Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
B49CE - Tutorial Topic 1 Questions v3
Caricato da
Buyu0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
202 visualizzazioni2 paginetut
Titolo originale
B49CE_Tutorial Topic 1 Questions v3
Copyright
© © All Rights Reserved
Formati disponibili
PDF, TXT o leggi online da Scribd
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentotut
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PDF, TXT o leggi online su Scribd
0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
202 visualizzazioni2 pagineB49CE - Tutorial Topic 1 Questions v3
Caricato da
Buyutut
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PDF, TXT o leggi online su Scribd
Sei sulla pagina 1di 2
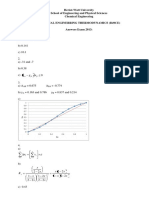
Tutorial Topic 1 Questions 1
1.4 Tutorial 1
1. Estimate the fugacity coefficient, and hence the fugacity, of ethylene at
50.5 bar and 24.95oC using the following data:
Molar mass of ethylene is 28.05 g/mol.
Table of P v T data for ethylene at 24.95oC
Pressure Specific Volume
(bar) ( 10 4 m 3 /mol )
1.0 245.00
5.1 47.80
10.1 23.20
15.2 15.00
20.2 10.80
25.3 8.34
30.3 6.66
35.4 5.44
40.4 4.52
45.5 3.78
50.5 3.17
Solutions:
0.73854
f 37.30 bar
2. Estimate the fugacity of gaseous ethane, temperature constant at
275K, using the following methods:
a) At 24.6 bar assuming ideal gas behaviour.
b) At 24.6 bar using the Z factor method.
c) At 24.6 bar using the generalised fugacity coefficient charts.
Critical properties for ethane are given below:
PC 48.72 bar
TC 32.17 o C
0.100
©HERIOT-W ATT UNIVERSITY B49CE December 2017 v3
Tutorial Topic 1 Questions 2
Use the pressure increments and thermodynamic data provided:
Pressure
(bar)
0.0
1.0
2.0
4.0
8.0
12.0
14.8
19.7
24.6
Solutions:
a) f 24.6 bar
b) f 18.43 bar
c) f 18.97 bar
3. Solid carbon dioxide at -59.11oC has a vapour pressure of 4.09918 bar
and specific volume of 0.000657 m3/kg. The molar mass of carbon
dioxide is 44.01 kg/kmol
Calculate the fugacity of solid carbon dioxide at 10 bar and -59.11oC
using the following the P v T data for gaseous carbon dioxide.
Pressure Specific Volume
3
(bar) (m / kg )
0.01020 33.36600
0.03061 13.02200
0.07483 5.32870
0.13605 2.92280
0.37415 1.05870
0.95236 0.41189
2.44897 0.15600
4.09918 0.09120
Solution:
f 2.83 bar
©HERIOT-W ATT UNIVERSITY B49CE December 2017 v3
Potrebbero piacerti anche
- Process Dynamics & Control ReportDocumento26 pagineProcess Dynamics & Control ReportDonna Joy MallariNessuna valutazione finora
- Assignment 2Documento2 pagineAssignment 2Ghulam MurtazaNessuna valutazione finora
- Patente Columna York - ScheibelDocumento6 paginePatente Columna York - Scheibeligor1991Nessuna valutazione finora
- 3Documento21 pagine3John100% (1)
- Deshidratación y Purificación (Alcohol Absoluto)Documento4 pagineDeshidratación y Purificación (Alcohol Absoluto)Jordan Venegas100% (1)
- Semibatch UniDocumento22 pagineSemibatch UniMelgi159100% (1)
- Evaluation of Michaelis Menten Parameters StudentsDocumento95 pagineEvaluation of Michaelis Menten Parameters StudentsAnonymous 0zrCNQ0% (1)
- L6 Pressure Drop in ReactorsDocumento21 pagineL6 Pressure Drop in ReactorsianharNessuna valutazione finora
- HW 4Documento2 pagineHW 4kimyakimya89Nessuna valutazione finora
- Fogler Solution Chapter 2 Problem 2.7Documento2 pagineFogler Solution Chapter 2 Problem 2.7MuhammadShoaibAnsariNessuna valutazione finora
- Distillation - Self Study QuestionsDocumento8 pagineDistillation - Self Study QuestionsEsther MaidenNessuna valutazione finora
- Transport Phenomena and Unit Operations A Combined Approach by Richard G. GriskeyDocumento5 pagineTransport Phenomena and Unit Operations A Combined Approach by Richard G. GriskeyRosaNessuna valutazione finora
- 1 Liquid-Liquid Equilibrium For The Ternary System Methanol + Acrylonitrile + WaterDocumento10 pagine1 Liquid-Liquid Equilibrium For The Ternary System Methanol + Acrylonitrile + WatersandraesiqNessuna valutazione finora
- Experimental and Modeling of A Non-Isothermal CSTRDocumento10 pagineExperimental and Modeling of A Non-Isothermal CSTRAlejandra SantosNessuna valutazione finora
- Homework 2-S15 (1) Chem E 178Documento1 paginaHomework 2-S15 (1) Chem E 178siegeddNessuna valutazione finora
- $RQ2A4QGDocumento377 pagine$RQ2A4QGKHANNessuna valutazione finora
- Vapor-Phase Chemical Equilibrium For The Hydrogenation of Benzene To Cyclohexane From Reaction-Ensemble GazDocumento13 pagineVapor-Phase Chemical Equilibrium For The Hydrogenation of Benzene To Cyclohexane From Reaction-Ensemble GazebrarNessuna valutazione finora
- Partial Molar Volumes From Refractive Index MeasurementsDocumento4 paginePartial Molar Volumes From Refractive Index MeasurementsFelipe Antonio Vasquez CarrascoNessuna valutazione finora
- ExercisesDocumento13 pagineExercisesRajpriya GuptaNessuna valutazione finora
- Co (NH3) 6Documento1 paginaCo (NH3) 6Ayotunde OnasanyaNessuna valutazione finora
- QDocumento1 paginaQSSLabNessuna valutazione finora
- FLASH ADIABÁTICO - Separation Process Principles - SEADER - 3rdedDocumento5 pagineFLASH ADIABÁTICO - Separation Process Principles - SEADER - 3rdedMaykkkowNessuna valutazione finora
- 2.10 Supplementary Reading ReferencesDocumento4 pagine2.10 Supplementary Reading ReferencesHarold Isai Silvestre GómezNessuna valutazione finora
- Enrtl-Rk Rate Based Dga ModelDocumento30 pagineEnrtl-Rk Rate Based Dga ModelsamandondonNessuna valutazione finora
- KineticsDocumento123 pagineKineticssamueloNessuna valutazione finora
- Weather DataDocumento3 pagineWeather DataWilmer Alexander0% (1)
- Tutorial 4 Solution PDFDocumento6 pagineTutorial 4 Solution PDFSalihah AbdullahNessuna valutazione finora
- Energy Conservation in DistillationDocumento4 pagineEnergy Conservation in DistillationRajat WadhwaniNessuna valutazione finora
- Cellic CTec3 HS Application Sheet NADocumento5 pagineCellic CTec3 HS Application Sheet NADaniel RivaldiNessuna valutazione finora
- Distillation Example 4 and 5Documento2 pagineDistillation Example 4 and 5DirkMyburghNessuna valutazione finora
- HW 01 SolutionDocumento12 pagineHW 01 Solutionmaulida rahmiNessuna valutazione finora
- Surface Tension of Water From: AlcoholDocumento4 pagineSurface Tension of Water From: AlcoholSkandar EverestNessuna valutazione finora
- CHEN3005 Process Instrumentation and ControlDocumento4 pagineCHEN3005 Process Instrumentation and ControlVincent Ys TanNessuna valutazione finora
- Chemical Engineering ThermodynamicsDocumento2 pagineChemical Engineering ThermodynamicsMadhuri Gupta25% (4)
- INDIVIDUAL REPORT - Keshrie Reddy1 PDFDocumento136 pagineINDIVIDUAL REPORT - Keshrie Reddy1 PDFBigNessuna valutazione finora
- Solutions Manual For Analysis Synthesis and Design of Chemical Processes 4th Edition PDFDocumento14 pagineSolutions Manual For Analysis Synthesis and Design of Chemical Processes 4th Edition PDFNathalia DelgadoNessuna valutazione finora
- Answers To ProblemsDocumento4 pagineAnswers To ProblemsSyed Bakhtyar AhmedNessuna valutazione finora
- Quiz Show FileDocumento18 pagineQuiz Show FileLeaniel SilvaNessuna valutazione finora
- CAP13Documento21 pagineCAP13LIma NetoNessuna valutazione finora
- Answer For TutorialDocumento7 pagineAnswer For TutorialFatur RohimNessuna valutazione finora
- Separations and Reaction Engineering Design Project Styrene ProductionDocumento10 pagineSeparations and Reaction Engineering Design Project Styrene ProductionLokesh EmandiNessuna valutazione finora
- A Novel Reverse Flow Strategy For Ethylbenzene Dehydrogenation in A Packed-Bed ReactorDocumento17 pagineA Novel Reverse Flow Strategy For Ethylbenzene Dehydrogenation in A Packed-Bed ReactorMuhammad Akbar FahleviNessuna valutazione finora
- Chapter 2Documento41 pagineChapter 2muhammad shahadat awanNessuna valutazione finora
- CRE1 PAPER - Alif - Farhandi - HannahDocumento53 pagineCRE1 PAPER - Alif - Farhandi - HannahFarhandi Fadillah FedrizalNessuna valutazione finora
- CH126P Lec 8 - Ch9Documento20 pagineCH126P Lec 8 - Ch9kumiristineNessuna valutazione finora
- Assignment 1Documento3 pagineAssignment 1imtiazNessuna valutazione finora
- CPD Group 16Documento9 pagineCPD Group 16iffatNessuna valutazione finora
- Cumene Process, Prod - CBIDocumento2 pagineCumene Process, Prod - CBIChris LindseyNessuna valutazione finora
- Aplicacion de Sowtfare para I.Q.Documento34 pagineAplicacion de Sowtfare para I.Q.Gabriel MenchuNessuna valutazione finora
- Khadem I 2017Documento12 pagineKhadem I 2017Michael KennedyNessuna valutazione finora
- Koretsky Thermodynamic Solutions For Fugacity, VLEDocumento11 pagineKoretsky Thermodynamic Solutions For Fugacity, VLEjgrav667Nessuna valutazione finora
- Assignment 2 With SolutionsDocumento4 pagineAssignment 2 With SolutionsVenkat MacharlaNessuna valutazione finora
- Calculation of UOP Characterization Factor and Estimation of Molecular Weight of Petroleum OilsDocumento14 pagineCalculation of UOP Characterization Factor and Estimation of Molecular Weight of Petroleum OilsLuis Ernesto Marin JaimesNessuna valutazione finora
- ProII Procesos BatchDocumento26 pagineProII Procesos Batcharmando0212-1Nessuna valutazione finora
- Question External Mass TransferDocumento1 paginaQuestion External Mass TransferMainul Haque0% (1)
- Thermodynamic & Chemical Equil PDFDocumento710 pagineThermodynamic & Chemical Equil PDFbitconcepts9781Nessuna valutazione finora
- Specification Sheet For Crystallizer Crystallizer Data SheetDocumento3 pagineSpecification Sheet For Crystallizer Crystallizer Data SheetAmar RazinNessuna valutazione finora
- Oxychlorination Reactor DesignDocumento45 pagineOxychlorination Reactor Designhinman714Nessuna valutazione finora
- Problema 12-10 TreybalDocumento1 paginaProblema 12-10 TreybalMiguel Angel Lugo CarvajalNessuna valutazione finora
- Reaction Engineering I-Problem Sheet IIDocumento7 pagineReaction Engineering I-Problem Sheet IISimay AydoganNessuna valutazione finora
- F17XB2 2014-15 Class Test Mathematics For Engineers and Scientists 2 Attempt All Questions (B)Documento2 pagineF17XB2 2014-15 Class Test Mathematics For Engineers and Scientists 2 Attempt All Questions (B)BuyuNessuna valutazione finora
- 1. A) Ln (F/P) = - 1/Rt ∫Αdp B) 0.141 C) 10.1 2. A) -31 And -7 B) 0.38 C) 3. A) A =-0.675 A = -0.774 B) Y = 0.163 And 0.786 Y = 0.837 And 0.214 C)Documento1 pagina1. A) Ln (F/P) = - 1/Rt ∫Αdp B) 0.141 C) 10.1 2. A) -31 And -7 B) 0.38 C) 3. A) A =-0.675 A = -0.774 B) Y = 0.163 And 0.786 Y = 0.837 And 0.214 C)BuyuNessuna valutazione finora
- B49CE - Tutorial Topic 2 Questions v3Documento6 pagineB49CE - Tutorial Topic 2 Questions v3BuyuNessuna valutazione finora
- Tutorial Answers - Separation B Tutorial - Combined Tutorial Solutions PDFDocumento38 pagineTutorial Answers - Separation B Tutorial - Combined Tutorial Solutions PDFBuyuNessuna valutazione finora
- Tutorial Answers - Separation B Tutorial - Combined Tutorial SolutionsDocumento38 pagineTutorial Answers - Separation B Tutorial - Combined Tutorial SolutionsBuyuNessuna valutazione finora
- Naming Organic Compounds B17CADocumento2 pagineNaming Organic Compounds B17CABuyuNessuna valutazione finora
- 2012 Dec SolutionsDocumento8 pagine2012 Dec SolutionsBuyu100% (1)
- 2014 DecDocumento5 pagine2014 DecBuyuNessuna valutazione finora
- Kelloway, E. Kevin Using Mplus For Structural Equation Modeling A Researchers GuideDocumento250 pagineKelloway, E. Kevin Using Mplus For Structural Equation Modeling A Researchers Guideintemperante100% (4)
- Impact of Agricultural Export On Economic Growth PDFDocumento28 pagineImpact of Agricultural Export On Economic Growth PDFAKINYEMI ADISA KAMORUNessuna valutazione finora
- PPT-Introduction To MELCOR and The RN PackageDocumento42 paginePPT-Introduction To MELCOR and The RN PackagejackleesjNessuna valutazione finora
- Investigation of Interparticle Breakage As Applied To Cone Crushing PDFDocumento16 pagineInvestigation of Interparticle Breakage As Applied To Cone Crushing PDFFrancisco Antonio Guerrero MonsalvesNessuna valutazione finora
- DS Lecture 5Documento29 pagineDS Lecture 5CH HASEEB AMJADNessuna valutazione finora
- FURTHER MATHEMATICS LOWER SIXTH RealDocumento3 pagineFURTHER MATHEMATICS LOWER SIXTH RealAlphonsius WongNessuna valutazione finora
- DXF FormatDocumento208 pagineDXF Formatthigopal100% (1)
- Big Rip EssayDocumento2 pagineBig Rip EssayBo BobNessuna valutazione finora
- Geometrical Formulation of Quantum MechanicsDocumento41 pagineGeometrical Formulation of Quantum Mechanicskipikos45Nessuna valutazione finora
- Modular - Gen Math11-Lesson 1.1 Functions As Mathematical Model of Real-Life Situations - BALLERAS, MDocumento12 pagineModular - Gen Math11-Lesson 1.1 Functions As Mathematical Model of Real-Life Situations - BALLERAS, MAyesha YusopNessuna valutazione finora
- Building Management System - BMS 1Documento31 pagineBuilding Management System - BMS 1jaffna100% (1)
- 1 - Petrobras - DeepWater Gas LiftDocumento36 pagine1 - Petrobras - DeepWater Gas LiftNisar KhanNessuna valutazione finora
- Bullying (Ijime) Among Japanese Hospital NursesDocumento9 pagineBullying (Ijime) Among Japanese Hospital NursesPedro Alberto Herrera LedesmaNessuna valutazione finora
- C++-Institute Certkiller CPA v2017-09-20 by Mark 105qDocumento96 pagineC++-Institute Certkiller CPA v2017-09-20 by Mark 105qعبدالله أبوشاويشNessuna valutazione finora
- Cbse Practical/Project Schedule: Class-Xi/Xii Mode S.NO. TopicDocumento26 pagineCbse Practical/Project Schedule: Class-Xi/Xii Mode S.NO. TopicGrand PlayerNessuna valutazione finora
- Research Paper - 1Documento18 pagineResearch Paper - 1Sunanda VinodNessuna valutazione finora
- Enhancing Color Photographic Images by The Modified Power Law (MPL)Documento14 pagineEnhancing Color Photographic Images by The Modified Power Law (MPL)Emmanuel TonyeNessuna valutazione finora
- C++ SPL Paper On Copy ConstructorsDocumento2 pagineC++ SPL Paper On Copy ConstructorsSanjay MunjalNessuna valutazione finora
- Presentation Introduction To FEA Using ProMechanicaDocumento40 paginePresentation Introduction To FEA Using ProMechanicasusanwebNessuna valutazione finora
- Extractor For Multi Value Class Characteristic Values Using Function ModuleDocumento13 pagineExtractor For Multi Value Class Characteristic Values Using Function ModuleEliseo Abad Camacho CNessuna valutazione finora
- Basics of Electrical MeasurementDocumento98 pagineBasics of Electrical MeasurementAshesh B VigneshNessuna valutazione finora
- Evaluation of Vertical Superimposed Stress in Subsoil Induced by Embankment LoadsDocumento9 pagineEvaluation of Vertical Superimposed Stress in Subsoil Induced by Embankment Loadscarlos moranteNessuna valutazione finora
- Quizizz - Probability-3rd GradeDocumento4 pagineQuizizz - Probability-3rd GradeDonovan KwanNessuna valutazione finora
- C3 PDFDocumento15 pagineC3 PDFANH NGUYỄN TRẦN ĐOANNessuna valutazione finora
- Using Deep Learning To Classify X-Ray Images of Potential Tuberculosis PatientsDocumento8 pagineUsing Deep Learning To Classify X-Ray Images of Potential Tuberculosis PatientsDr. Kaushal Kishor SharmaNessuna valutazione finora
- Nptel PDFDocumento12 pagineNptel PDFPhaní Tejâ RedlâNessuna valutazione finora
- Commands Guide Tutorial For SolidWorks 2012Documento64 pagineCommands Guide Tutorial For SolidWorks 2012Jelena Misimovic100% (1)
- CampusX DSMP SyllabusDocumento48 pagineCampusX DSMP Syllabussayantan palNessuna valutazione finora
- Ergo BrainDocumento159 pagineErgo BrainErnad HalilovićNessuna valutazione finora