Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
1PSC OverviewCurrent
Caricato da
Ega Dwi IfaafahTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
1PSC OverviewCurrent
Caricato da
Ega Dwi IfaafahCopyright:
Formati disponibili
NHSN Overview
National Healthcare Safety Network (NHSN) Overview
The NHSN is a secure, internet-based surveillance system that expands and integrates
former CDC surveillance systems, including the National Nosocomial Infections
Surveillance System (NNIS), National Surveillance System for Healthcare Workers
(NaSH), and the Dialysis Surveillance Network (DSN). In addition, facilities that
participate in certain reporting programs operated by the Centers for Medicare and
Medicaid Services (CMS) can do so through use of NHSN. Some U.S. states utilize
NHSN as a means for healthcare facilities to submit data on healthcare-associated
infections (HAIs) mandated through their specific state legislation.
NHSN enables healthcare facilities to collect and use data about healthcare-associated
infections, adherence to clinical practices known to prevent healthcare-associated
infections, the incidence or prevalence of multidrug-resistant organisms within their
organizations, trends and coverage of healthcare personnel safety and vaccination, and
adverse events related to the transfusion of blood and blood products.
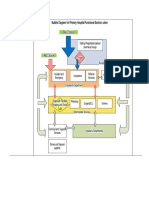
The NHSN includes three components: Patient Safety, Healthcare Personnel Safety, and
Biovigilance (Figure).
Figure NHSN Components
Within the Patient Safety Component, like-types of surveillance are grouped into
modules, each concerned with healthcare procedures, devices, or medications associated
with HAIs. Specific types of surveillance within the Patient Safety Component are
outlined below:
• Device-associated Module:
o CLABSI - Central line-associated bloodstream infection
o CLIP - Central line insertion practices adherence
o VAP - Ventilator-associated pneumonia
June, 2011 1-1
NHSN Overview
oCAUTI - Catheter-associated urinary tract infection
oDE - Dialysis Event
• Procedure-associated Module:
o SSI - Surgical site infection
o PPP - Post-procedure pneumonia
• Medication-associated Module:
o AUR - Antimicrobial use and resistance options
• Multidrug-Resistant Organism/Clostridium difficile Infection (MDRO/CDI)
Module
• Vaccination Module
Instructions and standardized surveillance methods and definitions for each module of the
Patient Safety Component are provided in this manual and on the NHSN website
(www.cdc.gov.gov/nhsn). Modules may be used singly or simultaneously and each
module has its own minimum time period for required participation (see individual
modules). Regardless of the combination of modules in which a facility chooses to
participate a total of 6 months of data must be reported annually to NHSN for continued
participation.
There are two modules within the Healthcare Personnel Safety (HPS) component of
NHSN: the Blood/Body Fluid Exposure Modules With and Without Exposure
Management and the Influenza Vaccination and Exposure Management Modules. The
Blood/Body Fluids Exposure and the Influenza Vaccination and Exposure Modules may
be used separately or simultaneously. Instructions and standardized surveillance methods
and definitions for each module are provided in the NHSN Manual: HPS Component
Protocol http://www.cdc.gov/nhsn/TOC_HPS_Manual.html
The Biovigilance Component of NHSN was developed in collaboration with the
transfusion and transplant communities. Biovigilance includes the collection of adverse
event data to improve outcomes in the use of blood products, organs, tissues, and cellular
therapies.
The Hemovigilance Module is the first part of the Biovigilance Component to be
developed in NHSN. This module is designed for staff in healthcare facility transfusion
services to track adverse events, including recipient adverse reactions and quality control
incidents, related to blood transfusion. Instructions and standardized surveillance method
and definitions for this module are provided in the NHSN Manual:
https://www.cdc.gov/nhsn/TOC-BIOManual.html.
Surveillance Techniques
Some of the options in the following modules require active, patient-based, prospective
surveillance of events and their corresponding denominator data by a trained Infection
June, 2011 1-2
NHSN Overview
Preventionist (IP). This means that the IP shall seek out infections during a patient’s stay
by screening a variety of data sources, such as laboratory, pharmacy,
admission/discharge/transfer, radiology/imaging, and pathology databases, and patient
charts, including history and physical exam notes, nurses/physicians notes, temperature
charts, etc. Others may be trained to screen data sources for these infections, but the IP
must make the final determination. Laboratory-based surveillance should not be used
alone, unless all possible criteria for identifying an infection are solely determined by
laboratory evidence (e.g., LabID event detection in the MDRO & CDI Module).
Retrospective chart reviews should be used only when patients are discharged before all
information can be gathered. NHSN forms should be used to collect all required data,
using the NHSN definitions of each data field. To minimize the IP’s data collection
burden, others may be trained to collect the denominator data and process of care data
(e.g., central line insertion and inpatient influenza vaccination information).
Procedure-Associated Module
Surgical site infection (SSI) and post-procedure pneumonia (PPP) monitoring is offered
through protocols in this module. Both protocols require active, patient-based,
prospective surveillance (see Surveillance Techniques above). PPP events are monitored
only for patients undergoing inpatient operative procedures and only during the patient’s
stay (i.e., post-discharge surveillance methods are not used for PPP). However both post-
discharge and ante-discharge surveillance methods should be used to detect SSIs
following in- and outpatient operative procedures. These methods include 1) direct
examination of patients’ wounds during follow-up visits to either surgery clinics or
physicians’ offices, 2) review of medical records or surgery clinic patient records, 3)
surgeon surveys by mail or telephone, and 4) patient surveys by mail or telephone
(though patients may have a difficult time assessing their infections). Any combination
of these methods is acceptable for use; however, CDC criteria for SSI must be used. To
minimize IPs’ workload of collecting denominator data, operating room data may be
downloaded (see file specifications at:
http://www.cdc.gov/nhsn/PDFs/ImportingProcedureData_current.pdf.
See the SSI and PPP protocols for detailed surveillance instructions.
Device-Associated Module
Medical instrumentation increases the risk of development of an HAI and most patients
admitted for health care are exposed to some kind of medical device in the course of their
treatment. Such devices include, but are not limited to, venous and urinary catheters, and
ventilators. NHSN enables facilities to monitor infectious complications associated with
the use of these devices and also to monitor processes related to their use which might
increase infection risk. Specifically, surveillance of Central Line-associated Bloodstream
Infection (CLABSI), Catheter-associated UTI (CAUTI), Dialysis Event (DE) and/or
Ventilator-associated Pneumonia (VAP) is possible using the NHSN. In addition,
June, 2011 1-3
NHSN Overview
Central Line Insertion Practices (CLIP) can be monitored to inform facilities of the
appropriateness of their processes and how they may relate to HAI development.
Device-associated denominator data should be collected at the same time each day.
When denominator data are available from electronic databases (e.g., ventilator days
from respiratory therapy), these sources may be used as long as the counts are not
substantially different (+/- 5%) from manually collected counts.
See the respective device-associated protocols for detailed surveillance instructions.
Medication-Associated Module
The use of antimicrobial agents has a direct effect on antimicrobial resistance patterns of
pathogens. The observed increase in multidrug resistance is in part due to inappropriate
prescription of, as well as incomplete completion of, courses of antibiotics.
The Medication-Associated Module allows facilities to collect information on the amount
of antimicrobials that are utilized for patient care within their systems, as well as to
collect data on the prevalence of drug-resistant organisms in their inpatient and outpatient
areas. Electronic capture of microbiology and pharmacy data is the available option for
this module.
See the Antimicrobial Use and Resistance (AUR) protocol for detailed surveillance
instructions.
Multidrug-resistant Organism & Clostridium difficile Infection (MDRO/CDI)
Module
The NHSN MDRO/CDI Module offers a means for facilities to meet criteria and metrics
that are outlined in several organizational guidelines to control and measure the spread of
MDROs and CDI within their healthcare system. The module has both required and
optional surveillance activities that can be tailored to the needs of the facility. Infection
surveillance and monitoring of proxy infection measures are choices available to facilities
choosing to participate in this program within NHSN.
In addition, process measures related to adherence to contact precautions when caring for
patients infected or colonized with an MDRO or C. difficile, and/or active surveillance
testing for such organisms, or outcome measurements of incidence and prevalence of
positive cultures of these organisms in patients can be undertaken.
See the MDRO/CDI protocol for detailed surveillance instructions in this manual.
June, 2011 1-4
NHSN Overview
Vaccination Module
Influenza continues to be associated with increased morbidity and mortality in certain
patient populations including the very young, elderly, immunocompromised, and
pregnant women. Hospitalization has been identified as a potential opportunity to
provide influenza immunization not only to these at-risk individuals, but also to any
patient.
The NHSN Vaccination module offers a means for facilities to track the success of
capitalizing on influenza vaccination opportunities. Two options are available related to
patient susceptibility and adherence to vaccination recommendations.
See the Vaccination protocol for detailed surveillance instructions.
June, 2011 1-5
Potrebbero piacerti anche
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (344)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (399)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (73)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- Confidential: Iom Minimum Medical Review QuestionnaireDocumento3 pagineConfidential: Iom Minimum Medical Review QuestionnairePochieNessuna valutazione finora
- Triad Color TestDocumento37 pagineTriad Color TestC.O.M.A research -stopalienabduction-0% (1)
- Fda VapDocumento34 pagineFda VapEga Dwi IfaafahNessuna valutazione finora
- Formula Untuk Surveilans HemodialisaDocumento4 pagineFormula Untuk Surveilans HemodialisaEga Dwi IfaafahNessuna valutazione finora
- Formula Untuk Surveilans HemodialisaDocumento4 pagineFormula Untuk Surveilans HemodialisaEga Dwi IfaafahNessuna valutazione finora
- Caut I Guideline 2009 FinalDocumento67 pagineCaut I Guideline 2009 FinalWendi LukeNessuna valutazione finora
- .PDF MRSA 1Documento24 pagine.PDF MRSA 1Ega Dwi IfaafahNessuna valutazione finora
- 2 HPS IntroDocumento1 pagina2 HPS IntroEga Dwi IfaafahNessuna valutazione finora
- 13vaksinasi Urrent PDFDocumento9 pagine13vaksinasi Urrent PDFEga Dwi IfaafahNessuna valutazione finora
- IHI Hand HygieneDocumento32 pagineIHI Hand HygieneIan Mizzel A. DulfinaNessuna valutazione finora
- Barang MasukDocumento1 paginaBarang MasukEga Dwi IfaafahNessuna valutazione finora
- 01 Central Lines How To Guide PDFDocumento40 pagine01 Central Lines How To Guide PDFEga Dwi IfaafahNessuna valutazione finora
- 2 HPS IntroDocumento1 pagina2 HPS IntroEga Dwi IfaafahNessuna valutazione finora
- .PDF MRSA 1Documento24 pagine.PDF MRSA 1Ega Dwi IfaafahNessuna valutazione finora
- Process Safety and Environmental Protection 2009 Volume 87, Number 1Documento2 pagineProcess Safety and Environmental Protection 2009 Volume 87, Number 1jenanboyzNessuna valutazione finora
- Dosage and SolutionsDocumento3 pagineDosage and SolutionsNicole PageNessuna valutazione finora
- ADHERENCE and INVASION ASSAYS A092Documento2 pagineADHERENCE and INVASION ASSAYS A092Samrah Anwar0% (2)
- Master in Public Administration (MPA)Documento106 pagineMaster in Public Administration (MPA)Mg OoNessuna valutazione finora
- Caries Risk Assesment: Disha Jandial Mds 1 Year Deptt. of Pediatrics and Preventive DentistryDocumento134 pagineCaries Risk Assesment: Disha Jandial Mds 1 Year Deptt. of Pediatrics and Preventive Dentistrydisha 146jandial100% (1)
- Advt. 4 Year 2022 - WebsiteDocumento6 pagineAdvt. 4 Year 2022 - WebsiteprakashNessuna valutazione finora
- Effects of Smoking and Alcohol On Complete Denture PatientsDocumento2 pagineEffects of Smoking and Alcohol On Complete Denture PatientsAshwin ThejaswiNessuna valutazione finora
- BR PDF Ad M2 2015Documento74 pagineBR PDF Ad M2 2015jamesNessuna valutazione finora
- MTP Petitioner FinalDocumento19 pagineMTP Petitioner FinalDeepu KumarNessuna valutazione finora
- Bereavement SupportDocumento26 pagineBereavement SupportAdiAri RosiuNessuna valutazione finora
- Project PrimaryDocumento7 pagineProject PrimaryLisanwork HonseboNessuna valutazione finora
- Osha Note Topic 1Documento49 pagineOsha Note Topic 1Hasnol HaiqalNessuna valutazione finora
- Purpose 1Documento4 paginePurpose 1Rizzah MagnoNessuna valutazione finora
- Intracranial Pressure: Current Perspectives On Physiology and MonitoringDocumento11 pagineIntracranial Pressure: Current Perspectives On Physiology and MonitoringWander ValentimNessuna valutazione finora
- Connections Issue 11Documento21 pagineConnections Issue 11Victoria University, Melbourne, AustraliaNessuna valutazione finora
- Drug Study SymbicortDocumento3 pagineDrug Study Symbicortaldwinng100% (3)
- PLLDocumento24 paginePLLjagannnathdNessuna valutazione finora
- AsthmaDocumento10 pagineAsthmaAcohCChaoNessuna valutazione finora
- Jurnal GadarDocumento6 pagineJurnal GadarLycia Dwi LindiyaniNessuna valutazione finora
- Tneb Nhis 2021 BP No 162 17721Documento166 pagineTneb Nhis 2021 BP No 162 17721Gnaneswaran SubramanianNessuna valutazione finora
- German Marine Agencies Inc Vs NLRC 142049 JanuaryDocumento14 pagineGerman Marine Agencies Inc Vs NLRC 142049 JanuaryJennyNessuna valutazione finora
- Notice, Caution, Warning Evaluation I Ix Graders: Acces For Residents OnlyDocumento2 pagineNotice, Caution, Warning Evaluation I Ix Graders: Acces For Residents OnlyRizqiyah SakinahNessuna valutazione finora
- ICNCDRS Blindness Registry FormDocumento4 pagineICNCDRS Blindness Registry FormAprilAngeliRobleNessuna valutazione finora
- Lung Collapse PDFDocumento65 pagineLung Collapse PDFRibhav GuptaNessuna valutazione finora
- Applying 5S ProceduresDocumento70 pagineApplying 5S ProceduresSanta Best100% (3)
- What's On - Abu Dhabi - August 2011Documento100 pagineWhat's On - Abu Dhabi - August 2011motivatepublishingNessuna valutazione finora
- Dr. Mohamed Ali Hamedh - DKA - 2023Documento25 pagineDr. Mohamed Ali Hamedh - DKA - 2023ÁýáFáŕőúgNessuna valutazione finora
- 2017-02-23 Calvert County TimesDocumento24 pagine2017-02-23 Calvert County TimesSouthern Maryland OnlineNessuna valutazione finora