Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
HSC
Caricato da
Pablo Rodrigo Acuña MardonesDescrizione originale:
Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
HSC
Caricato da
Pablo Rodrigo Acuña MardonesCopyright:
Formati disponibili
Haematopoietic stem cells, niches
and differentiation pathways
Thomas Graf and Andreas Trumpp
Haematopoietic stem cells (HSCs) continuously replenish blood cells that are lost by transcription factors, the activity of which is orchestrated by extrinsic and intrinsic signals.
attrition or tissue damage. They are capable of self-renewal and are currently the only The study of changes in regulatory networks during haematopoietic differentiation has
adult stem-cell type routinely used in clinical settings to replace lost cells. HSCs are mostly long served as a paradigm for basic processes of cell-fate specification and its aberrations,
quiescent but can be mobilized from their niche to proliferate and differentiate into such as those that occur in leukaemia. The easy accessibility and transplantability of normal
lineages of the innate and adaptive immune system, as well as into red blood cells and and leukaemic haematopoietic cells has led to the discovery of cytokines, oncogenes and
platelets. Cell-fate decisions are initiated and maintained by specific combinations of cancer stem cells and to some of the most celebrated successes of targeted drug design.
Guide through the poster
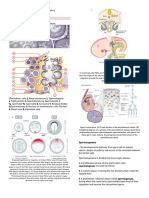
HSC niches The upper left region of the poster shows a mouse
Placenta Yolk sac Bone marrow
embryo with its haematopoietic anlagen. Although the
Sinusoid G-CSF
Mesenchymal HSC precursors
Megakaryocyte Erythrocyte Eosinophil Basophil C/EBPα Neutrophil extent of yolk-sac contribution to the formation of adult
Bone marrow Endothelial cell GFI1
HSCs are transiently present
TPO EPO IL-5 IL-3 HSCs is controversial, most HSCs are probably formed in
in the placenta (E11–E15) M-CSF PU.1hi
Thymic rudiment Vascular niche
GATA1 GATA1 GATA2 GATA2
PU.1hi
the aorta–gonad–mesonephros (AGM) region. From here,
FOG1 FOG1 C/EBPα C/EBPα EGR1/2 IRF8
Mouse embryo CXCL12-expressing ETS1 EKLF C/EBPα MAFB C/EBPα stem cells seed the fetal liver, where they expand and
From E10 onwards, AGM-derived
HSCs colonize the fetal liver, reticular cell differentiate into myeloid and lymphoid lineages.
where they expand and mature. MEP CMP GMP Monocyte/ Subsequently, HSCs exit the liver and populate
HSCs are generated in the Approximately at birth, HSCs start macrophage
AGM between E8.5 and E.11.5 to migrate to the bone marrow, specialized bone-marrow microenvironments (niches) in
which becomes the main site of IL-3 ID2
Activated LT-HSC ST-HSC MPP Mast cell GM-CSF which they gradually become quiescent. As shown in the
haematopoiesis in the adult. SPIB IL-4
LT-HSC
FLT3L
lower left panel, dormant HSCs are mostly located close
SCF ID2 to the lining of the trabecular bone, the endosteal niche.
PU.1 SPIB DC DC DC
Fetal liver Homeostasis (rare) This niche contains osteoblasts and CXC-chemokine
Injury (frequent) IL-7
F-HSC Dividing HSC SCF FLT3L E2A CXCL12 preBCR BCR IgM ligand 12 (CXCL12)-expressing reticular (CAR) cells.
FLT3L CXCL12 EBF PAX5 RAG RAG Several cell adhesion molecules, together with short-
Fibroblast
Endosteal niche
range signalling molecules, control HSC lodging and
ECM LMPP CLP Pre/pro- Pro-B cell Pre-B cell Immature B cell
MBT
MPP
B cell maintenance (lower left panel). In response to specific
Low O2 signals, it is thought that HSCs are recruited to vascular
Differentiation, CXCL12-expressing
Erythrocyte Neutrophil Macrophage Megakaryocyte CXCL12- NK cell
expressing migration and reticular cell niches — that are comprised of CAR cells and
expansion
reticular cell
Dormant
fenestrated sinusoidal endothelial cells — where they
MT NK MB TSP
LT-HSC Osteoblast begin to divide, differentiate and enter the circulation.
T-cell development B-cell development Bone-marrow-derived thymus-seeding progenitors
Osteocyte in the thymus Notch1 in the spleen
Ikaros (TSPs) seed the thymus and become committed to the
Bone IL-15 Ikaros
IL-2 ETS1 IgM+ T-cell lineage (lower right panel). In the thymus, the cells
ID2 PU.1 travel from the cortex through the subcapsular zone to
T cell Macrophage/ B cell ETP/ NK cell
neutrophil DN1 Immature the medulla, encountering different epithelial niches
B cell (T1) that guide them through several developmental stages.
IL-7 TCF
FLT3L PU.1 Fetal precursor Finally, CD4+ and CD8+ T cells enter the circulation and
E2A GATA3
CXCL12- Dormant HSC Nucleus differentiate into mature effector and helper T cells in
expressing Phenotypes of HSCs the spleen and lymph nodes. B-lineage cells become
reticular cell DN2
Mouse: Lin–Sca1+Kit+CD34–CD48–CD150+ Notch- IgMlow committed in the bone marrow and, after immature
Sonic Human: LIN–CD34+CD38–THY1lowKIT+ ligand TCRβ IgD+
hedgehog Patched
DLL4- GATA3 IL-7
B cells enter the circulation and home to the spleen,
B-1a/b cell Mature
Effect on self-renewal capacity
producing RAG RAG (In peritoneum Notch2 B cell (T2) they mature into antibody-secreting memory B cells and
cortical SOX13 as well as in spleen) BAFF
+ (Promote HSC maintenance and/or quiescence): BMI1, FOXO, GFI1, STAT5, ATM, p57, HOXB4 epithelial Weak BCR plasma cells (lower right panel).
DN3 γδ T cell
– (Promote HSC proliferation and/or differentiation): cMYC, cMYB, JUNB, β-catenin, p18, p27 cell signal The differentiation pathways are shown beginning
Smoothened TCRβ GATA3 with HSCs and ending with mature cells. Dotted lines
Notch1 RAG IgMhi
Self-renewal
Survival IgDlow represent transitions in which the origin of the more
MCL1 mature cell is not clear. Arrows indicate points of
DN4 Antigen BAFF
TPO stimulation, Strong BCR migration of circulating cells (with the exception of the
Dormancy Adhesion Self-renewal, Dormancy, Self-renewal Lodging Self-renewal Migration Marginal-zone
MPL helper T-cells B cell signal
Survival adhesion TCF GATA3 HSC synapse panel). It is likely that the early stages of
CXCL12 E2A RAG
Non-canonical HSC development represent a continuum. There are still
? ?
Migration RAC WNT signalling β-catenin ? uncertainties about several parts of the pathways. The
? DP TCRα TReg cell
CXCR4 GATA3 poster shows multipotent progenitors (MPPs) branching
Calcium IL-15
KIT TIE2 Notch receptor TCRα
ThPOK TGFβ into megakaryocyte/erythroid progenitors (MEPs) and
Frizzled SCA1 FOXP3
Short-lived IgMlow
TCRα plasma cell IgDhi lymphoid-primed multipotent progenitors (LMPPs).
RUNX3 RUNX1
VLA5 LMPPs either become common lymphoid progenitors
CD44 CD44 VLA4 Follicular B cell
LRP5 (CLPs) or common myeloid progenitors (CMPs) and
Cadherins
or LRP6 Antigen IL-6 granulocyte/macrophage progenitors (GMPs). However,
Ca2+ NKT cell SP CD8+ SP CD4+ stimulation, BLIMP1
helper T-cells STAT3 GMPs can also generate eosinophils, basophils and mast
CXCL12 WNT
OPN APRIL/BAFF NF-κB cells and it is possible that more than one pathway to
PAX5
Cadherins
BMP PTH T-cell development BCL6
Hyaluronic IFNγ granulocytes and macrophages exists. Finally, there are
in the periphery SP CD8+ IL-12 SP CD4+
ECM acid
(spleen and T-bet IL-6 controversies about the developmental origin of the
lymph node) STAT4 TGFβ different types of dendritic cells and B cells. The poster
ANG1 RUNX3 RORγt
IgG+ also shows lineage-instructive signals such as chemokines,
IL-4 Plasma
GATA3 cell cytokines and transcription factors at the points where
Osteoblast BMPR1A ICAM1 or Membrane- Jagged PTHR STAT6 Memory B cell they are required. The definition of a lineage-instructive
VCAM1 bound SCF HSC synapse Memory T cell Effector T cell TH1 cell TH2 cell TH17 cell factor is complex and is ideally based on gene ablation,
cell-type-specific gene inactivation and gain-of-function
experiments. Since this applies only to a fraction of the
factors shown here, the information provided is
Abbreviations ETS1, v-ets erythroblastosis virus E26 oncogene homologue 1; MPP, multipotent progenitor; MT, myeloid/T-cell precursor; somewhat speculative. Also, only transcription factors
Abcam Stem Cell antibodies you can rely on! We have over 30,000 antibodies in our catalogue.
Our Stem Cell Markers range includes over 2000 antibodies to:
Quality and honesty are our top priorities. Our Abpromise
guarantees full technical support from our experienced team.
F-HSC, fetal HSC; FLT3L, FMS-related-tyrosine-kinase-3 ligand; NF-κB, nuclear factor-κB; NK, natural killer; OPN, osteopontin;
that act dominantly during lineage commitment are
AGM, aorta–gonad–mesonephros; ANG1, angiopoietin 1; FOG1, friend of GATA 1; FOX, forkhead box; GATA1, GATA- PAX5, paired box gene/protein 5; PTH, parathyroid hormone;
Abcam supplies primary and secondary antibodies and reagents If an antibody doesn’t work as it says on our datasheet we will APRIL, a proliferation-inducing ligand; ATM, ataxia-telangiectasia binding protein 1; G-CSF, granulocyte colony-stimulating factor; PTHR, parathyroid hormone receptor; PU.1, transcription factor listed, but not those that need to be silenced.
to researchers worldwide. We ship direct to over 60 countries • Embryonic Stem Cells mutated; BAFF, B-cell-activating factor; BCL, B-cell lymphoma; GFI1, growth-factor independent 1; GM-CSF, granulocyte/ PU.1; RAG, recombination-activating gene 1; RORγt, retinoic-
give you a full refund or replacement if you tell us within BCR, B-cell receptor; BLIMP1, B-lymphocyte-induced macrophage CSF; GMP, granulocyte/macrophage progenitor; acid-receptor-related orphan receptor-γt; RUNX, runt-related
and have offices in the UK, US and Japan, as well as offering • Embryonic Germ Cells
90 days. We publish everything we know about every product maturation protein 1; BMP, bone morphogenetic protein; HOXB4, homeobox B4; HSC, haematopoietic stem cell; ICAM1, transcription factor; SCA1, stem-cell antigen 1; SCF, stem- Thomas Graf is an ICREA investigator at the Centre for Genomic Regulation,
customer support in English, French, German and Japanese. • Endothelial progenitors BMPR1A, bone morphogenetic protein receptor, type 1 A; intercellular adhesion molecule 1; ID2, inhibitor of DNA cell factor; SOX13, SRY box 13; SPIB, Spi-B transcription Dr Aiguader 88, E08003 Barcelona, Spain. e-mail: thomas.graf@crg.es
on our datasheets and our catalogue is web-based. This allows
• Hematopoietic progenitors C/EBPα, CCAAT/enhancer-binding protein α; CLP, common binding 2; IFNγ, interferon-γ; IL, interleukin; IRF8, interferon- factor; STAT, signal transducer and activator of transcription; Andreas Trumpp is at the Swiss Institute for Experimental Cancer Research (ISREC)
We are rapidly developing and expanding our range, looking for daily updates and far more information than in any printed lymphoid progenitor; CMP, common myeloid progenitor; regulatory factor 8; LIN, lineage; LMPP, lymphoid primed MPP; ST-HSC, short-term repopulating HSC; T1, transitional B cell 1; and the Ecole Polytechnique Fédérale de Lausanne (EPFL), Chemin des Boveresses
• Mesenchymal Stem Cells
new targets and improving our existing antibodies. To help us with catalogue, including customer reviews, technical enquiries and CXCL12, CXC-chemokine ligand 12; CXCR4, CXC-chemokine LRP5, low-density-lipoprotein-receptor-related protein 5; TCF, T-cell factor; TCR, T-cell receptor; TGFβ, transforming 155, 1066 Epalinges, Lausanne, Switzerland. e-mail: Andreas.Trumpp@isrec.ch
• Neural Stem Cells receptor 4; DC, dendritic cell; DLL4, delta-like ligand 4; LT-HSC, long-term repopulating HSC; MB, myeloid/B-cell growth factor-β; TH, T helper; ThPOK, T helper-inducing
this, we actively attend, support and help organise conferences on links to publication references. Visit our website today and see DN, double negative; DP, double positive; E2A, transcription precursor; MBT, myeloid, T- and B-cell precursor; MCL1, POZ/Kruppel factor; TIE2, tyrosine kinase receptor 2; Supplementary information (glossary terms and background reading) is associated
• Neural Crest Stem Cells with the online version of this poster. www.nature.com/nri/posters/hsc
Stem Cell research. Find out more on our Stem Cells resource page: for yourself: factor E2A; EBF, early B-cell factor; ECM, extracellular matrix; myeloid-cell leukaemia sequence 1; M-CSF, macrophage TPO, thrombopoietin; TReg, T regulatory; TSP, thymus-seeding
• Ectoderm, Mesoderm and Endoderm lineages EGR1, early-growth response; EKLF, erythroid Kruppel-like colony-stimulating factor; MEP, megakaryocyte/erythroid progenitor; VCAM1; vascular cell-adhesion molecule 1; Edited by Elaine Bell; copy edited by Marta Tufet; designed by Neil Smith and
www.abcam.com/stemcells • Wnt, TGF-β, Hedgehog and Notch signaling pathways www.abcam.com/stemcells factor; EPO, erythropoietin; ETP, early T-cell-lineage progenitor; progenitor; MPL, myeloproliferative leukaemia virus oncogene; VLA, very late antigen. Simon Fenwick. © 2007 Nature Publishing Group.
Glossary
Haematopoietic stem cells Bone-marrow and/or HSC transplantation
(HSCs). Cells that are capable of reconstituting all lineages of the A procedure involving injection of HSCs or bone marrow into
haematopoietic system after transplantation into lethally irradiated irradiated hosts to determine the cell’s biological potential. In the
mice. Long-term repopulating HSCs (LT-HSCs) do this life-long and clinic it is a life-saving procedure after chemotherapy or radiation
after secondary transplantations into irradiated hosts, whereas therapy that destroys the haematopoietic system. Bone-marrow
short-term repopulating HSCs (ST-HSCs) only show multilineage transplantation typically requires immunosuppression to prevent
repopulation for a few weeks. LT- and ST-HSCs can be identified graft rejection due to tissue mismatch.
and isolated by flow cytometry on the basis of their expression of
specific cell-surface antigens. Cord-blood stem cells
These are fetal HSCs present in the umbilical cord of newborns.
Lineage commitment They are used clinically as an alternative to bone-marrow-derived
Also known as cell-fate determination; a process whereby HSCs HSCs. If stored frozen and thawed years later, they can be useful for
become specialized while losing their self-renewal capacity. regenerating the haematopoietic system of the donor, obviating the
danger of tissue rejection of mismatched transplants.
Progenitor cells
Also known as precursor cells; are intermediates between HSCs and Dormancy and mobilization
differentiated cells with restricted differentiation potential, high Although HSCs self-renew, they are mostly quiescent, dividing
proliferative capacity, but little or no self-renewal capacity. They approximately once a month. Proliferation can be activated in
are probably equivalent to the transit amplifying cells of other adult response to injury or injection of cytokines, such as granulocyte
stem-cell systems. colony-stimulating factor (G-CSF). G-CSF also induces the exit
of HSCs from their niches and mobilization of the cells into the
Myeloid cells circulation, a procedure that is used clinically to obtain HSCs from a
A term variously used to define non-lymphoid cells or cells of the patient’s blood.
monocyte–macrophage compartment (known as myelomonocytic
cells). Cytokines and chemokines
Secreted proteins that stimulate cell growth, survival and
Granulocytes differentiation.
A group of cells including neutrophils, eosinophils, and basophils,
but the term is often used for neutrophils only. Colony-forming assay
If seeded in semi-solid medium, blood-cell precursors form colonies
Stem-cell niches in the presence of appropriate cytokines. This assay is widely
Specialized microenvironments that control stem-cell dormancy used to define a cell’s differentiation potential and to identify
and the balance between stem-cell self-renewal and differentiation. biologically active molecules.
Plasticity Cancer and/or leukaemia stem cells
The capacity of defined cell types to acquire new identities. The These are self-renewing cells capable of generating leukaemia
extent of plasticity of HSCs and haematopoietic progenitors under after transplantation. They can give rise to more differentiated
physiological conditions is controversial and probably quite limited. cells comprising the bulk of the leukaemia. Cancer stem cells,
However, re-specification of cell fate can be induced by enforced first identified in acute myeloid leukaemia, are critical targets for
transcription factor expression, cell fusion or nuclear transfer. intervention.
Self-renewal Lineage priming
The ability of cells to repeatedly generate at least one identical The promiscuous expression of myeloid–erythroid lineage
daughter cell. associated markers in HSCs.
Background reading
Wilson, A. & Trumpp, A. Bone-marrow haematopoietic-stem-cell Takahama Y. Journey through the thymus: stromal guides for T-cell
niches. Nature Rev. Immunol. 6, 93–106 (2006). development and selection. Nature Rev. Immunol. 6, 127–135 (2006).
Rothenberg E.V. Cell lineage regulators in B and T cell development. Cumano, A. & Godin, I. Ontogeny of the hematopoietic system.
Nature Immunol. 8, 441–444 (2007). Annu Rev. Immunol. 25, 745–785 (2007).
Laiosa C.V., Stadtfeld, M. & Graf, T. Determinants of lymphoid- Nagasawa T. Microenvironmental niches in the bone marrow
myeloid lineage diversification. Annu. Rev. Immunol. 24, 705–738 required for B-cell development. Nature Rev. Immunol. 6, 107–116
(2006). (2006).
Potrebbero piacerti anche
- Mitosis Worksheet PDFDocumento3 pagineMitosis Worksheet PDFநித்தியாமதி Nithi KalimuthuNessuna valutazione finora
- Atlas Diggs BiruDocumento128 pagineAtlas Diggs Biruninik yosida71% (7)
- Big Picture On The Cell PosterDocumento1 paginaBig Picture On The Cell PosterWellcome Trust100% (1)
- Genome Poster 2009Documento1 paginaGenome Poster 2009ISAAC LEWNessuna valutazione finora
- Human Chromosomes: An Illustrated Introduction to Human CytogeneticsDa EverandHuman Chromosomes: An Illustrated Introduction to Human CytogeneticsValutazione: 5 su 5 stelle5/5 (1)
- Hematopoietic Stem Cells: Gordon KellerDocumento7 pagineHematopoietic Stem Cells: Gordon KellerPisi MicaNessuna valutazione finora
- Cay, COAIFDocumento3 pagineCay, COAIFSapphire RedNessuna valutazione finora
- 1 Icrp Bab 1Documento59 pagine1 Icrp Bab 1YUNITA EKA PRATIWINessuna valutazione finora
- Icrp Bab 1Documento118 pagineIcrp Bab 1YUNITA EKA PRATIWINessuna valutazione finora
- Review: Hematopoiesis: An Evolving Paradigm For Stem Cell BiologyDocumento14 pagineReview: Hematopoiesis: An Evolving Paradigm For Stem Cell Biologywildan pragaNessuna valutazione finora
- (Review) (Biological Chemistry) Bernd Giebel Et Michael Punzel 2008Documento12 pagine(Review) (Biological Chemistry) Bernd Giebel Et Michael Punzel 2008Priscilla FreschuNessuna valutazione finora
- Mappa ImmunologiaDocumento1 paginaMappa ImmunologiaCarlotta RanalliNessuna valutazione finora
- Signaling Pathways Governing Stem-Cell Fate: Review ArticleDocumento12 pagineSignaling Pathways Governing Stem-Cell Fate: Review Articlesondoshagag311Nessuna valutazione finora
- Changes in The Cell Surface Markers During NormalDocumento10 pagineChanges in The Cell Surface Markers During NormalMaw BerryNessuna valutazione finora
- CMLS 2014Documento24 pagineCMLS 2014ИгорьNessuna valutazione finora
- Cell Cycle and Mitosis Notes H Name I. The Cell CycleDocumento3 pagineCell Cycle and Mitosis Notes H Name I. The Cell CycleNickNessuna valutazione finora
- HematopoeisisDocumento16 pagineHematopoeisisPauline SalvadorNessuna valutazione finora
- Hematology & Blood Bank TechniqueDocumento125 pagineHematology & Blood Bank Techniquemandawa786Nessuna valutazione finora
- Poster Caitlin BrennanDocumento1 paginaPoster Caitlin Brennansagun maharjanNessuna valutazione finora
- Artículo CRISPRDocumento14 pagineArtículo CRISPRAlejandra BonillaNessuna valutazione finora
- Cap 88-7 Fanaroff & Martin's Neonatal Perinatal MedicineDocumento22 pagineCap 88-7 Fanaroff & Martin's Neonatal Perinatal MedicineAlejandraSevillaNessuna valutazione finora
- Cap 88-7 Fanaroff & Martin's Neonatal Perinatal Medicine (01-10)Documento10 pagineCap 88-7 Fanaroff & Martin's Neonatal Perinatal Medicine (01-10)AlejandraSevillaNessuna valutazione finora
- Hematopoietic Stem and ProgenitorCellsDocumento10 pagineHematopoietic Stem and ProgenitorCellsRuxandra MesarosNessuna valutazione finora
- Evaluation Report On The Use of Stem Cells in The Treatment of Human DisordersDocumento5 pagineEvaluation Report On The Use of Stem Cells in The Treatment of Human DisordersRichard HampsonNessuna valutazione finora
- Signaling Pathways in Cancer and Embryonic Stem CellsDocumento12 pagineSignaling Pathways in Cancer and Embryonic Stem CellsMiftakhul fadliNessuna valutazione finora
- HomingDocumento14 pagineHomingMaría P SNessuna valutazione finora
- Week 1 - HEMATOPOIESISDocumento8 pagineWeek 1 - HEMATOPOIESISAcel Jone CayotNessuna valutazione finora
- Cardiac Myxoma. Contemporary Immunohistochemical (2020)Documento5 pagineCardiac Myxoma. Contemporary Immunohistochemical (2020)Yuri medranoNessuna valutazione finora
- ArtiDocumento13 pagineArtiValeria Dalay Avila LopezNessuna valutazione finora
- ApoptosisDocumento1 paginaApoptosisAqiena BalqisNessuna valutazione finora
- Connective TissueDocumento35 pagineConnective Tissues vNessuna valutazione finora
- Ha Ema To PoiesisDocumento38 pagineHa Ema To PoiesisEmma Joel OtaiNessuna valutazione finora
- Hema 1-Module 2 - Topic 2-Answer SheetDocumento3 pagineHema 1-Module 2 - Topic 2-Answer SheetKervy Jay AgraviadorNessuna valutazione finora
- Capitol 02 Cells+StructuresDocumento33 pagineCapitol 02 Cells+StructuresCiobotaru AlexandraNessuna valutazione finora
- Fishing For Answers To Hemostatic and Thrombotic DDocumento18 pagineFishing For Answers To Hemostatic and Thrombotic DaalmadasalazarNessuna valutazione finora
- Bases Celulares y TPHDocumento17 pagineBases Celulares y TPHpau_apbNessuna valutazione finora
- De Rybel 2015 Plant Vascular DevelopmentDocumento11 pagineDe Rybel 2015 Plant Vascular DevelopmentSantiagoNessuna valutazione finora
- CSC PosterDocumento1 paginaCSC PosteraiswaryadashNessuna valutazione finora
- Bone Marrow Niches HSC FatesDocumento1 paginaBone Marrow Niches HSC FatesAdelina PirvanNessuna valutazione finora
- Stemcell PosterDocumento1 paginaStemcell PosterhermannNessuna valutazione finora
- 2020 Frontiers Cell Dev Biol Soares-da-Silva F Etal Al.Documento20 pagine2020 Frontiers Cell Dev Biol Soares-da-Silva F Etal Al.PerpetuaNessuna valutazione finora
- BBA - Molecular Cell ResearchDocumento11 pagineBBA - Molecular Cell ResearchPUSKESMAS KECAMATAN KEPULAUAN SERIBU UTARANessuna valutazione finora
- Developmental Biology Notes 3Documento26 pagineDevelopmental Biology Notes 3PrezyNessuna valutazione finora
- Stem Cells, Cancer, and Cancer Stem Cells: InsightDocumento7 pagineStem Cells, Cancer, and Cancer Stem Cells: InsightMekki Lazir IlhdafNessuna valutazione finora
- Wharton's JellyDocumento13 pagineWharton's JellyZ MNessuna valutazione finora
- Law of Segregation and InteractionDocumento14 pagineLaw of Segregation and InteractionEmelinda CruzNessuna valutazione finora
- CH 16 BloodDocumento40 pagineCH 16 BloodShampa SenNessuna valutazione finora
- Stemcell MechanismDocumento9 pagineStemcell Mechanismlxhuy.d2023Nessuna valutazione finora
- Hair Follicle Stem Cells Provide A Functional Niche For Melanocyte Stem CellsDocumento1 paginaHair Follicle Stem Cells Provide A Functional Niche For Melanocyte Stem CellsDenySidiqMulyonoChtNessuna valutazione finora
- Fcell File 2Documento1 paginaFcell File 2holgermayerNessuna valutazione finora
- ?2021 - HematopoiesisDocumento2 pagine?2021 - HematopoiesissharonzechariaNessuna valutazione finora
- Liu2007 Sox17Documento7 pagineLiu2007 Sox17iulia andreeaNessuna valutazione finora
- Cancer-Associated Fibroblasts in Tumor Microenvironment - Accomplices in Tumor MalignancyDocumento11 pagineCancer-Associated Fibroblasts in Tumor Microenvironment - Accomplices in Tumor MalignancyJose EdgarNessuna valutazione finora
- General Stem Cell Biology BrochureDocumento32 pagineGeneral Stem Cell Biology BrochureSigma-AldrichNessuna valutazione finora
- Blood Cells: MicroreviewDocumento8 pagineBlood Cells: MicroreviewEyip SinayNessuna valutazione finora
- A Comparative Review of Canine and Human Rhabdomyosarcoma Con Enfasis en Clasificacion y Patogenesis - Caserto2013Documento21 pagineA Comparative Review of Canine and Human Rhabdomyosarcoma Con Enfasis en Clasificacion y Patogenesis - Caserto2013Jhoel Sebastian Torres GaonaNessuna valutazione finora
- Hematopoiesis (HEMA)Documento12 pagineHematopoiesis (HEMA)April Lady Faith P. PaundogNessuna valutazione finora
- Exercises of Reversible InjuriesDocumento6 pagineExercises of Reversible InjuriesRamel Yen CerantesNessuna valutazione finora
- Orchestration of Primary Hemostasis by Platelet and Endothelial Lysosome-Related OrganellesDocumento13 pagineOrchestration of Primary Hemostasis by Platelet and Endothelial Lysosome-Related OrganellesAndreea DanielaNessuna valutazione finora