Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Rotary Dryers
Caricato da
Agung SiswahyuTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Rotary Dryers
Caricato da
Agung SiswahyuCopyright:
Formati disponibili
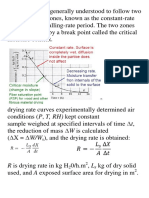
Rotary dryers, called the “workhorse of chemical dryers”, belong to the most widely
used class of continuous dryers in process industries. These dryers are suitable for
relatively free-flowing, non-sticky and granular materials; for example, almost all types
of crystals after crystallization and washing. Typical applications of rotary dryers are in
drying o table salt, sodium sulphate, ammonium sulphate, and many other salts, drying
of sand, minerals, organic solids, polymer resin beads, to mention a few. A rotary dryer
consists of a slowly rotating slightly inclined cylindrical shell fed with the moist solid at
the upper end. The material flows along the rotating shell, gets dried and leaves the
dryer at the lower end.
It is difficult, if not impossible, to design a rotary dryer on the basis of fundamental
principles only. The available design correlations are a few in number and may not
prove to be satisfactory for many systems. The design of a rotary dryer (and also of
most other dryers) is better done by using the pilot plant test data or the full-scale
operation data of a dryer of similar type together with the available correlations.
Problem Statement & Given Data:
A moist non-hygroscopic granular solid at 26°C is to be dried rom 20% initial moisture to
0.3% final moisture (wet basis) in a rotary dryer at a rate of 1500 kg/h. Hot air enters at
135°C with a humidity of 0.015. The exit solid temperature must not exceed 110°C and
the air velocity must not exceed 1.5 m/s in order to avoid dusting of the solid. Specific
heat of the dry solid is c ps = 0.85 kJ/kg.K. Suggest the diameter the length and the other
parameters of the dryer.
Solution:
Basis of calculation is 1 hour operation.
Mass of dry solid, LS = (1500) x (1 – 0.2) = 1200 kg/h; moisture in the wet solid
X1 = 20/80 = 0.25; moisture in the dry solid, X2 = 0.3/99.7 = 0.00301.
Water evaporated, mS = LS(X1 – X2) = 1200 x (0.25 – 0.00301) = 296.4 kg.
Figure 1 Solid and gas temperature profiles in a counter-current rotary dryer.
Refer to figure 1. Given: TS1 = 26°C; TG2 = 135°C; Y2 = 0.015
We assume that the exit temperature of the gas is T G1 = 60°C and that of the solid is T S2
= 100°C. These values are to be checked later on.
Calculation of enthalpy values of different streams (Reference temperature = 0°C)
H’S1 = [cps + (4.187).X1] (TS1 – 0) = [0.85 + (4.187) x 0.25] x (26 – 0)
= 49.31 kJ/kg dry solid
H’S2 = [cps + (4.187).X2] (TS2 – 0) = [0.85 + (4.187) x 0.00301] x (100 – 0)
= 86.2 kJ/kg dry solid
H’G2 = [1.005 + 1.88.Y2] (TG2 – 0) + Y2.λ0
= [1.005 + 1.88 x 0.015] x (135 – 0) + 0.015 x 2500 = 177 kJ/kg
H’G1 = [1.005 + 1.88.Y1] (TG1 – 0) + Y1.λ0
= [1.005 + 1.88 x Y1] x (60 – 0) + Y1 x 2500 = 60.3 + (2613 x Y1)
Overall mass balance
GS (Y1 – Y2) = LS (X1 - X2) = G2 (Y1 – 0.015) = 296.4 = GS = 296.4/(Y1 – 0.015)
LS (H’S2 – H’S1) = GS (H’G2 – H’G1) = 1200 x (86.2 – 49.31)
= [296.4/(Y1 – 0.015)] x (177 – 60.3 – 2613 x Y1)
Y1 = 0.04306 and GS = 296.4/(Y1 – 0.015) = 10,560 kg/h
Calculation of the shell diameter
Humid volume, VH = [(1/28.97) + (Y/18.02)] x 22.4 x [(TG + 273)/273]
Humid volume of the inlet gas (135°C, Y2 = 0.015), VH2 = 1.183 m3/kg dry air
Humid volume of the exit gas (60°C, Y1 = 0.04306), VH1 = 1.008 m3/kg dry air
The maximum volumetric gas flow rate (this occurs at the end 2 in figure 1) = G S x VH2
= 10,560 x 1.183 = 12,490 m3/h = 3.47 m3/s
Take the maximum superficial air velocity to be 1.2 m/s (this is 20% less than the
maximum allowable velocity since part of the dryer is filled with the moving solid, and
the entire cross-section is not available for gas flow). If d is the diameter,
(πd2/4) x (1.2) = 3.686 = d = 1.98. Select a 2 m diameter shell.
Calculation of the number of heat transfer units
The dryer is considered to consist of three zones as shown in figure 1. The temperature
and humidity or moisture content of the streams can be obtained by material and energy
balance.
Zone III: Only heating of the solid occurs in this zone; there is little water left for
vaporization. At the boundary between zones III and II, the solid is at T SB (= TSA) = 41°C
(this value is to be checked and modified later if necessary).
Enthalpy of the solid at the inlet to zone III,
H’SB = [0.85 + (4.187) x 0.00301] x (41 – 0) = 35.37 kJ/kg dry solid
Humid heat of the gas entering zone III, c HB = [1.005 + (1.88) x (0.015)] = 1.033 kJ/kg.K
(this remains constant in zone III, since the humidity does not change in this section).
Heat balance over zone III: LS (H’S2 – H’SB) = GS (cHB)III (TG2 - TGB)
= 1200 x (86.2 – 35.37) = 10,560 x 1.033 x (135 – T GB) = TGB = 129°C
Adiabatic saturation temperature of air entering zone II (129°C and humidity of 0.015) is
41.3°C. This is fairly close to the guess value of 41°C and T SA = TSB = 41°C is not
changed.
At the boundary B, ∆TB = 129 – 41 = 88°C, at end 2, ∆T2 = 135 – 100 = 35°C
Log mean temperature in zone III (∆T)m = (88 – 35)/[ln(88 – 35)] = 57.5°C
Number of heat transfer units, (NtG)III = (T2 – TGB)/(∆T)m = (135 – 129)/57.5 = 0.104
Zone II: In order to calculate (NtG)II, we need the value of TGA. This can be obtained by
heat balance.
H’GB = [1.005 + 1.88 x YB] x (129 – 0) + 2500 x YB = 170.8 kJ/kg. (since YB = 0.015)
H’SA = [0.85 + cPS x X1] x (TSA – 0) = [0.85 + 4.187 x 0.25] x (41 – 0) = 77.77 kJ/kg dry
solid
Enthalpy balance: LS x (H’SB – H’SA) = GS x (H’GB – H’GA)
= 1200 x (35.37 – 77.77) = 10,560 x (170.8 – H’ GA)
H’GA = 175.6 = [1.005 + 0.04306 x 1.88] x (TGA – 0) + 0.04306 x 2500 = TGA = 63°C
Temperature differences; At section A, (∆T)A = 63 – 41 = 22°C; (∆T)B = 88°C
(∆T)m = (88 – 22)/[ln(88/22)] = 47.6
Number of heat transfer units, (NtG)II = (TGB - TGA)/(∆T)m = (129 – 63)/47.6 = 1.386
Before calculating (NtG)I, let us check the validity of the assumed value of the exit gas
temperature, TG1 = 60°C, by making an energy balance over zone I.
GS (H’G2 – H’G1) = LS (H’S2 - H’S1)
= 10,560 (175.6 – H’G1) = 1200 (77.77 – 49.31) = H’ G1 = TG1 = 59.6°C, matches
the assumed value.
Zone I: (∆T)I = 60 – 26 = 34°C; (∆T)A = 22°C; (∆T)m = (34 – 22)/[ln(34/22)] = 27.5
Number of heat transfer units, (NtG)I = (TGA – TG1)/(∆T)m = (63 – 60)/27.5 = 0.109
Total number of heat transfer units N tG = 0.104 + 1.386 + 0.109 = 1.53 (this lies within
the usual range).
Length of a transfer unit
Average gas mass flow rate = [(10,560) x (1.015) + (10,560) x (1.04306)]/2 = 10,867
kg/h
The gas mass flow rate, G’ = (10,867/3600)/[(π/4) x 22] = 0.961 kg/m2 s
Volumetric heat transfer coefficient, Ua = [237 x (G’)0.67]/d =[237 x (0.961)0.67]/2
Ua = 115 W/m3 K
Humid heat at the ends: cH2 = 1.005 + 1.88 x 0.015 = 1.033
cH1 = 1.005 + 1.88 x 0.04306 = 1.083
Average humid heat, cH = (1.033 + 1.083)/2 = 1.058 kJ/kg K = 1058 J/kg dry air.K
Length of a heat transfer unit, LT = G’cH/Ua = (0.961 x 1058)/115 = LT = 8.84 m
Length of the dryer, L = (NtG) x (LT) = 1.56 x 8.84 = L = 13.8 m. Select 14 m
Select a 2 m diameter, 14 m long dryer
Potrebbero piacerti anche
- Drying - 5 - 29 Oct 2020 PDFDocumento25 pagineDrying - 5 - 29 Oct 2020 PDFshubhamNessuna valutazione finora
- FLUID MACHINERY - CENTRIFUGAL PUMP LAB REPORTDocumento31 pagineFLUID MACHINERY - CENTRIFUGAL PUMP LAB REPORTHarizx SaufixNessuna valutazione finora
- Study Refrigeration Cycle PerformanceDocumento10 pagineStudy Refrigeration Cycle PerformanceMeor Fitri SE100% (1)
- TRAY DRYER EXPERIMENT REPORTDocumento21 pagineTRAY DRYER EXPERIMENT REPORTamirulNessuna valutazione finora
- Natural Draft Tray DrierDocumento4 pagineNatural Draft Tray DrierAshish VermaNessuna valutazione finora
- Factors to Consider for Agitator and Mixer DesignDocumento4 pagineFactors to Consider for Agitator and Mixer DesignArun GuptaNessuna valutazione finora
- Dryers (Powerpt Presentation)Documento15 pagineDryers (Powerpt Presentation)mikogonzalveNessuna valutazione finora
- Boiler:: Working Principle of A BoilerDocumento22 pagineBoiler:: Working Principle of A BoilerZeshan AbdullahNessuna valutazione finora
- Lecture Note - Refrigeration CycleDocumento35 pagineLecture Note - Refrigeration CycleSuchi Suchi SuchiNessuna valutazione finora
- Experiment: Packed Distillation ColumnDocumento4 pagineExperiment: Packed Distillation Columnnhalieza1067Nessuna valutazione finora
- (Lab Report Operation Unit) Experiment 4: INTRODUCTION TO A BATCH PROCESS: SIMPLE BATCH DISTILLATIONDocumento10 pagine(Lab Report Operation Unit) Experiment 4: INTRODUCTION TO A BATCH PROCESS: SIMPLE BATCH DISTILLATIONFazsroul86% (7)
- Abstract, Intro, Objectives Tray DryerDocumento3 pagineAbstract, Intro, Objectives Tray DryerNawal DaBombNessuna valutazione finora
- Experiment: 1 Parallel Flow Heat ExchangerDocumento18 pagineExperiment: 1 Parallel Flow Heat ExchangerAnonymous QM0NLqZONessuna valutazione finora
- Agitation and Mixing (My Presentation)Documento22 pagineAgitation and Mixing (My Presentation)Aleem Choudhry100% (1)
- HEAT PUMP EXPERIMENT RESULTSDocumento11 pagineHEAT PUMP EXPERIMENT RESULTSNur AdlinaNessuna valutazione finora
- Batch Sedimentation Post-Laboratory Experiment 4Documento8 pagineBatch Sedimentation Post-Laboratory Experiment 4Kat-kat GrandeNessuna valutazione finora
- CycloneDocumento25 pagineCycloneAna Marie AllamNessuna valutazione finora
- DryersDocumento7 pagineDryersReNz ROMERONessuna valutazione finora
- Drying, and Drying Equipments 2Documento37 pagineDrying, and Drying Equipments 2Adheep DasNessuna valutazione finora
- Double Pipe Heat ExchangerDocumento7 pagineDouble Pipe Heat ExchangerPriyanshiVadaliaNessuna valutazione finora
- Drying Notes FinalDocumento34 pagineDrying Notes FinalKeith OmwoyoNessuna valutazione finora
- Expt 2 Performance of A Steam PlantDocumento8 pagineExpt 2 Performance of A Steam PlantAzim YusoffNessuna valutazione finora
- Double Pipe Heat Exchanger CalculationsDocumento11 pagineDouble Pipe Heat Exchanger Calculationsحسين عمريNessuna valutazione finora
- Practice Set 19 (Fins)Documento2 paginePractice Set 19 (Fins)Nibir SahaNessuna valutazione finora
- Solid Fuels Group 7 & 8Documento91 pagineSolid Fuels Group 7 & 8Jowel MercadoNessuna valutazione finora
- UO 6 Sedimentation Study UnitDocumento8 pagineUO 6 Sedimentation Study Uniteven lee100% (1)
- Duhring ChartDocumento4 pagineDuhring ChartCJ GoradaNessuna valutazione finora
- Drying PSDocumento10 pagineDrying PSVan Vesper DulliyaoNessuna valutazione finora
- Measurement of The Drag Coefficients of Spherical ParticlesDocumento10 pagineMeasurement of The Drag Coefficients of Spherical Particlessr3shNessuna valutazione finora
- DryingDocumento34 pagineDryingDhruv MotwaniNessuna valutazione finora
- Apparatus, Procedure, Recommendation Tray DryerDocumento4 pagineApparatus, Procedure, Recommendation Tray DryerillyzlNessuna valutazione finora
- 9 EvaporationDocumento82 pagine9 Evaporationsanjay YadavNessuna valutazione finora
- Drying 2 Class Notes PDFDocumento18 pagineDrying 2 Class Notes PDFFarouk Bassa100% (1)
- Double Pipe Heat Exchanger ExperimentDocumento7 pagineDouble Pipe Heat Exchanger ExperimentBenedicta Monis100% (1)
- Solution To Homework #2 For Chemical Engineering ThermodynamicsDocumento7 pagineSolution To Homework #2 For Chemical Engineering Thermodynamicsramesh pokhrel100% (1)
- Cold StoreDocumento33 pagineCold StorehanyassawyNessuna valutazione finora
- Experiment 14 - Radiation Heat TransferDocumento13 pagineExperiment 14 - Radiation Heat TransferMohamad Amin75% (4)
- Separation Processes Lab ReportDocumento15 pagineSeparation Processes Lab ReportArslanQureshi0% (1)
- Experiment 7 (Refrigeration Unit)Documento16 pagineExperiment 7 (Refrigeration Unit)fadhilahmad50% (2)
- Natural Convection Heat Transfer EquationsDocumento11 pagineNatural Convection Heat Transfer EquationstskNessuna valutazione finora
- Tutorial 1Documento4 pagineTutorial 1Unta Di PadAng PaSirNessuna valutazione finora
- Lecture-17: Multi-Stage Vapour Compression Refrigeration SystemsDocumento13 pagineLecture-17: Multi-Stage Vapour Compression Refrigeration SystemsMuhaamad TiloNessuna valutazione finora
- Variation In Refrigeration Coefficient Of PerformanceDocumento7 pagineVariation In Refrigeration Coefficient Of PerformanceFaez Feakry100% (1)
- Thermal Conductivity of Pipe Insulation Using Lagged PipeDocumento6 pagineThermal Conductivity of Pipe Insulation Using Lagged PipeanbuvrpNessuna valutazione finora
- Influence of Air Velocity on Drying Rate of Wet SandDocumento3 pagineInfluence of Air Velocity on Drying Rate of Wet SandJohanNessuna valutazione finora
- Design of Heat Exchanger Mini ProjectDocumento45 pagineDesign of Heat Exchanger Mini ProjectSuraya Afriyani100% (1)
- HTL-04 Thermal Conductivity of LiquidDocumento2 pagineHTL-04 Thermal Conductivity of Liquidvindiesel9222Nessuna valutazione finora
- Tray Drier Lab ReportDocumento10 pagineTray Drier Lab ReportFatinnnnnnNessuna valutazione finora
- CHAPTER 11: Dynamic Behaviour & Stability of Closed-Loop Control SystemsDocumento69 pagineCHAPTER 11: Dynamic Behaviour & Stability of Closed-Loop Control Systemshakita86Nessuna valutazione finora
- Oil Distillation ReportDocumento10 pagineOil Distillation ReportnisasoberiNessuna valutazione finora
- Heat Transfer Lab Manual 2015-16Documento99 pagineHeat Transfer Lab Manual 2015-16Harshit Sinha100% (1)
- Multicomponent Distillation CalculationsDocumento5 pagineMulticomponent Distillation CalculationsPatricia DavidNessuna valutazione finora
- Heat Exchangers LectureDocumento37 pagineHeat Exchangers LectureTerna Orlanda100% (2)
- What Is Humidification ProcessDocumento7 pagineWhat Is Humidification ProcessAqib ShahNessuna valutazione finora
- Perhitungan Rotary DryersDocumento5 paginePerhitungan Rotary DryersAgung SiswahyuNessuna valutazione finora
- Rotary Dryer CalculationDocumento5 pagineRotary Dryer Calculationsushant_jhawer100% (2)
- Lecture 3: Design Consideration of DriersDocumento6 pagineLecture 3: Design Consideration of DriersachalNessuna valutazione finora
- NPTEL Chemical Engineering Design Rotary DryerDocumento6 pagineNPTEL Chemical Engineering Design Rotary DryerganeshNessuna valutazione finora
- Calcium Based StabilizersDocumento4 pagineCalcium Based StabilizersAgung SiswahyuNessuna valutazione finora
- SIHI LEH Series Liquid Ring Vacuum Pump Features and BenefitsDocumento5 pagineSIHI LEH Series Liquid Ring Vacuum Pump Features and BenefitsAgung SiswahyuNessuna valutazione finora
- HTTP Alat Des Dry Evaporator SteamDocumento10 pagineHTTP Alat Des Dry Evaporator SteamAgung SiswahyuNessuna valutazione finora
- Technical Design Principles for Liquid Ring Vacuum PumpsDocumento18 pagineTechnical Design Principles for Liquid Ring Vacuum PumpsAgung SiswahyuNessuna valutazione finora
- Vacuum Technology: Sterling Fluid Systems GroupDocumento15 pagineVacuum Technology: Sterling Fluid Systems GroupAgung SiswahyuNessuna valutazione finora
- Falling Film Evaporators in The Food IndustryDocumento4 pagineFalling Film Evaporators in The Food IndustryAgung SiswahyuNessuna valutazione finora
- How To Control Liquid Ring Vacuum PumpsDocumento2 pagineHow To Control Liquid Ring Vacuum Pumpsjdgh1986Nessuna valutazione finora
- Ejector PDFDocumento5 pagineEjector PDFVirendra KumarNessuna valutazione finora
- Xchanger Suite Educational Order Form PDFDocumento1 paginaXchanger Suite Educational Order Form PDFAgung SiswahyuNessuna valutazione finora
- Nrstats PDFDocumento28 pagineNrstats PDFAgung SiswahyuNessuna valutazione finora
- Ejector PrincipleDocumento13 pagineEjector Principlekhoshya7100% (1)
- Self Guided Demo - Aspen Shell and Tube Exchanger V8.8Documento45 pagineSelf Guided Demo - Aspen Shell and Tube Exchanger V8.8HarySetiyawanNessuna valutazione finora
- Pelatihan Hysis TMIDocumento60 paginePelatihan Hysis TMIAgung SiswahyuNessuna valutazione finora
- Nama Pompa Pola Aliran Laju Alir (Kg/jam) Densitas (kg/m3) Q (m3/s) Kecepatan (M/S) Diameter (Inch)Documento2 pagineNama Pompa Pola Aliran Laju Alir (Kg/jam) Densitas (kg/m3) Q (m3/s) Kecepatan (M/S) Diameter (Inch)Agung SiswahyuNessuna valutazione finora
- TRAINING ADVANCE HYSYSDocumento9 pagineTRAINING ADVANCE HYSYSAgung SiswahyuNessuna valutazione finora
- Design and Study of Manufacturing of Polyester Plant Using Pta and MegDocumento8 pagineDesign and Study of Manufacturing of Polyester Plant Using Pta and MegUgurNessuna valutazione finora
- Aspen Energy Analyzer TutorialDocumento101 pagineAspen Energy Analyzer TutorialMcn Serg100% (3)
- Agung Speech 5 Feb 2018Documento1 paginaAgung Speech 5 Feb 2018Agung SiswahyuNessuna valutazione finora
- Kode-HS-2012 PDFDocumento190 pagineKode-HS-2012 PDFPungkyRamadhaniPamungkasAminotoNessuna valutazione finora
- Agung Siswahyu Summary ProfileDocumento1 paginaAgung Siswahyu Summary ProfileAgung SiswahyuNessuna valutazione finora
- Falling Film Evaporators in The Food IndustryDocumento4 pagineFalling Film Evaporators in The Food IndustryAgung SiswahyuNessuna valutazione finora
- HS CodeDocumento1 paginaHS CodeAgung SiswahyuNessuna valutazione finora
- Jurnal Mas Setyo PDFDocumento10 pagineJurnal Mas Setyo PDFAgung SiswahyuNessuna valutazione finora
- Design and Study of Manufacturing of Polyester Plant Using Pta and MegDocumento8 pagineDesign and Study of Manufacturing of Polyester Plant Using Pta and MegUgurNessuna valutazione finora
- EnvirontDocumento14 pagineEnvirontAgung SiswahyuNessuna valutazione finora
- CPE604 - Mini Project Plant Design Produ PDFDocumento136 pagineCPE604 - Mini Project Plant Design Produ PDFAgung Siswahyu0% (1)
- Oil RefDocumento6 pagineOil RefAgung SiswahyuNessuna valutazione finora
- E04413040 PDFDocumento11 pagineE04413040 PDFjoseNessuna valutazione finora
- Supply and Demand 2007 - 2017Documento1 paginaSupply and Demand 2007 - 2017Hasanuddin SellaNessuna valutazione finora
- HYSYS Distillation Column Simulation TutorialDocumento7 pagineHYSYS Distillation Column Simulation Tutorialcmlim100% (2)
- FEECO Rotary Dryers Coolers PDFDocumento13 pagineFEECO Rotary Dryers Coolers PDFFernando MilmanNessuna valutazione finora
- A ESS NCIA DA MENTE Usando o Seu Poder Interior paraDocumento3 pagineA ESS NCIA DA MENTE Usando o Seu Poder Interior paraalveroberNessuna valutazione finora
- Drying Costs of Woody Biomass in A Semi-Industrial Experimental Rotary Dryer - 2008Documento5 pagineDrying Costs of Woody Biomass in A Semi-Industrial Experimental Rotary Dryer - 2008Peter Kimbel100% (1)
- GEA Barr-Rosin - Industrial Drying Brochure - English - A4 - tcm11-16154Documento12 pagineGEA Barr-Rosin - Industrial Drying Brochure - English - A4 - tcm11-16154Daniel SouzaNessuna valutazione finora
- Rotary Sand DryerDocumento1 paginaRotary Sand DryerArun GuptaNessuna valutazione finora
- DheerajDocumento7 pagineDheerajSaksham KakarNessuna valutazione finora
- The Phosphates Processing HandbookDocumento29 pagineThe Phosphates Processing HandbookweiningwuNessuna valutazione finora
- Rotary Dryer CalculationDocumento5 pagineRotary Dryer Calculationsushant_jhawer100% (2)
- Chemical Technology Subject Code: CH2001Documento17 pagineChemical Technology Subject Code: CH2001U SANKAR TEJONessuna valutazione finora
- 43 Drying of Coal PDFDocumento25 pagine43 Drying of Coal PDFAdi Gunawan PrasetiaNessuna valutazione finora
- Steps To Process Palm Efb PelletDocumento25 pagineSteps To Process Palm Efb PelletNata MiharjaNessuna valutazione finora
- SEO Tray Dryer Guide - Drying Methods & Mechanisms ExplainedDocumento21 pagineSEO Tray Dryer Guide - Drying Methods & Mechanisms ExplainedConquer ConquerNessuna valutazione finora
- Rotary Dryer BrochureDocumento1 paginaRotary Dryer Brochurejanob7Nessuna valutazione finora
- Trojosky2019 PDFDocumento21 pagineTrojosky2019 PDFHector CabezasNessuna valutazione finora
- Rotary Dryer Design Calculation For Wood ChipsDocumento8 pagineRotary Dryer Design Calculation For Wood ChipsShankar AcharNessuna valutazione finora
- Rotary Dryer: DUEX Industrial SystemsDocumento7 pagineRotary Dryer: DUEX Industrial SystemssilygierlNessuna valutazione finora
- Rotary Dryer Handbook - PREVIEW - 2Documento7 pagineRotary Dryer Handbook - PREVIEW - 2HARKULVINDER SINGHNessuna valutazione finora
- Pharmaceutical DryersDocumento2 paginePharmaceutical Dryersf_azarNessuna valutazione finora
- Combustion Chambers: Steel Shell RefractoryDocumento1 paginaCombustion Chambers: Steel Shell RefractoryCarlos PerezNessuna valutazione finora
- Airstream Dryer PDFDocumento12 pagineAirstream Dryer PDFAsikinNessuna valutazione finora
- MD ND MDG Series Leaflet EN - LRDocumento16 pagineMD ND MDG Series Leaflet EN - LRMahesh MishraNessuna valutazione finora
- Rotary Dryer Handbook PDFDocumento43 pagineRotary Dryer Handbook PDFAhmad NilNessuna valutazione finora
- Combustion Chambers PDFDocumento1 paginaCombustion Chambers PDFkosmc123Nessuna valutazione finora
- 4 - Rotary DryerDocumento24 pagine4 - Rotary DryerkhawarNessuna valutazione finora
- Rotary DryerDocumento11 pagineRotary DryerRiska Ristiyanti100% (1)
- Computer Simulation of A Rotary Dryer Part IDocumento6 pagineComputer Simulation of A Rotary Dryer Part IJhonatan33xNessuna valutazione finora
- Rotary Dryer Handbook PDFDocumento34 pagineRotary Dryer Handbook PDFNichol Salas ManioNessuna valutazione finora
- EPSRC Thermal Management Sheffield Drying Tech Feb 2010Documento50 pagineEPSRC Thermal Management Sheffield Drying Tech Feb 2010Anonymous VlKfgxLNessuna valutazione finora
- Rotary Dryer HandbookDocumento34 pagineRotary Dryer Handbook11331490% (10)
- Rotary KilnDocumento12 pagineRotary KilnNasjilah Muhayati100% (1)