Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Manual 057 Trending of Stability Data
Caricato da
Haroon RasheedCopyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Manual 057 Trending of Stability Data
Caricato da
Haroon RasheedCopyright:
Formati disponibili
Manual 057 Trending of Stability Data
1 Purpose
The purpose of this international guideline is to outline minimum mandatory
requirements as well as recommendations for the identification and reaction to
trends found in stability data.
2 Scope and Applicability
This guideline is applicable to all Commercial Stability Sites and contractors
performing stability studies on commercial drug substances and drug products.
3 Definitions
3.1 Out of Specification (OOS) Result
A laboratory test result that is outside its regulatory or compendial limits.
3.2 Trend
A pattern of data that indicates change over time. This data may demonstrate
either an increasing or decreasing trend (change of mean) for the stability
indicating parameter over time or the data may indicate no discernible change at
all. The change may be linear or non-linear.
3.3 Trending in Stability Studies
The evaluation of stability data (not necessarily statistical) in order to identify
trends and their impact on the stability of a product.
3.3 Significant Trend
An average typical trend for a parameter that, in relation to release result
variability and specification limits, may lead to an OOS result before or at end of
shelf life for any batch released. The definition of the criterion for significant
trend might differ between products, but could for example be defined as a
certain proportion of the specification interval.
3.4 Out of Trend (OOT)
A single result or a number of results that do not follow the expected trend, for a
particular batch or series of batches, either in comparison with other stability
studies or with respect to previous results collected during a stability study. There
are three types of OOT situations identified in this guideline: Atypical result,
Atypical trend and Adverse trend.
3.5 Atypical Result
A single result that does not follow the expected trend for a stability indicating
parameter compared to previous results from the same study.
Copyright©www.gmpsop.com. All rights reserved
Unauthorized copying, publishing, transmission and distribution of any part of the content by
electronic means are strictly prohibited. Page 2 of 16
Manual 057 Trending of Stability Data
Inform QP/senior QA Management at the site managing the contractor, if a
significant trend has been observed
Report adverse trends according to QA Agreement
4.3 Lead Site
It is the responsibility of the Lead Team/Site (acting as the Commercial Stability Site)
assigned to a contractor to ensure that the contractor has procedures in place to
comply with this guideline. This responsibility shall be clearly documented in the
relevant Quality Assurance Agreement.
4.4 QP/Senior QA Management
It is the responsibility of QP/senior QA Management at the formulation site, or
site managing the contractor to:
Review the outcome of the trending reports issued by the Commercial
Stability Site or contractor
Ensure that the product release specification is constructed in a way that
provides a high probability that any batch released will remain within the
registered specification throughout the retest period/shelf-life of the drug
substance/drug product when stored according to the labeled storage
conditions
React to reported adverse trends shown by a single batch or series of
batches according to procedure.
5 Guideline
5.1 Introduction
The registered retest period/shelf life of a drug substance/product will have been
set taking into account the specification to be registered and the trends seen in
stability studies completed or ongoing at the time of new product and/or new
primary pack registration. However, these studies will have been conducted on
relatively few batches, some made only at pilot scale.
Therefore, the Commercial Stability Site must use trending of stability data
to support the retest period/shelf-life of a drug substance/product as well as
to indicate when a change to retest period/shelf-life and/or cautionary labeling
statement is required. Trending must also be used as a tool to identify significant
trends and recommend release alert limits when necessary.
The formulation site must take appropriate action to ensure that drug substance/
product release procedures are updated to take into account the latest stability trend
analysis reports issued by the Commercial Stability Site.
Copyright©www.gmpsop.com. All rights reserved
Unauthorized copying, publishing, transmission and distribution of any part of the content by
electronic means are strictly prohibited. Page 4 of 16
Manual 057 Trending of Stability Data
post change stability data must,
when sufficient data is available, be considered to replace the reference data
as the basis for setting or revising registered release limits or release alert
limits.
5.4 Identification of OOTs
When new results from a stability study are available, the data must be evaluated as
typical or atypical against the reference data. A visual inspection of the data, for
example by using a scatter plot, may be sufficient to identify typical or atypical trends
and to predict adverse trends. If a visual inspection is not sufficient, further statistical
evaluation should be initiated.
It is important to take into consideration the amount of data that is being evaluated, as
it may be too early in the study to have sufficient data to provide confidence that OOT
predictions are accurate.
5.4.1 Atypical result
The review of individual results should be performed as soon as possible after the
results have been obtained and ideally by the responsible laboratory. By doing so
it is possible to either confirm an atypical result or identify the probable cause of
the atypical result.
An atypical result is observed when a single result, while within specification
limits, is aberrant, i.e. outside normal analytical and sampling variation as well as
exhibiting a difference in the typical change over time. (See figure 1-2, appendix 1).
When an analytical result appears to be atypical during evaluation of stability data,
the actions must follow the steps outlined in appendix 2. As the first step, the
analytical result must be reviewed to confirm the result as atypical or due to an
analytical error. If an atypical result is confirmed, the probability of an OOS must be
evaluated. The minimum action required when an atypical result is identified, is to
monitor the next time point. The need for adding an extra time point ahead of the next
scheduled time point must also be considered.
5.4.2 Atypical trend and/or adverse trend
Examples of atypical trend situations are when one or more of the following
events occur:
1. A series of results within a study show a trend that is different in
comparison with the trend of other studies
2. At least two consecutive results within a study are confirmed as atypical
3. An atypical result occurs in at least two studies
Examples of atypical and adverse trends are illustrated in simple scatter plots in
appendix 3.
When an atypical trend is identified, the actions must follow the steps outlined in
Copyright©www.gmpsop.com. All rights reserved
Unauthorized copying, publishing, transmission and distribution of any part of the content by
electronic means are strictly prohibited. Page 6 of 16
Manual 057 Trending of Stability Data
according to the specification, especially for parameters like degradation products
and impurities.
5.4.4 Statistical evaluation
The data should be presented initially as a scatter plot, allowing simple visual
inspection of the characteristics of the parameter over time. Make sure that the
time axis is correct. This method can be used to identify both atypical results and
atypical trends by comparison with normal/typical stability results both within
and between studies.
If appropriate, the individual results or batches that are identified as atypical
during visual inspection may be more closely evaluated using more sophisticated
statistical methods.
Comparisons with other data at the corresponding time point or comparisons of
slopes or other parameters that quantify the change rate are some examples. The
model used depends on the data that is being evaluated.
5.4.5 Stability Study Alert Limits
Alert limits for stability data could be either non-statistical (absolute settings) or
statistical (based on reference data). Alert limits, based on reference data, can be
used as an aid in identifying atypical results or atypical trends.
When setting alert limits it is recommended that a statistical approach is used.
There are several different methods, although none of them can be generally
Applied.
The following items should however generally be considered:
Appropriate reference data (representative for the normal stability profile
of the product’s stability indicating parameter).
Availability and amount of data. Can data from several product/package
combinations be used as reference? Type of data (single, multiple, near
detection limits).
Appropriate model fitted to the normal degradation profile (e.g. linear or
non-linear).
Time points. When can action be taken?
Choosing a suitable level of risk (avoiding false alarms).
Logistics of implementation (different market specifications, pack sizes,
strengths, time points etc.).
Adequate precision, i.e. enough decimal places to enable evaluation.
Once stability study alert limits have been implemented, they must be
Copyright©www.gmpsop.com. All rights reserved
Unauthorized copying, publishing, transmission and distribution of any part of the content by
electronic means are strictly prohibited. Page 8 of 16
Manual 057 Trending of Stability Data
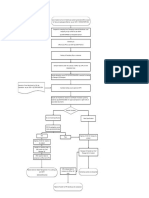
6.2 Appendix 2 Atypical Result investigation
Manual for OOS result
investigation
Copyright©www.gmpsop.com. All rights reserved
Unauthorized copying, publishing, transmission and distribution of any part of the content by electronic means are strictly prohibited. Page 11 of 16
Manual 057 Trending of Stability Data
Copyright©www.gmpsop.com. All rights reserved
Unauthorized copying, publishing, transmission and distribution of any part of the content by
electronic means are strictly prohibited. Page 13 of 16
Manual 057 Trending of Stability Data
Copyright©www.gmpsop.com. All rights reserved
Unauthorized copying, publishing, transmission and distribution of any part of the content by
electronic means are strictly prohibited. Page 15 of 16
Potrebbero piacerti anche
- Practical Approaches to Method Validation and Essential Instrument QualificationDa EverandPractical Approaches to Method Validation and Essential Instrument QualificationNessuna valutazione finora
- Handling of Oot ResultsDocumento5 pagineHandling of Oot ResultstghonsNessuna valutazione finora
- Hold Time Stability Studies in Pharmaceutical Industry Review 2167 7689.1000104Documento8 pagineHold Time Stability Studies in Pharmaceutical Industry Review 2167 7689.1000104iloveit52252Nessuna valutazione finora
- Stability Studies Assessment ExperienceDocumento61 pagineStability Studies Assessment ExperienceDANIBATANessuna valutazione finora
- Data Integrity and Compliance: A Primer for Medical Product ManufacturersDa EverandData Integrity and Compliance: A Primer for Medical Product ManufacturersNessuna valutazione finora
- CHECKLIST Q3-2 2013b PDFDocumento13 pagineCHECKLIST Q3-2 2013b PDFJoe Luis Villa MedinaNessuna valutazione finora
- Dissolution Case StudiesDocumento70 pagineDissolution Case Studieslhthang1990100% (1)
- Pharmaceutical Product Quality Assurance Through CMC Drug Development ProcessDocumento20 paginePharmaceutical Product Quality Assurance Through CMC Drug Development Processpharmashri5399Nessuna valutazione finora
- Temperature Mapping Protocol: Lincoln Parenteral LTDDocumento7 pagineTemperature Mapping Protocol: Lincoln Parenteral LTDRajender SinghNessuna valutazione finora
- USP-NF 1251 Weighing On An Analytical BalanceDocumento6 pagineUSP-NF 1251 Weighing On An Analytical BalanceMinh LêNessuna valutazione finora
- 01 Cleaning Validation of Vibratory Sifter CLV 01Documento2 pagine01 Cleaning Validation of Vibratory Sifter CLV 01Ravi YadavNessuna valutazione finora
- Hold Time Study 1Documento3 pagineHold Time Study 1aboemadaldeenNessuna valutazione finora
- 01 LC Vibratory Sifter 01Documento2 pagine01 LC Vibratory Sifter 01Ravi YadavNessuna valutazione finora
- Operating Procedure of Fume Hood Model No.: EDU1200ABP, ChinaDocumento4 pagineOperating Procedure of Fume Hood Model No.: EDU1200ABP, ChinaBejoy KarimNessuna valutazione finora
- Omgoing Stability Testing - Innovations - in - Pharmaceutical - TechnologyDocumento3 pagineOmgoing Stability Testing - Innovations - in - Pharmaceutical - TechnologyJuan RojasNessuna valutazione finora
- 2 Process Validation QandA Version 4 (June 2011) - Adopted 18th PPWG Meeting PDFDocumento4 pagine2 Process Validation QandA Version 4 (June 2011) - Adopted 18th PPWG Meeting PDFVishal SomaniNessuna valutazione finora
- Stability Register 01Documento1 paginaStability Register 01kanji63Nessuna valutazione finora
- Stability Studies - An Overview: - What Is Stability? - Why It Is Required? - What Are The Guidelines?Documento24 pagineStability Studies - An Overview: - What Is Stability? - Why It Is Required? - What Are The Guidelines?VidyaNessuna valutazione finora
- Amlodipine and Celecoxib Tablets PDFDocumento38 pagineAmlodipine and Celecoxib Tablets PDFNarongchai PongpanNessuna valutazione finora
- Analytical Report For Ketofast 10 TabletDocumento20 pagineAnalytical Report For Ketofast 10 TabletShagorShagorNessuna valutazione finora
- LAB 130 Stability Management Procedure SampleDocumento3 pagineLAB 130 Stability Management Procedure SampleOdunlamiNessuna valutazione finora
- Annex 4 WHO Guidelines 4 Sampling of Pharmaceutical ProductsDocumento23 pagineAnnex 4 WHO Guidelines 4 Sampling of Pharmaceutical ProductsJakobus Benny SalimNessuna valutazione finora
- Oos SopDocumento10 pagineOos SopSolomonNessuna valutazione finora
- OOS调差Documento17 pagineOOS调差Smartishag BediakoNessuna valutazione finora
- 3-Test Report of Amlodipine Besilate 3Documento1 pagina3-Test Report of Amlodipine Besilate 3ShagorShagorNessuna valutazione finora
- Stability Data - ConcordDocumento127 pagineStability Data - Concordтатьяна васильковаNessuna valutazione finora
- Stability ReportDocumento9 pagineStability ReportShagorShagorNessuna valutazione finora
- DEC Study in Formulation DevelopmentDocumento9 pagineDEC Study in Formulation Developmentfad12345Nessuna valutazione finora
- Cream & Ointment Process ValidationDocumento3 pagineCream & Ointment Process Validationtajmir haqueNessuna valutazione finora
- Guide To Inspections of Pharmaceutical Quality Control LaboratoriesDocumento16 pagineGuide To Inspections of Pharmaceutical Quality Control Laboratoriessubrata1Nessuna valutazione finora
- Pharmaceutical Master Validation Plan The Syed Imtiaz 57743816Documento3 paginePharmaceutical Master Validation Plan The Syed Imtiaz 57743816Dandung RuskarNessuna valutazione finora
- Yield Investigation ReportDocumento1 paginaYield Investigation ReportNishant ShresthaNessuna valutazione finora
- 04 Cleaning Validation of Empty Capsule Loader 04Documento2 pagine04 Cleaning Validation of Empty Capsule Loader 04Ravi YadavNessuna valutazione finora
- 20.SOP-Code Structure For IndicatorDocumento3 pagine20.SOP-Code Structure For IndicatorBejoy Karim50% (2)
- SOP For Reduce Testing For Raw MaterialDocumento3 pagineSOP For Reduce Testing For Raw MaterialMubarak Patel100% (1)
- Forced DegradationDocumento8 pagineForced DegradationBiyaya San PedroNessuna valutazione finora
- QBD AnalyticalDocumento17 pagineQBD Analyticalqbdresearch labNessuna valutazione finora
- TEM 115 ProtocolRework Manufactured Finished Goods SampleDocumento1 paginaTEM 115 ProtocolRework Manufactured Finished Goods SampleOmnia ElshafieNessuna valutazione finora
- Annual Product Quality ReviewDocumento3 pagineAnnual Product Quality ReviewPharmacist100% (1)
- QCD-036-01 Good Chromatography PracticesDocumento12 pagineQCD-036-01 Good Chromatography Practicesarnab rayNessuna valutazione finora
- Example RA For Transport To Regulated MarketsDocumento7 pagineExample RA For Transport To Regulated MarketsDoan Chi ThienNessuna valutazione finora
- #2 ISPE Schedule L1Documento48 pagine#2 ISPE Schedule L1ananthNessuna valutazione finora
- Forced Degradation - Mass BalanceDocumento8 pagineForced Degradation - Mass BalanceppiccoliniNessuna valutazione finora
- SMF LAW Online2017 enDocumento31 pagineSMF LAW Online2017 enAnonymous cZ0Sn4hxF100% (1)
- Report Esomeprazole IV Inj (Guide Batch 03)Documento60 pagineReport Esomeprazole IV Inj (Guide Batch 03)Sari Widya Astuti SelianNessuna valutazione finora
- Asean Process Validation GuidelineDocumento6 pagineAsean Process Validation GuidelineWilliam Chandra100% (1)
- Pharmaceutical Quality Control Labs (7 - 93) - FDADocumento15 paginePharmaceutical Quality Control Labs (7 - 93) - FDAdnalokeshNessuna valutazione finora
- The Sex Influence On PharmacokineticDocumento106 pagineThe Sex Influence On PharmacokineticIuliaCNessuna valutazione finora
- Handling of Out of Specification ResultsDocumento39 pagineHandling of Out of Specification ResultsDevang GondaliyaNessuna valutazione finora
- Sop 003 For Analysis of Rinse SampleDocumento3 pagineSop 003 For Analysis of Rinse Samplevasant ugale100% (1)
- Lean Stability: Global Regulatory Reception - Successes and Challenges of Recent Case StudiesDocumento19 pagineLean Stability: Global Regulatory Reception - Successes and Challenges of Recent Case StudiesMartin CelestinoNessuna valutazione finora
- Out of SpecificationDocumento13 pagineOut of SpecificationBhupendra Tomar0% (1)
- Understand The Difference Between Verification and ValidationDocumento6 pagineUnderstand The Difference Between Verification and ValidationAsel Juárez ViteNessuna valutazione finora
- 02 LC of Double Cone Blender 02Documento2 pagine02 LC of Double Cone Blender 02Ravi YadavNessuna valutazione finora
- Pharmacogenomics: Learning ObjectivesDocumento16 paginePharmacogenomics: Learning ObjectivesJamilNessuna valutazione finora
- Consern Pharma Limited, Ludhiana: Focal Point, V.P.O. Tibba, District Ludhiana-141120, (Punjab), India 1 of 7Documento7 pagineConsern Pharma Limited, Ludhiana: Focal Point, V.P.O. Tibba, District Ludhiana-141120, (Punjab), India 1 of 7ASHIMA SHARMANessuna valutazione finora
- In-Use Stability TestingDocumento3 pagineIn-Use Stability TestingIsabelLópezNessuna valutazione finora
- Hold Time Study Protocol OF Cleaned Manufacturing Equipment Awaiting For UseDocumento11 pagineHold Time Study Protocol OF Cleaned Manufacturing Equipment Awaiting For UseMarwa AhmedNessuna valutazione finora
- 2 CGMP Meeting d1s3 Production Shah v3.0Documento58 pagine2 CGMP Meeting d1s3 Production Shah v3.0vsvsuresh2099100% (1)
- Alacc Guide 2008Documento18 pagineAlacc Guide 2008Karen Kawanishi100% (1)
- Delta Quality Presentation PDFDocumento13 pagineDelta Quality Presentation PDFAmanjot singh boparaiNessuna valutazione finora
- Quality Manual (ISO-9001)Documento32 pagineQuality Manual (ISO-9001)Haroon RasheedNessuna valutazione finora
- Quality ManualDocumento7 pagineQuality ManualFA KhanNessuna valutazione finora
- Calculation Tool For The PVT of Dissolution AssembliesDocumento1 paginaCalculation Tool For The PVT of Dissolution AssembliesMeylinda Kartika SariNessuna valutazione finora
- ChemicalsDocumento1 paginaChemicalsHaroon RasheedNessuna valutazione finora
- Photostability BujjiKanchiDocumento26 paginePhotostability BujjiKanchiHaroon RasheedNessuna valutazione finora
- Cleaning Validation Michael Payne PDFDocumento47 pagineCleaning Validation Michael Payne PDFAhmad ZaidiNessuna valutazione finora
- Assay of LH0003 T Limits: Stage 1 Stage 2: WT (MG) 10 Units WT (MG) 10 Units WT (MG) 10 UnitsDocumento4 pagineAssay of LH0003 T Limits: Stage 1 Stage 2: WT (MG) 10 Units WT (MG) 10 Units WT (MG) 10 UnitsHaroon RasheedNessuna valutazione finora
- Auditing Qms p1Documento53 pagineAuditing Qms p1Ahmad Imran100% (1)
- PV CalculatorDocumento18 paginePV CalculatorHaroon RasheedNessuna valutazione finora
- Trend Analysis Sheet OOSDocumento11 pagineTrend Analysis Sheet OOSHaroon RasheedNessuna valutazione finora
- Assay of LH0003 T Limits: Stage 1 Stage 2: WT (MG) 10 Units WT (MG) 10 Units WT (MG) 10 UnitsDocumento4 pagineAssay of LH0003 T Limits: Stage 1 Stage 2: WT (MG) 10 Units WT (MG) 10 Units WT (MG) 10 UnitsHaroon RasheedNessuna valutazione finora
- Assay of LH0003 T Limits: Stage 1 Stage 2: WT (MG) 10 Units WT (MG) 10 Units WT (MG) 10 UnitsDocumento4 pagineAssay of LH0003 T Limits: Stage 1 Stage 2: WT (MG) 10 Units WT (MG) 10 Units WT (MG) 10 UnitsHaroon RasheedNessuna valutazione finora
- Annex A Step by Step Guide For ISO 9001 2015 NG FG AGDocumento39 pagineAnnex A Step by Step Guide For ISO 9001 2015 NG FG AGRt Saragih100% (1)
- Cleaning Validation Michael Payne PDFDocumento47 pagineCleaning Validation Michael Payne PDFAhmad ZaidiNessuna valutazione finora
- PV CalculatorDocumento18 paginePV CalculatorHaroon RasheedNessuna valutazione finora
- LCMS Trouble ShootingDocumento68 pagineLCMS Trouble ShootingLuis VilchezNessuna valutazione finora
- Trend Analysis Sheet OOSDocumento11 pagineTrend Analysis Sheet OOSHaroon RasheedNessuna valutazione finora
- Assay of LH0003 T Limits: Stage 1 Stage 2: WT (MG) 10 Units WT (MG) 10 Units WT (MG) 10 UnitsDocumento4 pagineAssay of LH0003 T Limits: Stage 1 Stage 2: WT (MG) 10 Units WT (MG) 10 Units WT (MG) 10 UnitsHaroon RasheedNessuna valutazione finora
- PT Protocol HPLCDocumento11 paginePT Protocol HPLCHaroon RasheedNessuna valutazione finora
- Annex A Step by Step Guide For ISO 9001 2015 NG FG AGDocumento39 pagineAnnex A Step by Step Guide For ISO 9001 2015 NG FG AGRt Saragih100% (1)
- PMDocumento2 paginePMHaroon RasheedNessuna valutazione finora
- LCMS Trouble ShootingDocumento68 pagineLCMS Trouble ShootingLuis VilchezNessuna valutazione finora
- Handling of Out of Specification Results: International Journal of Pharmaceutical Quality Assurance April 2015Documento8 pagineHandling of Out of Specification Results: International Journal of Pharmaceutical Quality Assurance April 2015Haroon RasheedNessuna valutazione finora
- GC 2010 ShimadzuDocumento0 pagineGC 2010 ShimadzuAlfonso MartínezNessuna valutazione finora
- PM V2 PDFDocumento1 paginaPM V2 PDFHaroon RasheedNessuna valutazione finora
- PM V2 PDFDocumento1 paginaPM V2 PDFHaroon RasheedNessuna valutazione finora
- GC 2010 ShimadzuDocumento0 pagineGC 2010 ShimadzuAlfonso MartínezNessuna valutazione finora
- Stata Book - Manual - Panel Data AnalysisDocumento35 pagineStata Book - Manual - Panel Data AnalysisIzaniey Ismail100% (4)
- The Forrester Wave Enterprise Architecture Management Suites Q1 2021Documento15 pagineThe Forrester Wave Enterprise Architecture Management Suites Q1 2021Beatriz CostaNessuna valutazione finora
- Researching and Writing A Dissertation An Essential Guide For Business Students PDFDocumento8 pagineResearching and Writing A Dissertation An Essential Guide For Business Students PDFPaperHelperUKNessuna valutazione finora
- Has The COVID-19 Pandemic Affected The SusceptibilDocumento9 pagineHas The COVID-19 Pandemic Affected The SusceptibilSofiJohanaNessuna valutazione finora
- Maternal and Child Undernutrition 1 LancetDocumento18 pagineMaternal and Child Undernutrition 1 LancetAnonymous QLxHwZ0LTKNessuna valutazione finora
- Detailed Urban Land Use Land Cover ClassificationDocumento24 pagineDetailed Urban Land Use Land Cover ClassificationZaman RaiNessuna valutazione finora
- The Theory of KnowledgeDocumento22 pagineThe Theory of KnowledgeLuna Hernandez Garcia100% (1)
- List of Topics For Middle School Research PapersDocumento7 pagineList of Topics For Middle School Research Papersfwuhlvgkf100% (1)
- Graduation ProjectDocumento20 pagineGraduation ProjectSylvia Al-a'maNessuna valutazione finora
- Mahindra Tractors - Promotion of New Product Yuvraj215 - Summer Training ReportDocumento86 pagineMahindra Tractors - Promotion of New Product Yuvraj215 - Summer Training Reportneha satish pawarNessuna valutazione finora
- S - 1263873971.02 mkt00720 - SG - E06 - EnabledDocumento142 pagineS - 1263873971.02 mkt00720 - SG - E06 - EnablednazruNessuna valutazione finora
- Control System Design - An Introduction To State-Space Methods - FriedlandDocumento97 pagineControl System Design - An Introduction To State-Space Methods - FriedlandAnderson F Miranda SilvaNessuna valutazione finora
- 130 339 1 PBDocumento12 pagine130 339 1 PBSari YantiNessuna valutazione finora
- Gated Communities Social Sustainability 10Documento157 pagineGated Communities Social Sustainability 10Gabriela Souza67% (3)
- The Use of Indonesian and Slang in The Younger Generation: 2021 Laguage Aducation Study ProgramDocumento22 pagineThe Use of Indonesian and Slang in The Younger Generation: 2021 Laguage Aducation Study Programandi fachrulNessuna valutazione finora
- VS 4 S/Na 3 Poor 2 Not Satisfactory 1Documento4 pagineVS 4 S/Na 3 Poor 2 Not Satisfactory 1Anonymous 7BpT9OWPNessuna valutazione finora
- Bid Evaluation PDFDocumento3 pagineBid Evaluation PDFCUTto1122Nessuna valutazione finora
- Impact of Buying Behavior of Youth Towards Cosmetic Products in Perambalur TownDocumento10 pagineImpact of Buying Behavior of Youth Towards Cosmetic Products in Perambalur TownDeepuNessuna valutazione finora
- Learning-Plan-in-Law-144 - Labor Law 1 - SJARVDocumento7 pagineLearning-Plan-in-Law-144 - Labor Law 1 - SJARVSteps RolsNessuna valutazione finora
- S. B. No. 2573: Second Regular SessionDocumento13 pagineS. B. No. 2573: Second Regular SessionmkoguicoNessuna valutazione finora
- Shsa1105 - Unit-III Course MaterialDocumento57 pagineShsa1105 - Unit-III Course MaterialMaadhavaNessuna valutazione finora
- Hovercraft-Lab ReportDocumento2 pagineHovercraft-Lab ReportRudi HartonoNessuna valutazione finora
- Parasocial by Salwa AminDocumento17 pagineParasocial by Salwa AminSalwa AminNessuna valutazione finora
- 485Documento80 pagine485Bqdcc6Nessuna valutazione finora
- Forgiveness, Personality and Gratitude: Short CommunicationDocumento11 pagineForgiveness, Personality and Gratitude: Short CommunicationOana MariaNessuna valutazione finora
- Artificial Intelligence For Clinical OncologyDocumento23 pagineArtificial Intelligence For Clinical OncologyJuan Fernando Méndez RamírezNessuna valutazione finora
- Information Systems Analysis: Topic 7: Process-Oriented IS MethodologiesDocumento24 pagineInformation Systems Analysis: Topic 7: Process-Oriented IS MethodologiesAkuzike NgukuNessuna valutazione finora
- Thesis Ibanag) )Documento17 pagineThesis Ibanag) )Rahc Engam Gahyadap100% (20)
- 15 Kasprzak PDFDocumento4 pagine15 Kasprzak PDFLavanya KalapalaNessuna valutazione finora
- Researchpaper Improving Customers Service at IKEA Using Six Sigma MethodologyDocumento9 pagineResearchpaper Improving Customers Service at IKEA Using Six Sigma MethodologyM Nadeem Baig100% (1)
- Internal Combustion: How Corporations and Governments Addicted the World to Oil and Subverted the AlternativesDa EverandInternal Combustion: How Corporations and Governments Addicted the World to Oil and Subverted the AlternativesValutazione: 4 su 5 stelle4/5 (2)
- A Practical Handbook for Drilling Fluids ProcessingDa EverandA Practical Handbook for Drilling Fluids ProcessingNessuna valutazione finora
- Abrasive Water Jet Perforation and Multi-Stage FracturingDa EverandAbrasive Water Jet Perforation and Multi-Stage FracturingNessuna valutazione finora
- Practical Reservoir Engineering and CharacterizationDa EverandPractical Reservoir Engineering and CharacterizationValutazione: 4.5 su 5 stelle4.5/5 (3)
- Pocket Guide to Flanges, Fittings, and Piping DataDa EverandPocket Guide to Flanges, Fittings, and Piping DataValutazione: 3.5 su 5 stelle3.5/5 (22)
- Fundamentals and Applications of Bionic Drilling FluidsDa EverandFundamentals and Applications of Bionic Drilling FluidsNessuna valutazione finora
- Casing and Liners for Drilling and Completion: Design and ApplicationDa EverandCasing and Liners for Drilling and Completion: Design and ApplicationValutazione: 5 su 5 stelle5/5 (3)
- Well Testing Project Management: Onshore and Offshore OperationsDa EverandWell Testing Project Management: Onshore and Offshore OperationsNessuna valutazione finora
- Machine Learning Guide for Oil and Gas Using Python: A Step-by-Step Breakdown with Data, Algorithms, Codes, and ApplicationsDa EverandMachine Learning Guide for Oil and Gas Using Python: A Step-by-Step Breakdown with Data, Algorithms, Codes, and ApplicationsValutazione: 4 su 5 stelle4/5 (4)
- Case Studies of Material Corrosion Prevention for Oil and Gas ValvesDa EverandCase Studies of Material Corrosion Prevention for Oil and Gas ValvesNessuna valutazione finora
- The Petroleum Engineering Handbook: Sustainable OperationsDa EverandThe Petroleum Engineering Handbook: Sustainable OperationsValutazione: 3.5 su 5 stelle3.5/5 (5)
- Gas and Oil Reliability Engineering: Modeling and AnalysisDa EverandGas and Oil Reliability Engineering: Modeling and AnalysisValutazione: 4.5 su 5 stelle4.5/5 (6)
- Heavy and Extra-heavy Oil Upgrading TechnologiesDa EverandHeavy and Extra-heavy Oil Upgrading TechnologiesValutazione: 4 su 5 stelle4/5 (2)
- Advanced Biomass Gasification: New Concepts for Efficiency Increase and Product FlexibilityDa EverandAdvanced Biomass Gasification: New Concepts for Efficiency Increase and Product FlexibilityValutazione: 3 su 5 stelle3/5 (2)
- Well Integrity for Workovers and RecompletionsDa EverandWell Integrity for Workovers and RecompletionsValutazione: 5 su 5 stelle5/5 (3)
- Advanced Production Decline Analysis and ApplicationDa EverandAdvanced Production Decline Analysis and ApplicationValutazione: 3.5 su 5 stelle3.5/5 (4)
- Heat Exchanger Equipment Field Manual: Common Operating Problems and Practical SolutionsDa EverandHeat Exchanger Equipment Field Manual: Common Operating Problems and Practical SolutionsValutazione: 4 su 5 stelle4/5 (6)
- Petroleum Production Engineering, A Computer-Assisted ApproachDa EverandPetroleum Production Engineering, A Computer-Assisted ApproachValutazione: 4.5 su 5 stelle4.5/5 (11)
- Guide to the Practical Use of Chemicals in Refineries and PipelinesDa EverandGuide to the Practical Use of Chemicals in Refineries and PipelinesValutazione: 5 su 5 stelle5/5 (1)