Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Konsep Utama p53
Caricato da
Iffah MardhiyahTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Konsep Utama p53
Caricato da
Iffah MardhiyahCopyright:
Formati disponibili
Key concepts
• Organisms attempt to block the development of cancer through the actions of the
p53 alarm protein, which can cause cells to enter quiescence or apoptosis in the
event that the machinery regulating cell proliferation is malfunctioning or the cell
is exposed to various types of physiologic stress.
• p53 is a nuclear protein that normally exists as a tetramer and functions as a transcription
factor.
The mutant p53 found in many human tumors usually carries amino acid substitutions
in its DNA-binding domain. When mutant p53 forms tetrameric complexes
with wild-type p53, it interferes with the normal functions of the wild-type subunits
and thus with functions of the tetramer as a whole.
• p53 can impose cell cycle arrest through its ability to induce expression of p21 Cip1,
and apoptosis through its ability to induce expression of a variety of pro-apoptotic
proteins.
• p53 normally turns over rapidly. This turnover is blocked when a variety of signals
indicate cell-physiologic stress, including anoxia, damage to the genome, and signaling
imbalances in the intracellular growth-regulating machinery.
• p53 becomes functionally activated when its normally rapid degradation is
blocked. In addition, covalent modifications of the resulting accumulated p53
protein modulate its activity as a transcription factor, directing it to activate the
expression of genes involved in various cellular responses, notably apoptosis,
cytostasis, and senescence.
• p53 levels are controlled by two critical upstream regulators, Mdm2 and p19ARF.
Mdm2 works to destroy p53, while ARF inhibits Mdm2 from acting.
• Excessive activity of E2Fs, which is triggered by deregulation of the pRb pathway,
results in activation of ARF and thus p53.
• Apoptosis involves the activation of a cascade of caspases that results in the
destruction of a cell, usually within an hour. It can be activated by p53 as well as
signals impinging on the cell from the outside, notably those transduced by cell
surface death receptors.
• The apoptotic caspase cascade can be triggered through the opening of a channel
in the outer membrane of mitochondria, which releases several pro-apoptotic
proteins, notably cytochrome c.
• Opening of the mitochondrial membrane channel is determined by the relative
levels of Bcl-2–related anti-apoptotic and pro-apoptotic proteins.
• Loss of apoptotic functions allows cancer cells to survive a variety of cell-physiologic
stresses, including anoxia, signaling imbalances, DNA damage, and loss of
anchorage.
• Cancer cells invent numerous ways to inactivate the apoptotic machinery in order
to survive and thrive. Included among these are activation of Akt/PKB firing,
increase in the levels of anti-apoptotic Bcl-2–related proteins, inactivation of p53
through changes in the p53 gene or the upstream regulators of p53, methylation of
the promoters of a variety of pro-apoptotic genes, interference with cytochrome c
release from mitochondria, and inhibition of caspases.
Potrebbero piacerti anche
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5795)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (74)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (400)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- Swiss Target PredictionDocumento5 pagineSwiss Target PredictionDishank PNessuna valutazione finora
- Targeting WNT Signaling in Cancer Opportunities Abound If We Can Avoid The Sword of DamoclesDocumento300 pagineTargeting WNT Signaling in Cancer Opportunities Abound If We Can Avoid The Sword of DamoclesGris CortezNessuna valutazione finora
- Biochemistry Lecture Notes (Protein Structure 1)Documento6 pagineBiochemistry Lecture Notes (Protein Structure 1)MarcChang75% (4)
- B&B Medical Physiology - II. Physiology of Cells and Molecules. 2. Functional Organization of The Cell. BDocumento4 pagineB&B Medical Physiology - II. Physiology of Cells and Molecules. 2. Functional Organization of The Cell. BbahlasaNessuna valutazione finora
- Current State of Alzheimer'S Disease Research and TherapeuticsDocumento382 pagineCurrent State of Alzheimer'S Disease Research and TherapeuticsyusufNessuna valutazione finora
- 5) PPT On Mechanism of Action of Pyruvate Dehydrogenase Complex (PDH)Documento11 pagine5) PPT On Mechanism of Action of Pyruvate Dehydrogenase Complex (PDH)Subha MaheswariNessuna valutazione finora
- Amino AcidDocumento22 pagineAmino AcidRamya KrishnakumarNessuna valutazione finora
- ProteinsDocumento20 pagineProteinsJin Chung Kuan100% (2)
- Seminars in Cancer Biology: Avaniyapuram Kannan MuruganDocumento20 pagineSeminars in Cancer Biology: Avaniyapuram Kannan MuruganMurugan Avaniyapuram KannanNessuna valutazione finora
- 2017 Book SH2DomainsDocumento546 pagine2017 Book SH2DomainsMario Alfredo Loeza CabreraNessuna valutazione finora
- Bulletin 6040Documento1 paginaBulletin 6040Jorge Antonio Pérez LainesNessuna valutazione finora
- Mechanism and Regulation of FibrinolysisDocumento8 pagineMechanism and Regulation of FibrinolysisNalini RooplalNessuna valutazione finora
- HEMOSTASISDocumento12 pagineHEMOSTASISRyan PedregosaNessuna valutazione finora
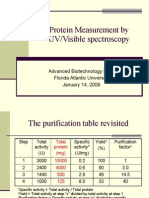
- Protein Measurement by UV/Visible Spectroscopy: Advanced Biotechnology Lab I Florida Atlantic University January 14, 2008Documento10 pagineProtein Measurement by UV/Visible Spectroscopy: Advanced Biotechnology Lab I Florida Atlantic University January 14, 2008Gina ZhouNessuna valutazione finora
- EnzymesDocumento6 pagineEnzymesSarah Farhah2000100% (1)
- Biophysics Assignment - Sohum Muley 2020B1AA2184HDocumento4 pagineBiophysics Assignment - Sohum Muley 2020B1AA2184HpolsaagyapolsNessuna valutazione finora
- Mechanism of Blood Clotting Extensic Pathway Factors Affecting Blood ClottingDocumento18 pagineMechanism of Blood Clotting Extensic Pathway Factors Affecting Blood ClottingRaunak TripathiNessuna valutazione finora
- Nicotine Spectra IRDocumento8 pagineNicotine Spectra IRYohanNugrahaNessuna valutazione finora
- Art Deco AssignmentDocumento20 pagineArt Deco AssignmentAmerNessuna valutazione finora
- BEU11403 Biomaterials - Lecture 2 PDFDocumento40 pagineBEU11403 Biomaterials - Lecture 2 PDFKin HamzahNessuna valutazione finora
- Hemostasis Reagent Portfolio PDFDocumento6 pagineHemostasis Reagent Portfolio PDFAlma FloresNessuna valutazione finora
- 04 Vesicle TransportDocumento35 pagine04 Vesicle TransportAnonymous vhjGsvpNessuna valutazione finora
- Blast2go TutorialDocumento48 pagineBlast2go TutorialBharatbioinormaticshauNessuna valutazione finora
- Blood CoagulationDocumento10 pagineBlood CoagulationgauravkokraNessuna valutazione finora
- Protein KinasesDocumento494 pagineProtein KinaseslguerreroNessuna valutazione finora
- Term-Project-Ii-Bch3125 ADocumento8 pagineTerm-Project-Ii-Bch3125 APure PureNessuna valutazione finora
- Bp404t Unit 2Documento102 pagineBp404t Unit 2Atul SharmaNessuna valutazione finora
- CollagenDocumento18 pagineCollagenAhsan AliNessuna valutazione finora
- Chapter 7 Enzyme Mechanism & ControlDocumento37 pagineChapter 7 Enzyme Mechanism & ControlLiana ChowdhuryNessuna valutazione finora
- Skeletal Muscle From Myogenesis To Clinical RelationsDocumento380 pagineSkeletal Muscle From Myogenesis To Clinical RelationsIndera VyasNessuna valutazione finora