Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
The Periodic Table of Elements Research
Caricato da
Cawf KgfTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
The Periodic Table of Elements Research
Caricato da
Cawf KgfCopyright:
Formati disponibili
See discussions, stats, and author profiles for this publication at: https://www.researchgate.
net/publication/278393189
The Periodic Table of Elements
Research · June 2015
DOI: 10.13140/RG.2.1.4509.7129
CITATION READS
1 79,466
1 author:
Daniel Lundberg
Swedish University of Agricultural Sciences
52 PUBLICATIONS 571 CITATIONS
SEE PROFILE
Some of the authors of this publication are also working on these related projects:
On the size of lanthanoid and actinoid ionic radii View project
Research graphics View project
All content following this page was uploaded by Daniel Lundberg on 15 July 2018.
The user has requested enhancement of the downloaded file.
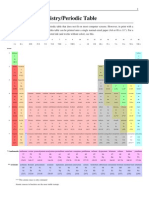
The periodic table of elements
View publication stats
1 18
with conventional atomic weights scaled to A r(12C) = 12 and physical state (at STP 25 °C and 1 atm)*
1 2
H 2 13 14 15 16 17 He
HYDROGEN HELIUM
1.008 class metal metalloid non-metal likely metal unknown sub-group 4.0026
3 4 atomic number 80 33 54 105 118 57-71 5 6 7 8 9 10
Li Be chemical symbol
Hg As Xe Ln B C N O F Ne
LITHIUM BERYLLIUM name MERCURY ARSENIC XENON DUBNIUM OGANESSON LANTHANOIDS BORON CARBON NITROGEN OXYGEN FLUORINE NEON
6.94 9.0122 standard atomic weight 200.59 74.9216 131.293 (268) (294) 10.81 12.011 14.007 15.999 18.9984 20.1797
11 12 physical state liquid solid gas probably solid unknown 13 14 15 16 17 18
Na Mg 3 4 5 6 7 8 9 10 11 12 Al Si P S Cl Ar
SODIUM MAGNESIUM ALUMINUM SILICON PHOSPHORUS SULFUR CHLORINE ARGON
22.9898 24.305 26.9815 28.085 30.9738 32.06 35.45 39.948
19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36
K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr
POTASSIUM CALCIUM SCANDIUM TITANIUM VANADIUM CHROMIUM MANGANESE IRON COBALT NICKEL COPPER ZINC GALLIUM GERMANIUM ARSENIC SELENIUM BROMINE KRYPTON
39.0983 40.078 44.9559 47.867 50.9415 51.9961 54.9380 55.845 58.9332 58.6934 63.546 65.38 69.723 72.630 74.9216 78.971 79.904 83.798
37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54
Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe
RUBIDIUM STRONTIUM YTTRIUM ZIRCONIUM NIOBIUM MOLYBDENUM TECHNETIUM RUTHENIUM RHODIUM PALLADIUM SILVER CADMIUM INDIUM TIN ANTIMONY TELLURIUM IODINE XENON
85.4678 87.62 88.9058 91.224 92.9064 95.95 (98) 101.07 102.9055 106.42 107.8682 112.414 114.818 118.710 121.760 127.60 126.9045 131.293
55 56 57-71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86
Cs Ba Ln Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn
CESIUM BARIUM LANTHANOIDS HAFNIUM TANTALUM TUNGSTEN RHENIUM OSMIUM IRIDIUM PLATINUM GOLD MERCURY THALLIUM LEAD BISMUTH POLONIUM ASTATINE RADON
132.9055 137.327 178.49 180.9479 183.84 186.207 190.23 192.217 195.084 196.9666 200.592 204.38 207.2 208.9804 (209) (210) (222)
87 88 89-103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118
Ra An
FRANCIUM RADIUM ACTINOIDS RUTHERFORDIUM DUBNIUM SEABORGIUM BOHRIUM HASSIUM MEITNERIUM DARMSTADTIUM ROENTGENIUM COPERNICIUM NIHONIUM FLEROVIUM MOSCOVIUM LIVERMORIUM TENNESSINE OGANESSON
(223) (226) (267) (268) (269) (270) (269) (278) (281) (282) (285) (286) (289) (289) (293) (294) (294)
57 58 59 60 61 62 63 64 65 66 67 68 69 70 71
Ln La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
LANTHANOIDS LANTHANUM CERIUM PRASEODYMIUM NEODYMIUM PROMETHIUM SAMARIUM EUROPIUM GADOLINIUM TERBIUM DYSPROSIUM HOLMIUM ERBIUM THULIUM YTTERBIUM LUTETIUM
138.9055 140.116 140.9077 144.242 (145) 150.36 151.964 157.25 158.9254 162.500 164.9303 167.259 168.9342 173.054 174.9668
89 90 91 92 93 94 95 96 97 98 99 100 101 102 103
An Ac Th Pa U Np Pu Am Cm Bk Cf Es
ACTINOIDS ACTINIUM THORIUM PROTACTINIUM URANIUM NEPTUNIUM PLUTONIUM AMERICIUM CURIUM BERKELIUM CALIFORNIUM EINSTEINIUM FERMIUM MENDELEVIUM NOBELIUM LAWRENCIUM
(227) 232.0377 231.0359 238.0289 (237) (244) (243) (247) (247) (251) (252) (257) (258) (259) (266)
* The symbols listed are those recommended by the International Union of Pure and Applied Chemistry (IUPAC). The atomic masses for hydrogen, lithium, boron, carbon, nitrogen, oxygen, silicon, chlorine, and thallium are the suggested conventional
atomic weights regardless of the respective element's terrestrial origin, with the remaining limited to four decimals (whenever needed). Elements with their atomic weight in parenthesis have no stable isotopes, and the value refers to the most long-
lived, non-metastable one. Bismuth, thorium, protactinium, and uranium have no stable isotopes either, but these have a characteristic terrestrial isotopic composition allowing a standard atomic mass to be tabulated. For a more detailed description
of the determination of atomic masses, se J. Meija et al., Pure Appl. Chem. 2016, 88 , 265-291. Please cite this article as D. Lundberg, The periodic table of elements (DOI · 10.13140/RG.2.1.4509.7129). Last updated: May 7, 2018.
Potrebbero piacerti anche
- The Periodic Table of Elements: Daniel LundbergDocumento2 pagineThe Periodic Table of Elements: Daniel LundbergOgunbowale Olatayo BodunrinNessuna valutazione finora
- The Periodic Table of Elements: Daniel LundbergDocumento2 pagineThe Periodic Table of Elements: Daniel LundbergAHNAF AJMAINNessuna valutazione finora
- The Periodic Table of Elements: Daniel LundbergDocumento2 pagineThe Periodic Table of Elements: Daniel LundbergEZLYEN AZLINNessuna valutazione finora
- The Periodic Table of Elements ResearchDocumento2 pagineThe Periodic Table of Elements ResearchAayush GuptaNessuna valutazione finora
- IUPAC Periodic Table-28Nov16 PDFDocumento1 paginaIUPAC Periodic Table-28Nov16 PDFAryan GuptaNessuna valutazione finora
- Understanding Radioactivity FukushimaDocumento31 pagineUnderstanding Radioactivity FukushimamapsingerNessuna valutazione finora
- Periodic Table of The Elements: Atomic Number Symbol NameDocumento1 paginaPeriodic Table of The Elements: Atomic Number Symbol Namechen haoNessuna valutazione finora
- 1 The Periodic Table of ElementsDocumento1 pagina1 The Periodic Table of Elementshau qi hongNessuna valutazione finora
- Periodic TableDocumento1 paginaPeriodic TableAmelie GarciaNessuna valutazione finora
- Jadual BerkalaDocumento2 pagineJadual BerkalaRohani Yusof75% (4)
- Grade 7 Science Practice Test 2019-20Documento70 pagineGrade 7 Science Practice Test 2019-20ggNessuna valutazione finora
- ChemistryDocumento3 pagineChemistryMurphy_AMDNessuna valutazione finora
- IUPAC Periodic Table-22Jun07bDocumento1 paginaIUPAC Periodic Table-22Jun07bAdnan Ali100% (2)
- Periodic Table Color 2017Documento1 paginaPeriodic Table Color 2017yahooincNessuna valutazione finora
- Periodic TableDocumento1 paginaPeriodic TableAutumn EverettNessuna valutazione finora
- Periodic Table of ElementsDocumento1 paginaPeriodic Table of Elementsadefuwa kuroliNessuna valutazione finora
- B & W Periodic TableDocumento1 paginaB & W Periodic Tableshubham dagaleNessuna valutazione finora
- PERIODIC TABLE TITLEDocumento1 paginaPERIODIC TABLE TITLElingarajugowdaNessuna valutazione finora
- Chemistry STD - VIIDocumento3 pagineChemistry STD - VIIPrem GomesNessuna valutazione finora
- SssssssDocumento1 paginaSssssssWillian Fredy Peñasco ApazaNessuna valutazione finora
- Tadashi Okuyama, Mark Maskill - Organic Chemistry - A Mechanistic Approach-Oxford University Press (2013)Documento681 pagineTadashi Okuyama, Mark Maskill - Organic Chemistry - A Mechanistic Approach-Oxford University Press (2013)Sooraj Srinivasan100% (12)
- Periodic TableDocumento1 paginaPeriodic TableIbrahim DesoukyNessuna valutazione finora
- Periodic Table of ElementsDocumento1 paginaPeriodic Table of ElementsaaminahcNessuna valutazione finora
- Tableau Periodiquedes Elements 2017Documento1 paginaTableau Periodiquedes Elements 2017Abdulia Musa MarahNessuna valutazione finora
- Mokeur Periodic TablecolDocumento1 paginaMokeur Periodic TablecolDaniel Jay KutzikNessuna valutazione finora
- First Mock Exam As Chem MCQ Nov 23Documento16 pagineFirst Mock Exam As Chem MCQ Nov 23shashibhushan.classroomNessuna valutazione finora
- Chemistry P - 2Documento17 pagineChemistry P - 2shezin rahmanNessuna valutazione finora
- Periodic Table of The ElementsDocumento1 paginaPeriodic Table of The ElementslebchemNessuna valutazione finora
- Periodic Table OF THE ElementsDocumento4 paginePeriodic Table OF THE ElementsAkshat ArchitNessuna valutazione finora
- 2023 Periodic Table (9729)Documento1 pagina2023 Periodic Table (9729)lyddd xiaNessuna valutazione finora
- Solid State PhysicsDocumento417 pagineSolid State Physicsapi-377220450% (2)
- Mock 1 Chemistry Nov 23 MCQ Questions OnlyDocumento15 pagineMock 1 Chemistry Nov 23 MCQ Questions Onlyshashibhushan.classroomNessuna valutazione finora
- In Kèm Với Đề - Periodic TableDocumento1 paginaIn Kèm Với Đề - Periodic TableNguyen (Harry) Xuan HoangNessuna valutazione finora
- Periodic TableDocumento2 paginePeriodic TableSaheed KalliyadanpoyilNessuna valutazione finora
- Periodic TableDocumento1 paginaPeriodic Tablejames.woodNessuna valutazione finora
- Periodic Table of Elements: SymbolDocumento1 paginaPeriodic Table of Elements: SymbolKhadijahMadhadzirNessuna valutazione finora
- CHEM SPM Periodic Table BWDocumento1 paginaCHEM SPM Periodic Table BWangie081250% (2)
- Periodic Table of the Elements: An SEO-Optimized TitleDocumento1 paginaPeriodic Table of the Elements: An SEO-Optimized TitleMwinde SimbezaNessuna valutazione finora
- Pink Lined Periodic Table PosterDocumento1 paginaPink Lined Periodic Table PosterrizzesdarNessuna valutazione finora
- Periodic Table of The Elements: Darmstadtium RutherfordiumDocumento1 paginaPeriodic Table of The Elements: Darmstadtium RutherfordiumLJ Asencion CacotNessuna valutazione finora
- Periodic Table With AnnotationsDocumento2 paginePeriodic Table With AnnotationsleehongxuanzarielNessuna valutazione finora
- Ncea Chemistry Level 1 ResourceDocumento4 pagineNcea Chemistry Level 1 ResourceUmiNessuna valutazione finora
- 0620/42/M/J/16 © Ucles 2016Documento1 pagina0620/42/M/J/16 © Ucles 2016esmat ZedanNessuna valutazione finora
- 0620/42/M/J/16 © Ucles 2016Documento1 pagina0620/42/M/J/16 © Ucles 2016esmat ZedanNessuna valutazione finora
- Periodic TableDocumento1 paginaPeriodic TableGerard Hung Yourui (Chs)Nessuna valutazione finora
- Chemistry Lesson 3Documento11 pagineChemistry Lesson 3Vinod Varadan SNessuna valutazione finora
- Periodic TableDocumento1 paginaPeriodic TableRubaNessuna valutazione finora
- Periodic TableDocumento1 paginaPeriodic Tablejax stykerNessuna valutazione finora
- Regular Chemistry 11 DataBookletDocumento10 pagineRegular Chemistry 11 DataBookletdNessuna valutazione finora
- Periodic Table of The Elements (Used For Grade 8 and High School)Documento1 paginaPeriodic Table of The Elements (Used For Grade 8 and High School)EricNessuna valutazione finora
- The Periodic Table of ElementsDocumento1 paginaThe Periodic Table of ElementsFatimah RehmanNessuna valutazione finora
- 2016 Specimen Data BookletDocumento1 pagina2016 Specimen Data BookletCarra Putri 7H 06Nessuna valutazione finora
- IUPAC Periodic Table of The Elements: Ti CRDocumento1 paginaIUPAC Periodic Table of The Elements: Ti CRMargaux HidalgoNessuna valutazione finora
- Book 2 - Atoms The Periodic Table Bohr Models 1Documento40 pagineBook 2 - Atoms The Periodic Table Bohr Models 1PuraniNessuna valutazione finora
- Newer Redox Titrants: International Series of Monographs in Analytical ChemistryDa EverandNewer Redox Titrants: International Series of Monographs in Analytical ChemistryNessuna valutazione finora
- Fossil Hydrocarbons: Chemistry and TechnologyDa EverandFossil Hydrocarbons: Chemistry and TechnologyValutazione: 3 su 5 stelle3/5 (1)
- Handbook of Coordination Catalysis in Organic ChemistryDa EverandHandbook of Coordination Catalysis in Organic ChemistryNessuna valutazione finora
- Electrogas Welding Process PDFDocumento8 pagineElectrogas Welding Process PDFsaravananNessuna valutazione finora
- Fracture and Fracture Toughness of Cast Irons: W. L. Bradley and M. N. SrinivasanDocumento33 pagineFracture and Fracture Toughness of Cast Irons: W. L. Bradley and M. N. SrinivasanNarasimha Murthy InampudiNessuna valutazione finora
- Documentacion 5549393Documento4 pagineDocumentacion 5549393Marcelo San MartinNessuna valutazione finora
- Solidification ProcessingDocumento14 pagineSolidification ProcessingTrupti Ranjan DasNessuna valutazione finora
- Trifluoromethanesulfonyl Chloride: Safety Data Sheet 6162601Documento7 pagineTrifluoromethanesulfonyl Chloride: Safety Data Sheet 6162601saravanaknNessuna valutazione finora
- VIVAX Cool 2014 PDFDocumento36 pagineVIVAX Cool 2014 PDFFeritFazliu100% (1)
- Transport Phenomena - MSC - Lecture 11Documento17 pagineTransport Phenomena - MSC - Lecture 11showravNessuna valutazione finora
- Defining High Fiber Ingredient Terminology Chapter 1Documento27 pagineDefining High Fiber Ingredient Terminology Chapter 1Horacio Cuevas100% (1)
- Exploratory drilling methods overviewDocumento12 pagineExploratory drilling methods overviewArvind MishraNessuna valutazione finora
- Concrete Pavement RepairDocumento10 pagineConcrete Pavement Repairgorafd449Nessuna valutazione finora
- Phase Rule FundamentalsDocumento30 paginePhase Rule FundamentalsjacNessuna valutazione finora
- ICI Pakistan Limited Annual Report 2016 17Documento296 pagineICI Pakistan Limited Annual Report 2016 17aliNessuna valutazione finora
- HA - Product Guide - Industrial Fixed Gas and Flame DetectionDocumento141 pagineHA - Product Guide - Industrial Fixed Gas and Flame DetectionJavier OrnelasNessuna valutazione finora
- Soil PH and Soil Acidity PDFDocumento16 pagineSoil PH and Soil Acidity PDFManuel EscobarNessuna valutazione finora
- Glass-Ceramic Glazes For Ceramic Tiles PDFDocumento53 pagineGlass-Ceramic Glazes For Ceramic Tiles PDFKristanto WahyudiNessuna valutazione finora
- GB Es Hu BG Ru Ua KZDocumento84 pagineGB Es Hu BG Ru Ua KZstool72Nessuna valutazione finora
- Daftar Formularium ObatDocumento6 pagineDaftar Formularium ObatRina HerlinaNessuna valutazione finora
- Chem Principles 7e ISM Focus 01 Even FINALDocumento26 pagineChem Principles 7e ISM Focus 01 Even FINALSelma MeloNessuna valutazione finora
- Main SeminarDocumento27 pagineMain SeminarPallavi Patil100% (1)
- Maintaining Refractory MaterialsDocumento5 pagineMaintaining Refractory MaterialsE.Caglar BugraNessuna valutazione finora
- Nafion ConductivityDocumento9 pagineNafion ConductivityAli Alipor NajmiNessuna valutazione finora
- American Machinists Handbook 1000165626Documento586 pagineAmerican Machinists Handbook 1000165626emocca100% (2)
- Heat Exchanger Design OptimizationDocumento26 pagineHeat Exchanger Design OptimizationEhsan MoemeniNessuna valutazione finora
- CEB1112Documento5 pagineCEB1112oscar horacio floresNessuna valutazione finora
- High-Performance Debris Filter: PR-BW 800Documento15 pagineHigh-Performance Debris Filter: PR-BW 800shelbyds100% (1)
- Found Support - Reading - Lesson 8Documento8 pagineFound Support - Reading - Lesson 8Hiếu HiếuNessuna valutazione finora
- Diggy Diggy Hole VoixDocumento4 pagineDiggy Diggy Hole VoixastiChantillyNessuna valutazione finora
- Soap and SaponificationDocumento3 pagineSoap and Saponificationemmet11Nessuna valutazione finora
- Arterial Blood Gas AnalysisDocumento8 pagineArterial Blood Gas AnalysisMichael NapoleonNessuna valutazione finora
- First Boiler Light Up: Akdas DGM (Os) NTPC LTDDocumento49 pagineFirst Boiler Light Up: Akdas DGM (Os) NTPC LTDrajan_me083Nessuna valutazione finora