Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Jama Ference 2020 It 200013
Caricato da
miguelalmenarezTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Jama Ference 2020 It 200013
Caricato da
miguelalmenarezCopyright:
Formati disponibili
Clinical Review & Education
JAMA Insights | CLINICAL UPDATE
Lipids and Lipoproteins in 2020
Brian A. Ference, MD, MPhil, MSc; John J. P. Kastelein, MD, PhD; Alberico L. Catapano, PhD
This JAMA Insights explains how recent studies have clarified the Guideline Recommendations
role of lipids and lipoproteins in the development of atheroscle- Current guidelines recommend lowering LDL-C with statins, ezeti-
rotic cardiovascular disease (ASCVD) and led to changes in clinical mibe, and proprotein convertase subtilisin/kexin type 9 inhibitors,
practice guidelines for the management of dyslipidemia.1 because these therapies have been shown to reduce the risk of
ASCVD events in randomized trials.1,5 Each of these therapies re-
Lipids and Lipoproteins in the Development of ASCVD duces LDL-C by reducing the number of circulating LDL particles
Cholesterolandtriglyceridesarethemajorlipidsinhumansandaretrans- through upregulation of the LDL receptor. In contrast, the guide-
ported in plasma by lipoproteins. A lipoprotein is composed of choles- lines do not recommend therapies that reduce LDL-C by a mecha-
terol, triglycerides, and a single apolipoprotein B100 molecule (apoB) nism other than clearing LDL particles through the LDL receptor or
when secreted into plasma by the liver, and is referred to as a very low- that primarily reduce plasma triglyceride levels, because the clini-
density lipoprotein (VLDL). The triglycerides are rapidly removed by the cal benefit of these therapies is uncertain.
enzyme lipoprotein lipase and used for energy consumption and stor-
age. As triglycerides are being progressively removed, the lipoprotein Role of apoB in Estimating the Clinical Benefit
is referred to as a VLDL remnant particle. After most of the triglycerides of Novel Lipid-Lowering Therapies
have been removed, the lipoprotein becomes denser and is referred to Recent studies have helped clarify the role of lipids and lipopro-
as a low-density lipoprotein (LDL). However, it is important to recognize teins in the development of atherosclerosis. For example, a choles-
that a VLDL particle, a remnant particle, and an LDL particle are merely teryl ester transfer protein inhibitor blocks the transfer of choles-
different names for the same circulating apoB lipoprotein at different terol esters to LDL particles, thus reducing plasma LDL-C by reducing
stages in its lifecycle, depending on the lipid content that it carries. the amount of cholesterol carried by LDL particles. However, men-
At any point in its lifecycle, regardless of its lipid content, an apoB delian randomization studies and randomized trials have demon-
lipoprotein less than 70 nm in diameter can flux across the endothe- strated that the clinical benefit of a cholesteryl ester transfer pro-
lial barrier, where it may be returned to circulation via the lymphatic tein inhibitor is proportional to the absolute reduction in circulating
system or become trapped in the artery wall. The trapping of an apoB LDL particles as measured by apoB, rather than the reduction in the
lipoprotein in the artery wall and subsequent release of its choles- cholesterol carried by those particles as measured by LDL-C.6,7 An-
terol content to macrophages is the necessary step for the initiation other mendelian randomization study demonstrated that genetic
and progression of an atherosclerotic plaque.2 Over time, the athero- variants that mimic LDL-C–lowering therapies and triglyceride-
sclerotic plaque slowly enlarges as more apoB-containing VLDL, rem- lowering therapies were associated with the same reduction in
nant, and LDL particles become trapped in the artery wall (Figure).3 ASCVD risk for the same change in apoB concentration, despite being
associated with markedly different changes in plasma LDL-C and tri-
Measurement of Plasma Lipids and Lipoproteins glyceride concentrations.8 These data strongly suggest that the risk
Each apoB-containing lipoprotein has a single apoB molecule. There- of ASCVD is determined by the total concentration of circulating apoB
fore, plasma apoB concentration is a direct measure of the total num- particles regardless of the lipid content they carry, and therefore the
ber of circulating atherogenic apoB particles that can become clinical benefit of any lipid-lowering therapy should be propor-
trapped in the artery wall. However, the standard lipid panel does tional to the absolute achieved reduction in apoB concentration re-
not typically measure apoB levels. Instead, the number of circulat- gardless of the corresponding changes in LDL-C or triglycerides.
ing apoB particles is indirectly estimated by measuring plasma LDL
cholesterol (LDL-C) and triglyceride concentration. Implications for Clinical Practice
LDL-C is an estimate of the total cholesterol content carried by The emerging evidence suggests that the optimal lipid-lowering
LDL particles, which is an estimate of the concentration of circulating therapy for any person will be the one that produces the greatest
LDL particles.4 Similarly, plasma triglyceride concentration (mg/dL) absolute reduction in apoB. Under most circumstances, 90% of cir-
divided by 5 is an estimate of the cholesterol content carried by culating apoB particles are LDL particles.3 Therefore, a therapy that
triglyceride-rich lipoproteins, which is an estimate of the concentra- reduces LDL-C by reducing LDL particles through upregulation of the
tion of circulating VLDL and remnant particles.4 These estimates can LDL receptor, such as a statin, will likely be the optimal first lipid-
be combined to derive an estimate of the total cholesterol content car- lowering therapy for most people. If additional lipid lowering is re-
ried by all apoB particles, referred to as non–high-density lipoprotein quired, another therapy that reduces LDL-C by reducing LDL par-
cholesterol, which in turn is an estimate of the total circulating concen- ticles will likely produce the greatest absolute reduction in apoB for
tration of all apoB particles. However, the practice of measuring LDL-C most people without markedly elevated triglycerides levels, and thus
and triglycerides to indirectly estimate the concentration of circulat- will be the preferred next therapy. In contrast, for some people with
ing atherogenic apoB particles has caused confusion about the role markedly elevated triglycerides and low LDL-C levels, triglyceride-
oflipidsandlipoproteinsinthedevelopmentofASCVDanduncertainty rich VLDL remnant particles may compose a greater proportion of
about the benefit of different types of lipid-lowering therapies. This circulating apoB particles than LDL. For these individuals, a novel
uncertainty is reflected in current clinical practice guidelines. agent that substantially lowers plasma triglycerides could produce
jama.com (Reprinted) JAMA Published online July 22, 2020 E1
© 2020 American Medical Association. All rights reserved.
Downloaded From: https://jamanetwork.com/ by Miguel Almenarez Garcia on 07/22/2020
Clinical Review & Education JAMA Insights
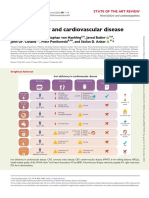
Figure. Role of Apolipoprotein B100 Molecule (apoB)–Containing Lipoproteins in the Development of Atherosclerosis
Lifecycle of a single apolipoprotein B100 (apoB)–containing lipoprotein
1 The liver combines a single apoB molecule, 2 Once in the circulation, triglycerides are 3 When most triglycerides are removed,
triglycerides, and cholesterol into an apoB removed from VLDL by lipoprotein lipase, the now dense apoB lipoprotein is called
lipoprotein and secretes it into plasma as and the apoB lipoprotein is now called a low-density lipoprotein (LDL).
a very low-density lipoprotein (VLDL). a VLDL remnant particle.
HEPATOCYTE
apoB VLDL remnant
Cholesterol VLDL particle LDL
Lipoprotein
Triglycerides
lipase
VLDL to LDL conversion occurs in 6
hours. An LDL is in the circulation for
48 hours total, so an apoB lipoprotein
Energy use spends 90% of its lifecycle as an LDL.
LDL receptor PLASMA ENDOTHELIAL CELL and storage
Any apoB lipoprotein <70 nm in diameter
LDL is removed from the circulation can cross the endothelial barrier
by LDL receptors on hepatocytes
VLDL Remnant LDL
ARTERY LUMEN
Macrophage
Most apoB lipoproteins
Atherosclerotic plaque are returned to the circulation
via the lymphatic system
Some apoB lipoproteins can
Over time, the atherosclerotic plaque grows as more apoB–containing become trapped in the artery wall
VLDL, remnant, and LDL particles become trapped in the artery wall.
ARTERY WALL
The goal of lipid-lowering therapy therefore is to reduce the number of
circulating apoB lipoproteins that can become trapped in the artery wall. LYMPHATIC DUCT
a greater absolute reduction in apoB and therefore would be the pre- looking statement that anticipates the availability of novel lipid-
ferred next therapy to add. lowering therapies in the future and the need to measure apoB to
Based on the evidence described above, the 2019 European help guide selection of the optimal therapy for each person, as out-
Society of Cardiology/European Atherosclerosis Society guidelines lined above. Therefore, because future clinical practice guidelines
for the management of dyslipidaemia1 became the first major inter- may recommend using apoB measurements to help select the ap-
national guideline to state that measurement of apoB levels “is rec- propriate therapy for each patient, clinicians should be aware of the
ommended” to help assess ASCVD risk and to estimate the ex- central role of apoB-containing lipoproteins in the development and
pected clinical benefit from lipid-lowering therapy. This is a forward- management of ASCVD.
ARTICLE INFORMATION College of Cardiology, the European Atherosclerosis cardiovascular disease. Eur Heart J. 2017;38(32):
Author Affiliations: Centre for Naturally Society, and the Physicians Academy for Continuing 2459-2472.
Randomized Trials, University of Cambridge, Education outside the submitted work. Dr Kastelein 4. Friedewald WT, Levy RI, Fredrickson DS.
Cambridge, United Kingdom (Ference); reported receiving personal fees from Akcea, Estimation of the concentration of low-density
Department of Vascular Medicine, Academic AstraZeneca, Daiichi Sankyo, Esperion, Medicines lipoprotein cholesterol in plasma, without use of
Medical Center, University of Amsterdam, Company, Novartis, Novo Nordisk, and Regeneron the preparative ultracentrifuge. Clin Chem. 1972;18
Amsterdam, the Netherlands (Kastelein); during the conduct of the study. Dr Catapano (6):499-502. doi:10.1093/clinchem/18.6.499
Department of Pharmacological and Biomolecular reported receiving grants from Mediolanum; grants
and personal fees from Amgen, Pfizer, Merck, 5. Grundy SM, Stone NJ, Bailey AL, et al. 2018
Sciences, University of Milan and Multimedica
Regeneron, and Sanofi; nonfinancial support and AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/
IRCCS, Milano, Italy (Catapano).
personal fees from Menarini, Eli Lilly, Recordati, APhA/ASPC/NLA/PCNA guideline on the
Corresponding Author: Brian A. Ference, MD, management of blood cholesterol: executive
Sigma-Tau Pharmaceuticals, and Kowa; and
MPhil, MSc, Centre for Naturally Randomized Trials, summary. J Am Coll Cardiol. 2019;73(24):3168-3209.
personal fees from AstraZeneca, Aegerion, Amaryt,
University of Cambridge, 2 Worts' Causeway,
Medco, and Genzyme outside the submitted work. 6. Ference BA, Kastelein JJP, Ginsberg HN, et al.
Cambridge, United Kingdom, CB1 8RN (baf29@
Association of genetic variants related to CETP
medschl.cam.ac.uk).
REFERENCES inhibitors and statins with lipoprotein levels and
Published Online: July 22, 2020. cardiovascular risk. JAMA. 2017;318(10):947-956.
1. Mach F, Baigent C, Catapano AL. ESC/EAS
doi:10.1001/jama.2020.5685
guidelines for the management of dyslipidaemias. 7. Bowman L, Hopewell JC, Chen F, et al. Effects of
Conflict of Interest Disclosures: Dr Ference Eur Heart J. 2020;41(1):111-188. anacetrapib in patients with atherosclerotic
reported receiving grants and personal fees from vascular disease. N Engl J Med. 2017;377(13):1217-1227.
2. Skålén K, Gustafsson M, Rydberg EK, et al.
Amgen and Merck; grants from Novartis and
Subendothelial retention of atherogenic 8. Ference BA, Kastelein JJP, Ray KK, et al.
Esperion Therapeutics; and personal fees from
lipoproteins in early atherosclerosis. Nature. 2002; Association of triglyceride-lowering LPL variants
Regeneron, Sanofi, Pfizer, Eli Lilly, Novo Nordisk,
417(6890):750-754. doi:10.1038/nature00804 and LDL-C-lowering LDLR variants with risk of
Ionis Pharmaceuticals, dalCOR, Medicines
3. Ference BA, Ginsberg HN, Graham I, et al. coronary heart disease. JAMA. 2019;321(4):364-373.
Company, Mylan, CiVi Biopharma, Silence
Therapeutics, Krka Pharmaceuticals, the American Low-density lipoproteins cause atherosclerotic
E2 JAMA Published online July 22, 2020 (Reprinted) jama.com
© 2020 American Medical Association. All rights reserved.
Downloaded From: https://jamanetwork.com/ by Miguel Almenarez Garcia on 07/22/2020
Potrebbero piacerti anche
- The HDL Handbook: Biological Functions and Clinical ImplicationsDa EverandThe HDL Handbook: Biological Functions and Clinical ImplicationsNessuna valutazione finora
- Case 260Documento6 pagineCase 260Spencer KrollNessuna valutazione finora
- Peter Attia Measuring Cardiovascular Disease Risk and The Importance of ApoBDocumento14 paginePeter Attia Measuring Cardiovascular Disease Risk and The Importance of ApoBabhimanyu50% (2)
- Beyond Low Density in CholesterolDocumento8 pagineBeyond Low Density in CholesterolenriqueavilazamoraNessuna valutazione finora
- ApoB 2Documento7 pagineApoB 2Alex AlexNessuna valutazione finora
- Case Study: Chapter 35: Agents Used in DyslipidemiaDocumento28 pagineCase Study: Chapter 35: Agents Used in DyslipidemiaNguyen Huu TinNessuna valutazione finora
- Biomarker 3Documento7 pagineBiomarker 3Raluca Andreea AxinteNessuna valutazione finora
- Antioxidants 10 00784 v2Documento28 pagineAntioxidants 10 00784 v2NatasyaNessuna valutazione finora
- Low Density LipoproteinDocumento8 pagineLow Density LipoproteinZiedTrikiNessuna valutazione finora
- Lipoprotein Paper Academia EtaDocumento15 pagineLipoprotein Paper Academia Etapawovem755Nessuna valutazione finora
- Lipoprotein Paper Academia DeltaDocumento30 pagineLipoprotein Paper Academia Deltapawovem755Nessuna valutazione finora
- Lipoprotein Paper Academia ZetaDocumento8 pagineLipoprotein Paper Academia Zetapawovem755Nessuna valutazione finora
- Lipid DisordersDocumento65 pagineLipid DisordersNguyễn Minh Phương UyênNessuna valutazione finora
- Diabetic Dyslipidaemia (DD)Documento63 pagineDiabetic Dyslipidaemia (DD)SamarNessuna valutazione finora
- LDL InggDocumento7 pagineLDL InggDuti AprilniNessuna valutazione finora
- Role of Brown Fat in Lipoprotein Metabolism and AtherosclerosisDocumento10 pagineRole of Brown Fat in Lipoprotein Metabolism and Atherosclerosisgina inNessuna valutazione finora
- Presentation - LipoproteinDocumento31 paginePresentation - Lipoproteinrahulshrmaa2222Nessuna valutazione finora
- Cholesterol Factors Determining Blood Cholesterol LevelsDocumento7 pagineCholesterol Factors Determining Blood Cholesterol LevelsAnonymous bKm5eCtNessuna valutazione finora
- LDLR Biosynthesis and TransportDocumento10 pagineLDLR Biosynthesis and TransportElyza AimanNessuna valutazione finora
- ApoB 1Documento17 pagineApoB 1Alex AlexNessuna valutazione finora
- Lipid Profile Disease and DiagnosisDocumento31 pagineLipid Profile Disease and DiagnosisGeetanjali JhaNessuna valutazione finora
- Apo BDocumento13 pagineApo BcydolusNessuna valutazione finora
- Caso Clinico 30Documento8 pagineCaso Clinico 30Ariel SanNessuna valutazione finora
- Diabetes and Vascular Disease Research 2010 Younis 289 95Documento8 pagineDiabetes and Vascular Disease Research 2010 Younis 289 95Marthin HotangNessuna valutazione finora
- Jurnal MaglitinideDocumento7 pagineJurnal MaglitinideWanda Novia P SNessuna valutazione finora
- Lipid ProfileDocumento23 pagineLipid Profilekyawswakyawswa100% (1)
- 2006-4254b - 16 - 02 - KP Atorvastatin Label FDA 8-7-06Documento24 pagine2006-4254b - 16 - 02 - KP Atorvastatin Label FDA 8-7-06Dikdik AbdullahNessuna valutazione finora
- Lipids Case StudiesDocumento6 pagineLipids Case Studiesgogo29% (7)
- Annals of Clinical Biochemistry: International Journal of Laboratory MedicineDocumento93 pagineAnnals of Clinical Biochemistry: International Journal of Laboratory MedicinePaula VillaNessuna valutazione finora
- Cholesterol, Triglycerides, and Associated Lipoproteins - Clinical Methods - NCBI BookshelfDocumento30 pagineCholesterol, Triglycerides, and Associated Lipoproteins - Clinical Methods - NCBI BookshelfNeha MasarkarNessuna valutazione finora
- Lipoprotein MetabolismDocumento23 pagineLipoprotein MetabolismDarien LiewNessuna valutazione finora
- Lipoprotein MetabolismDocumento21 pagineLipoprotein MetabolismEllah GutierrezNessuna valutazione finora
- Lipitor: (Atorvastatin Calcium) TabletsDocumento29 pagineLipitor: (Atorvastatin Calcium) Tabletsbmartindoyle6396Nessuna valutazione finora
- LDL y AterogenesisDocumento6 pagineLDL y AterogenesisMEDICOBLASTO EN PROSESONessuna valutazione finora
- Kohli Giugliano 2013 Low Density Lipoprotein Lowering in 2013 by Nonstatin Agents The Discovery and Development ofDocumento12 pagineKohli Giugliano 2013 Low Density Lipoprotein Lowering in 2013 by Nonstatin Agents The Discovery and Development ofOana MihaelaNessuna valutazione finora
- Abstract and IntroductionDocumento14 pagineAbstract and Introductiondavid_lg6179Nessuna valutazione finora
- FNSC 3002 Practical 1 Determination of Plasma Ldl-Cholesterol Hdl-Cholesterol Triacylglycerol and ApolipoproteinsDocumento7 pagineFNSC 3002 Practical 1 Determination of Plasma Ldl-Cholesterol Hdl-Cholesterol Triacylglycerol and ApolipoproteinsPurple DecibelNessuna valutazione finora
- Elevated TG LDL CV Risk Editorial 2023Documento3 pagineElevated TG LDL CV Risk Editorial 2023Roxana MariaNessuna valutazione finora
- Pathophysiology of Diabetic Dyslipidaemia: Where Are We?: ReviewDocumento14 paginePathophysiology of Diabetic Dyslipidaemia: Where Are We?: ReviewApril ApriliantyNessuna valutazione finora
- LDLC3Documento4 pagineLDLC3Michael HenzuNessuna valutazione finora
- At or Vast at inDocumento22 pagineAt or Vast at inVelpandian PathyNessuna valutazione finora
- Hegel e 2009Documento13 pagineHegel e 2009Tina MonroyNessuna valutazione finora
- Apo BDocumento9 pagineApo BmiguelalmenarezNessuna valutazione finora
- Giglio Et Al 2023 Treatment With Proprotein Convertase Subtilisin Kexin Type 9 Inhibitors Pcsk9i Current Evidence ForDocumento13 pagineGiglio Et Al 2023 Treatment With Proprotein Convertase Subtilisin Kexin Type 9 Inhibitors Pcsk9i Current Evidence ForOana MihaelaNessuna valutazione finora
- D-Dyslipidemia by Prof. Abbas OrabiDocumento38 pagineD-Dyslipidemia by Prof. Abbas OrabiIbrahim NegmNessuna valutazione finora
- Lip It orDocumento16 pagineLip It orluvly_nurulNessuna valutazione finora
- Proprotein Convertase Subtilisin Kexin 9 InhibitorsDocumento16 pagineProprotein Convertase Subtilisin Kexin 9 InhibitorsTony Miguel Saba SabaNessuna valutazione finora
- Dyslipidemia 140710032036 Phpapp01Documento90 pagineDyslipidemia 140710032036 Phpapp01Elena EllaNessuna valutazione finora
- Sirera, DyslipidemiaDocumento68 pagineSirera, Dyslipidemiarsirera6409Nessuna valutazione finora
- HDL - C Methods: Preparative UltracentrifugationDocumento13 pagineHDL - C Methods: Preparative UltracentrifugationanggaririnNessuna valutazione finora
- Austprescr 39 164Documento4 pagineAustprescr 39 164dika CaritaNessuna valutazione finora
- Relationship Between High-Density Lipoprotein-Cholesterol and Malondialdehyde-Modified Low-Density Lipoprotein ConcentrationsDocumento7 pagineRelationship Between High-Density Lipoprotein-Cholesterol and Malondialdehyde-Modified Low-Density Lipoprotein ConcentrationsAnonymous tG35SYROzENessuna valutazione finora
- Jurnal ProfDocumento13 pagineJurnal ProfkevinNessuna valutazione finora
- Lipitor: (Atorvastatin Calcium) TabletsDocumento29 pagineLipitor: (Atorvastatin Calcium) TabletsEvelinaĐulbićNessuna valutazione finora
- Pathophysiology of Diabetic Dyslipidaemia: Where Are We?: Diabetologia March 2015Documento15 paginePathophysiology of Diabetic Dyslipidaemia: Where Are We?: Diabetologia March 2015Dilly NizaNessuna valutazione finora
- Biochemistry LipoproteinDocumento84 pagineBiochemistry LipoproteiniqiqiqiqiqNessuna valutazione finora
- Comparison of Two Homogeneous LDL-Cholesterol Assays Using Fresh Hypertriglyceridemic Serum and Quantitative Ultracentrifugation Fractions PDFDocumento10 pagineComparison of Two Homogeneous LDL-Cholesterol Assays Using Fresh Hypertriglyceridemic Serum and Quantitative Ultracentrifugation Fractions PDFmagendi indra muktiNessuna valutazione finora
- Disorders of Lipid Metabolism & AtherosclerosisDocumento16 pagineDisorders of Lipid Metabolism & AtherosclerosisMuthiaranifsNessuna valutazione finora
- Analisis DiscordDocumento7 pagineAnalisis DiscordSergio GonzalezNessuna valutazione finora
- High-Density Lipoprotein Cholesterol - The Next Battle Front in The Fight Against Hear T DiseaseDocumento8 pagineHigh-Density Lipoprotein Cholesterol - The Next Battle Front in The Fight Against Hear T DiseaseRainolNessuna valutazione finora
- SHR Red - IraDocumento105 pagineSHR Red - IramiguelalmenarezNessuna valutazione finora
- MRFITDocumento3 pagineMRFITmiguelalmenarezNessuna valutazione finora
- Biomarkers of Aging and Relevant Evaluation Techniques - A Comprehensive ReviewDocumento29 pagineBiomarkers of Aging and Relevant Evaluation Techniques - A Comprehensive ReviewmiguelalmenarezNessuna valutazione finora
- Nejmoa Tremendo!!!Documento14 pagineNejmoa Tremendo!!!miguelalmenarezNessuna valutazione finora
- An Update On VEXAS SyndromeDocumento14 pagineAn Update On VEXAS SyndromemiguelalmenarezNessuna valutazione finora
- Fluid Therapy and Traumatic Brain Injury: A Narrative ReviewDocumento6 pagineFluid Therapy and Traumatic Brain Injury: A Narrative ReviewmiguelalmenarezNessuna valutazione finora
- Antibiotics 12 00413Documento12 pagineAntibiotics 12 00413miguelalmenarezNessuna valutazione finora
- Association of Treatment With Metformin Vs SulfonylureaDocumento11 pagineAssociation of Treatment With Metformin Vs SulfonylureamiguelalmenarezNessuna valutazione finora
- Thrombosis Research: J. Chin-Hon, L. Davenport, J. Huang, M. Akerman, A. HindenburgDocumento6 pagineThrombosis Research: J. Chin-Hon, L. Davenport, J. Huang, M. Akerman, A. HindenburgmiguelalmenarezNessuna valutazione finora
- 1 s2.0 S2468125323001917 MainDocumento11 pagine1 s2.0 S2468125323001917 MainmiguelalmenarezNessuna valutazione finora
- Maddox Et Al 2024 2024 Acc Expert Consensus Decision Pathway For Treatment of Heart Failure With Reduced EjectionDocumento45 pagineMaddox Et Al 2024 2024 Acc Expert Consensus Decision Pathway For Treatment of Heart Failure With Reduced EjectionmiguelalmenarezNessuna valutazione finora
- AnalgesiaDocumento43 pagineAnalgesiamiguelalmenarezNessuna valutazione finora
- METADocumento11 pagineMETAmiguelalmenarezNessuna valutazione finora
- Iron Deficiency and Cardiovascular DiseaseDocumento14 pagineIron Deficiency and Cardiovascular DiseasemiguelalmenarezNessuna valutazione finora
- Cana y GotaDocumento9 pagineCana y GotamiguelalmenarezNessuna valutazione finora
- Review: Are Metabolically Healthy Overweight and Obesity Benign Conditions?Documento17 pagineReview: Are Metabolically Healthy Overweight and Obesity Benign Conditions?miguelalmenarezNessuna valutazione finora
- ACROMEGALIADocumento14 pagineACROMEGALIAmiguelalmenarezNessuna valutazione finora
- Macrovascular Complications in DiabetesDocumento31 pagineMacrovascular Complications in DiabetesmiguelalmenarezNessuna valutazione finora
- HipofosfatemiaDocumento12 pagineHipofosfatemiamiguelalmenarezNessuna valutazione finora
- Decision Study SorafenibDocumento10 pagineDecision Study SorafenibmiguelalmenarezNessuna valutazione finora
- Immune ThrombocytopeniaDocumento11 pagineImmune ThrombocytopeniamiguelalmenarezNessuna valutazione finora
- Elder AbuseDocumento10 pagineElder AbusemiguelalmenarezNessuna valutazione finora
- Control GlicemicoDocumento20 pagineControl GlicemicomiguelalmenarezNessuna valutazione finora
- Reviews: Liver Regeneration: Biological and Pathological Mechanisms and ImplicationsDocumento16 pagineReviews: Liver Regeneration: Biological and Pathological Mechanisms and ImplicationsmiguelalmenarezNessuna valutazione finora
- Relacion Colesterol Total A HDL y Colesterol No HD PDFDocumento9 pagineRelacion Colesterol Total A HDL y Colesterol No HD PDFJoseAbdalaNessuna valutazione finora
- Jaring, Josephine Rivera 1816085500Documento3 pagineJaring, Josephine Rivera 1816085500Analiza Rivera JaringNessuna valutazione finora
- D-Dyslipidemia by Prof. Abbas OrabiDocumento38 pagineD-Dyslipidemia by Prof. Abbas OrabiIbrahim NegmNessuna valutazione finora
- Jumlah Pemeriksaan Hematologi Dan KimiaDocumento46 pagineJumlah Pemeriksaan Hematologi Dan KimiaFernando PkpNessuna valutazione finora
- Dyslipidemia PDFDocumento40 pagineDyslipidemia PDFRISNessuna valutazione finora
- 150422009-Lipids CalibratorDocumento8 pagine150422009-Lipids CalibratorFahmiNessuna valutazione finora
- Perbedaan Kadar Kolesterol Sebelum Dan Sesudah Dilakukan Brisk Walking Pada SiswaDocumento8 paginePerbedaan Kadar Kolesterol Sebelum Dan Sesudah Dilakukan Brisk Walking Pada SiswaEva RahmadaniNessuna valutazione finora
- Hyperlipidemia 1Documento54 pagineHyperlipidemia 1MarrkNessuna valutazione finora
- Waktu Tunggu Hasil Lab MutuDocumento42 pagineWaktu Tunggu Hasil Lab MutuDwinoorNessuna valutazione finora
- RandoxDocumento32 pagineRandoxIta MaghfirahNessuna valutazione finora
- Asupan Gizi Dengan Pengendalian Diabetes Pada Diabetisi Tipe Ii Rawat Jalan Di Blu Prof - Dr.R.D.Kandou ManadoDocumento11 pagineAsupan Gizi Dengan Pengendalian Diabetes Pada Diabetisi Tipe Ii Rawat Jalan Di Blu Prof - Dr.R.D.Kandou Manadochafeb febiNessuna valutazione finora
- Metabolisme Lipoprotein Modul 2.2Documento72 pagineMetabolisme Lipoprotein Modul 2.2Rizky AkbarNessuna valutazione finora
- Papob GB-D 21 001 03.01 2013-01-02Documento4 paginePapob GB-D 21 001 03.01 2013-01-02Darko MaksimovicNessuna valutazione finora
- Introduction To Lipids and LipoproteinsDocumento24 pagineIntroduction To Lipids and LipoproteinsYoungFanjiensNessuna valutazione finora
- Agatabo K'umurwayi WordDocumento4 pagineAgatabo K'umurwayi WordMANIRAKIZA LUCNessuna valutazione finora
- 8 LipoproteinsDocumento12 pagine8 LipoproteinsSubhi Mishra100% (1)
- Lampiran Berita Acara Pemusnahan Reagen Dan Bahan Medis Habis Pakai Tanggal 28 Januari 2019Documento2 pagineLampiran Berita Acara Pemusnahan Reagen Dan Bahan Medis Habis Pakai Tanggal 28 Januari 2019HendroNessuna valutazione finora
- Pricelist AMSDocumento1 paginaPricelist AMSMimi AnugrahNessuna valutazione finora
- JURNALDocumento9 pagineJURNALAnnisa HasanahNessuna valutazione finora
- 91 158 1 SMDocumento6 pagine91 158 1 SMReyna AlshiraNessuna valutazione finora
- Mapping Urologi 26 November 2019Documento4 pagineMapping Urologi 26 November 2019ikkelNessuna valutazione finora
- ICU Report SheetDocumento1 paginaICU Report SheetjejadayoNessuna valutazione finora
- (UMA) ERBA XL-640 Basic Performance DataDocumento38 pagine(UMA) ERBA XL-640 Basic Performance DataKo KyoNessuna valutazione finora
- Lipoproteins: SummaryDocumento3 pagineLipoproteins: SummaryEzekiel Morena RiveraNessuna valutazione finora
- 1 PDFDocumento2 pagine1 PDFUnni Krishnan R GNessuna valutazione finora
- PPLDocumento20 paginePPLkcpamuletyNessuna valutazione finora
- HiperlipidemiaDocumento25 pagineHiperlipidemiaMarbungaran Putra GalinggingNessuna valutazione finora
- Plasma Lipoproteins - METABOLISM PDFDocumento4 paginePlasma Lipoproteins - METABOLISM PDFRishabhNessuna valutazione finora
- Abetalipoproteinemia Powerpoint ReportDocumento43 pagineAbetalipoproteinemia Powerpoint Reportpurpleflurp23Nessuna valutazione finora
- Instructor's Presentation-Lipids and LipoproteinsDocumento43 pagineInstructor's Presentation-Lipids and Lipoproteinsjomel rondinaNessuna valutazione finora