom Labs CatalogJuan Carlos Naranjo67% (3)
- Chords RegionsDocumento3 pagineChords RegionswillygopeNessuna valutazione finora
- Geometry BasicsDocumento2 pagineGeometry BasicswillygopeNessuna valutazione finora
- A Tiling of The Plane With TrianglesDocumento6 pagineA Tiling of The Plane With TriangleswillygopeNessuna valutazione finora
- Putnam InequalitiesDocumento15 paginePutnam InequalitieswillygopeNessuna valutazione finora
- A 10-Year Old Girl With Neck Pain: Clinical HistoryDocumento4 pagineA 10-Year Old Girl With Neck Pain: Clinical HistorywillygopeNessuna valutazione finora
- Trig SubstitutionDocumento13 pagineTrig SubstitutionwillygopeNessuna valutazione finora
- A 45-Year Old Male With Left-Sided Hemihypesthesia: Clinical HistoryDocumento4 pagineA 45-Year Old Male With Left-Sided Hemihypesthesia: Clinical HistorywillygopeNessuna valutazione finora
- 1009 Case 2Documento4 pagine1009 Case 2willygopeNessuna valutazione finora
- A 7-Year-Old Boy Dying of Acute Encephalopathy: Case History Autopsy FindingsDocumento4 pagineA 7-Year-Old Boy Dying of Acute Encephalopathy: Case History Autopsy FindingswillygopeNessuna valutazione finora
- 1208 Case 2Documento4 pagine1208 Case 2willygopeNessuna valutazione finora
- 1109 Case 1Documento4 pagine1109 Case 1willygopeNessuna valutazione finora
- 1108 Case 2Documento4 pagine1108 Case 2willygopeNessuna valutazione finora
- 1109 Case 2Documento4 pagine1109 Case 2willygopeNessuna valutazione finora
- 1208 Case 1Documento2 pagine1208 Case 1willygopeNessuna valutazione finora
- 1108 Case 1Documento2 pagine1108 Case 1willygopeNessuna valutazione finora
- 1008 Case 2Documento4 pagine1008 Case 2willygopeNessuna valutazione finora
- 1009 Case 1Documento4 pagine1009 Case 1willygopeNessuna valutazione finora
- 0909 Case 1Documento4 pagine0909 Case 1willygopeNessuna valutazione finora
- 1008 Case 1Documento4 pagine1008 Case 1willygopeNessuna valutazione finora
- 0610case1 Metastatic MelanomaDocumento4 pagine0610case1 Metastatic MelanomawillygopeNessuna valutazione finora
- 0809 Case 2Documento4 pagine0809 Case 2willygopeNessuna valutazione finora
- 0909 Case 2Documento2 pagine0909 Case 2willygopeNessuna valutazione finora
- 0709 Case 2Documento4 pagine0709 Case 2willygopeNessuna valutazione finora
- 0509 Case 1Documento4 pagine0509 Case 1willygopeNessuna valutazione finora
- A 24-Year-Old Male With Headaches: Clinical HistoryDocumento4 pagineA 24-Year-Old Male With Headaches: Clinical HistorywillygopeNessuna valutazione finora
- 0609 Case 2Documento2 pagine0609 Case 2willygopeNessuna valutazione finora
- 0609 Case 1Documento4 pagine0609 Case 1willygopeNessuna valutazione finora
- 0510 Case 1Documento2 pagine0510 Case 1willygopeNessuna valutazione finora
- 0410case1 GBM With PNETDocumento4 pagine0410case1 GBM With PNETwillygopeNessuna valutazione finora
- Influence of Soil Structure Interaction On Seismic Demands - 2019 - EngineeringDocumento15 pagineInfluence of Soil Structure Interaction On Seismic Demands - 2019 - Engineeringabubaker ahmedNessuna valutazione finora
- Calculation of Electric Field DistributiDocumento9 pagineCalculation of Electric Field DistributiAbouZakariaNessuna valutazione finora
- Project 2020 1 IT02 KA229 079474Documento3 pagineProject 2020 1 IT02 KA229 079474RECAİ TAŞNessuna valutazione finora
- Compact NSX - 630A - LV429797 - 40ADocumento4 pagineCompact NSX - 630A - LV429797 - 40ASenthil SankarasubramanianNessuna valutazione finora
- TextbookList2021-2022 ST Paul CoventDocumento17 pagineTextbookList2021-2022 ST Paul Coventkww3007Nessuna valutazione finora
- Determining The Location of The Sustainable Fishing Industry With Center of Gravity Method, Case Study Northeast Coast of JavaDocumento6 pagineDetermining The Location of The Sustainable Fishing Industry With Center of Gravity Method, Case Study Northeast Coast of JavaInternational Journal of Innovative Science and Research TechnologyNessuna valutazione finora
- Plac908 Dap Record 5 2023Documento3 paginePlac908 Dap Record 5 2023api-706977810Nessuna valutazione finora
- Republic Act No. 8560 February 26, 1998 An Act Regulating The Practice of Geodetic Engineering in The PhilippinesDocumento15 pagineRepublic Act No. 8560 February 26, 1998 An Act Regulating The Practice of Geodetic Engineering in The PhilippinesJazmin Shane Buates BellezaNessuna valutazione finora
- The Origins of Genome Complexity: Science December 2003Documento6 pagineThe Origins of Genome Complexity: Science December 2003Ramadhan AkmalNessuna valutazione finora
- LAC Reflection JournalDocumento6 pagineLAC Reflection JournalEDITHA QUITO100% (1)
- US Declaration of Interdependence 1976Documento10 pagineUS Declaration of Interdependence 1976FranSwizzle SimsNessuna valutazione finora
- 10C21Documento26 pagine10C21Trần ThịnhNessuna valutazione finora
- Session 4 Stephanie CicchiniDocumento20 pagineSession 4 Stephanie CicchiniMarinos GounaridisNessuna valutazione finora
- IUCAA ReportDocumento4 pagineIUCAA ReportRADHIKA BHARADIYANessuna valutazione finora
- Thickness Determination For Spray-Applied Fire Protection MaterialsDocumento6 pagineThickness Determination For Spray-Applied Fire Protection MaterialsAntonNessuna valutazione finora
- Introduction: Digital Controller Design: SystemDocumento13 pagineIntroduction: Digital Controller Design: Systembala_aeroNessuna valutazione finora
- Dhyg 415 Study Guide Yezi Pang - 1Documento117 pagineDhyg 415 Study Guide Yezi Pang - 1api-473128642Nessuna valutazione finora
- Glencoe Algebra Unit 1 ppt3Documento25 pagineGlencoe Algebra Unit 1 ppt3zeynepNessuna valutazione finora
- Current Trends On Deep Learning Models For Brain Tumor Segmentation and Detection - A ReviewDocumento5 pagineCurrent Trends On Deep Learning Models For Brain Tumor Segmentation and Detection - A ReviewPadmavathy VelayudhamNessuna valutazione finora
- Dragline Cycle TimeDocumento11 pagineDragline Cycle TimeanfiboleNessuna valutazione finora
- Work, Energy and PowerDocumento56 pagineWork, Energy and PowerAnu MakhijaNessuna valutazione finora
- Lesson Plan Grade 7 3rd QuarterDocumento3 pagineLesson Plan Grade 7 3rd QuarterDanny EspañolaNessuna valutazione finora
- 5 SLS RequiermentsDocumento13 pagine5 SLS RequiermentssuniljayaNessuna valutazione finora
- Factoring Using Common Monomial FactorDocumento26 pagineFactoring Using Common Monomial FactorHappyNessuna valutazione finora
- MOSFET - Working, Types, Operation, Advantages & ApplicationsDocumento28 pagineMOSFET - Working, Types, Operation, Advantages & ApplicationsgezahegnNessuna valutazione finora
- ATMAN 2023 (Responses)Documento265 pagineATMAN 2023 (Responses)gmfinanceNessuna valutazione finora
- Tic Tac Toe CodeDocumento4 pagineTic Tac Toe CodeluisrogaNessuna valutazione finora
- Legaledge Test Series: Mock Common Law Admission Test 2024 Mock Clat - 03Documento40 pagineLegaledge Test Series: Mock Common Law Admission Test 2024 Mock Clat - 03Ashutosh MalviyaNessuna valutazione finora
- 4D Printing Technology: A Seminar Report OnDocumento29 pagine4D Printing Technology: A Seminar Report OnInvento BuddhiNessuna valutazione finora