Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Catalyst Increases The Rate of Reaction
Caricato da
ZakyAlFatony0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
14 visualizzazioni2 pagine1. The document discusses the development of Fischer-Tropsch catalysts for converting syngas into liquid hydrocarbons to help reduce Indonesia's dependence on fuel oil imports. It outlines methods for catalyst preparation and characterization to optimize the selectivity toward diesel and gasoline range hydrocarbons.
2. Key steps in catalyst development included support pretreatment to modify pore size, addition of copper and potassium promoters, and characterization using XRD, TPD, and activity testing. Pretreatment and promoters were found to influence particle size, surface area, and acidity in ways that increased hydrocarbon selectivity.
3. Testing showed the external pore formation on the modified supports enhanced catalytic activity and selectivity for C5+ hydrocarbons due
Descrizione originale:
Microteaching
Titolo originale
Narasi Micro Teaching
Copyright
© © All Rights Reserved
Formati disponibili
DOCX, PDF, TXT o leggi online da Scribd
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documento1. The document discusses the development of Fischer-Tropsch catalysts for converting syngas into liquid hydrocarbons to help reduce Indonesia's dependence on fuel oil imports. It outlines methods for catalyst preparation and characterization to optimize the selectivity toward diesel and gasoline range hydrocarbons.
2. Key steps in catalyst development included support pretreatment to modify pore size, addition of copper and potassium promoters, and characterization using XRD, TPD, and activity testing. Pretreatment and promoters were found to influence particle size, surface area, and acidity in ways that increased hydrocarbon selectivity.
3. Testing showed the external pore formation on the modified supports enhanced catalytic activity and selectivity for C5+ hydrocarbons due
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato DOCX, PDF, TXT o leggi online su Scribd
0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
14 visualizzazioni2 pagineCatalyst Increases The Rate of Reaction
Caricato da
ZakyAlFatony1. The document discusses the development of Fischer-Tropsch catalysts for converting syngas into liquid hydrocarbons to help reduce Indonesia's dependence on fuel oil imports. It outlines methods for catalyst preparation and characterization to optimize the selectivity toward diesel and gasoline range hydrocarbons.
2. Key steps in catalyst development included support pretreatment to modify pore size, addition of copper and potassium promoters, and characterization using XRD, TPD, and activity testing. Pretreatment and promoters were found to influence particle size, surface area, and acidity in ways that increased hydrocarbon selectivity.
3. Testing showed the external pore formation on the modified supports enhanced catalytic activity and selectivity for C5+ hydrocarbons due
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato DOCX, PDF, TXT o leggi online su Scribd
Sei sulla pagina 1di 2
1 • Ok, Good Morning everyone, Assalamualaikum Wr.Wb..
• My name is Zaky and i'm one of a lecturer candidate of Pertamina University
• Today i’d like to be talking about my expertise in Fischer Tropsch Technology and see..how somewhere in the near
future it's gonna help us deals with our nation problem in fuel oil import.
• I'll begin by outlining the FTS Technology, then I'll go on to highlight what this technology can offer, and finally i'll
discuss what can we take a part in develop this technology
2 • Acording to the Indonesia Ministry of Energy and Mineral Resources, fuel oil imports are projected to have
reached 71.8% of the total demand, or around 2.5 times the national fuel production
• Indonesia actually has potential resources such as coal, natural gas, and biomass that hasn’t been utilized
optimally
• One of the major problems faced related to the utilization of these resources is the conversion technology
• And through the conversion of initial resources into syngas, we can address the overall issue using Fischer-Tropsch
Process
3 • FTS is a catalytic process in which syngas or mixture of carbon monoxide and hydrogen are converted into liquid
hydrocarbons
• Well, this process are often found to yield a wide-range of hydrocarbon distribution from methane to heavy wax
products.
• Right now, It’s still difficult to obtain specific range of hydrocarbon
• In order to narrowing the range of hydrocarbon product we need to develop LTFT technology
• This process has many advantages. Besides of having low operating conditions (it’s means very feasible to do), this
process often produces middle destilate to heavy wax products
• so we can still use our refinery infrastructure
• In order to develop LTFT technology, we have to first develop the catalyst.
• The main goals of catalyst development is high syngas conversion and high product selectivity towards heavy wax
products C5+
4. • For those who doesn't quite familiar with catalyst, let me give a briefing introduction on Catalyst
• Catalyst is a substance that increases the rate of the reaction, without being substantially consumed in the process
• How it works, the catalyst changes the reaction path by lowering its activation energy and consequently the
catalyst increases the rate of reaction.
• Catalyst is consist of 3 component, active metal, promoter, and support.
• Catalyst can be prepared by impregnation, coprecipitation, and sol gel method
5 • Next, Let me go back into some more detail about how to develop the catalyst
• 1st. We use pretreatment method to modify the γ-Al2O3 support, the pore size of the support would expand, then
the the activity and selectivity for C5+ formation increased. It apears that the pore size of the support playing role
as a template for Co particle size formation.
• We could use this techniques In order to get desired Co particle size.
6 • 2nd. We use promoter addition, Cu and K.
• Promotion of support pretreatment-modified catalyst with Cu and K promoter have similar effects.
• Addition of low loadings of both promoter enhances the CO conversion and the selectivity of C5+, compare to the
standard Co. while the further addition will actually give negative effects.
• The only difference is that the decline and the rise in the selectivity of C 1 and of C5+ respectively, are much more
pronounced in K promoter, while the rise in CO conversion more pronounced in Cu promoter.
• The incerased activity due to the ease of reduction that arise from formation of Co particle size above 6 nm.
• While high yields of C5+ hydrocarbon arise due to suppression of cracking properties.
•
• Further characterization using TPD reveals that the surface acid amount was decreased
• So our next research topic is, the catalytic performance of the catalyst, is possibly influenced by the surface acidity
properties
7 • Catalytic activity test was carried out in a tubular fixed-bed reactor (O.D. = 20 mm) with a catalyst of 1 g.
• Prior to the reaction, the catalyst was reduced in a flow of H2 (90 NTP ml/min) at 400oC.
• After the reduction is completed, the catalyst was then cooled down to 250oC in flowing H2, slowly pressurized to
the reaction pressure of 20 bar, switched to syngas (molar ratio of H2/CO = 2 : 1), then the feed gas flow rate was
adjusted to 25 ml/min.
• The gas phase sample at input and output points were taken every 1 hour and analyzed using a gas chromatograph
(GC)
8. • Next, to ensure that there are no diffusion barriers in the catalytic activity test, a diffusion limitation test was
carried out.
• The external diffusion limitation test is carried out by varying the linear flow rate at a fixed residence time.
• While The internal diffusion limitation test is carried out by varying the catalyst particle size at a linear flow rate
which has free external diffusion limitation.
• Data was taken at Q > 25 mL/min with particle size between 30 – 50 mesh”
9 • This is the calculation methods
10 • Fischer-Tropsch product consist of 2 type, wich is light hydrocarbon and heavy hydrocarbon
• Briefly, from chromatogram there are more than C21+ , 42 precisely C carbon that identified
11 • After impregnation of the cobalt species on the modified support, the pore diameter were slightly decreased, but
the surface area and pore volume were increased, suggests that some external pore formation has occurred.
• Further promotion with Cu promoter increased the surface area, pore volume and pore diameter as compared
with those of the unpromoted modified catalyst. The most probable interpretation of this phenomenon is again
related to the external pore formation.
• On the other side, promotion with K promoter has a drastic reverse effect, suggest that some massive pore
blockage and collapse phenomenon.
12 • From XRD diffractogram there are two compound that can be identified, there are gamma alumina and cobalt
oxide
• Co species all existed in the form of Co3O4 crystalline phase, no diffraction peak of CoO, Cobalt aluminate or other
Co species were found.
• The particle size of Co3O4 is calculated by using the X-ray line broadening method with the help of Debye-Scherrer
equation. While the particle size of cobalt, is estimated using Schanke equation with consideration of changes in
molar volume occurring during Co3O4 transition to Coo.
• average cobalt particle size (dp Co) obtained is in the range of 8.6-11 nm.
• Which can be considered as a suitable particle size that will bring a positive synergy
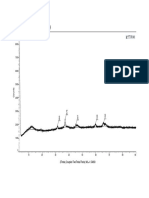
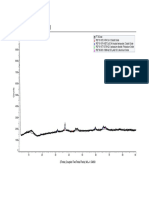
13 • The surface acidity of catalyst was examined by NH3-TPD
• From the picture on the left, There are two desorption peak observed, the low temperature is assigned to weak
acidic sites (below 300 oC) and the high temperature (above 400 oC) to strong acidic sites.
• The catalytic performance of the catalyst, especially the product distribution is possibly influenced by surface
acidity properties as a result of suppression of cracking properties.
• it was observed that almost all of the surface acidity of prepared catalysts are expected to have a beneficial effects
on activity since there are shifts in both desorption peak to the lower temperature.
• that support pretreatment with NH4NO3 solution decrease the catalyst relative acidity.
• Promotion with Cu promoter increased the relative acidity, while promotion with K promoter decreased the
relative acidity as compared with those of the unpromoted support pretreatment-modified catalyst.
14 •
Potrebbero piacerti anche
- FCC 8Documento28 pagineFCC 8bhupengeraNessuna valutazione finora
- ECBMDocumento25 pagineECBMShubham PrakashNessuna valutazione finora
- Project Presentation On Butadiene SulfoneDocumento69 pagineProject Presentation On Butadiene SulfoneDinesh DinnuNessuna valutazione finora
- Alptekin TDA CO2 Conversion To FuelDocumento20 pagineAlptekin TDA CO2 Conversion To Fueltiti neamtuNessuna valutazione finora
- Catalytic Cracking 3Documento10 pagineCatalytic Cracking 3SagarGuptaNessuna valutazione finora
- Fluid Catalytic Cracking 2Documento27 pagineFluid Catalytic Cracking 2PAWAR ROHAN RAMESHNessuna valutazione finora
- Cracking ReactionsDocumento15 pagineCracking ReactionssalemNessuna valutazione finora
- Chap 3B PetroDocumento39 pagineChap 3B Petrokishoreprithika100% (1)
- Lecture 8 - Metal Recovery From Leach SolutionsDocumento26 pagineLecture 8 - Metal Recovery From Leach SolutionsTeererai KaguraNessuna valutazione finora
- Gail Research and Development Noida: Guide Mr. N.K. Pande D.G.M (R&D) Vaibhav Raj ECHE16034 RgiptDocumento19 pagineGail Research and Development Noida: Guide Mr. N.K. Pande D.G.M (R&D) Vaibhav Raj ECHE16034 RgiptVaibhav RajNessuna valutazione finora
- Fluid Catalytic Cracking Unit Recent Advancements: Submitted byDocumento18 pagineFluid Catalytic Cracking Unit Recent Advancements: Submitted byprateek kumarNessuna valutazione finora
- 1 and 2 Lecture, رابع بوليمرات - د.زينبDocumento30 pagine1 and 2 Lecture, رابع بوليمرات - د.زينبMr. nobodyNessuna valutazione finora
- Isomerization ProcessDocumento35 pagineIsomerization ProcessAsim Memon100% (1)
- Reforming Process - Catalyst Advancement: A Presentation OnDocumento23 pagineReforming Process - Catalyst Advancement: A Presentation OnSiddharth Sharma100% (1)
- Presentation On Slurry ReactorsDocumento13 paginePresentation On Slurry ReactorsRaihanNessuna valutazione finora
- Module 4 (KTU)Documento118 pagineModule 4 (KTU)Aravind G100% (1)
- Catalysis and Catalytic ReactionsDocumento77 pagineCatalysis and Catalytic Reactionsdian_2108Nessuna valutazione finora
- Reforming and IsomerizationDocumento17 pagineReforming and Isomerizationhala mrayanNessuna valutazione finora
- Catalytic Reforming ProcessDocumento28 pagineCatalytic Reforming ProcessSiddesh PatilNessuna valutazione finora
- CT4485 Exam 31-01-2012 With AnswersDocumento7 pagineCT4485 Exam 31-01-2012 With AnswersVali100% (1)
- Chemical Oxygen Demand PDFDocumento8 pagineChemical Oxygen Demand PDFYajNessuna valutazione finora
- Presentation of Critical ReviewDocumento35 paginePresentation of Critical Reviewandresfm419Nessuna valutazione finora
- Catalysis and Catalytic Reactions: A. Sarath BabuDocumento77 pagineCatalysis and Catalytic Reactions: A. Sarath Babuazmigalaxy8955Nessuna valutazione finora
- Catalysis and Catalytic Reactions: A. Sarath BabuDocumento77 pagineCatalysis and Catalytic Reactions: A. Sarath BabuvamsiakellaNessuna valutazione finora
- The Diesel Catalytic ConverterDocumento4 pagineThe Diesel Catalytic ConvertersayeemNessuna valutazione finora
- Cat Con.Documento20 pagineCat Con.Hussain AhmedNessuna valutazione finora
- Fischer Tropsch SynthesisDocumento18 pagineFischer Tropsch Synthesisdeion29Nessuna valutazione finora
- CrackingDocumento28 pagineCrackingAlhaj MassoudNessuna valutazione finora
- Lecture9 - Hydrogen Generation Unit (HGU)Documento3 pagineLecture9 - Hydrogen Generation Unit (HGU)Bipradeep GhoshNessuna valutazione finora
- Unit 2 Refining Process - 2Documento25 pagineUnit 2 Refining Process - 2prathamesh singhNessuna valutazione finora
- A Vital Element of Our Low-Carbon Energy Future: Carbon Capture and StorageDocumento42 pagineA Vital Element of Our Low-Carbon Energy Future: Carbon Capture and StorageAsad KhanNessuna valutazione finora
- Catalytic Reforming and IsomerizationDocumento41 pagineCatalytic Reforming and IsomerizationAli Daniyal AwanNessuna valutazione finora
- Base Chemicals From Biomass and Intensification of CarbonDocumento10 pagineBase Chemicals From Biomass and Intensification of CarbonProbablyсвдсNessuna valutazione finora
- On IC EnginesDocumento29 pagineOn IC EnginesBabu JonnalagaddaNessuna valutazione finora
- Catalyst Synthesis TechniquesDocumento19 pagineCatalyst Synthesis Techniquescoolcupidguy100% (1)
- Processes 1:: Hydrode Alkylation UnitDocumento5 pagineProcesses 1:: Hydrode Alkylation UnitRay RomeyNessuna valutazione finora
- HydrotreatingPoster PDFDocumento1 paginaHydrotreatingPoster PDFekosmind100% (2)
- Effect of Coke Deposition On The Effective Diffusivity of Catalyst PelletDocumento12 pagineEffect of Coke Deposition On The Effective Diffusivity of Catalyst PelletJose NNessuna valutazione finora
- Reactor Design M.SCDocumento48 pagineReactor Design M.SCAquafina BaghdadNessuna valutazione finora
- Advanced Reactor Design: Slurry ReactorsDocumento23 pagineAdvanced Reactor Design: Slurry ReactorsBhushan Zade100% (1)
- HydroprocessingDocumento18 pagineHydroprocessinghala mrayanNessuna valutazione finora
- Week 5Documento91 pagineWeek 5Çiğdem Burçin IşlerNessuna valutazione finora
- Leonid SurguchevDocumento12 pagineLeonid SurguchevkglorstadNessuna valutazione finora
- Fluidized Bed Reactor 2Documento11 pagineFluidized Bed Reactor 2Mahdi SalihNessuna valutazione finora
- Methanol Recovery SystemDocumento17 pagineMethanol Recovery SystemJoao JesusNessuna valutazione finora
- Cre Seminar PPT 2Documento14 pagineCre Seminar PPT 2Shreya DatirNessuna valutazione finora
- Optimization of Wastewater Facilities To Address Biological Nutrient Reduction RequirementsDocumento19 pagineOptimization of Wastewater Facilities To Address Biological Nutrient Reduction RequirementscristyryeNessuna valutazione finora
- The Venturi Aeration Process:: Understanding Oxygen Transfer and Wastewater ConditioningDocumento23 pagineThe Venturi Aeration Process:: Understanding Oxygen Transfer and Wastewater ConditioningKumarNessuna valutazione finora
- Technical Paper PresentationDocumento27 pagineTechnical Paper PresentationBhagyashree BachhavNessuna valutazione finora
- Dry Reformation Reaction Using Ni-Co CatalystDocumento10 pagineDry Reformation Reaction Using Ni-Co CatalystGanesh VenkateshNessuna valutazione finora
- Literature Review CO2 ConversionsDocumento28 pagineLiterature Review CO2 ConversionscfmonarquiaNessuna valutazione finora
- Catalytic ReactionDocumento8 pagineCatalytic ReactionAzhari JahinNessuna valutazione finora
- Summer Research ProjectDocumento23 pagineSummer Research ProjectJim LivingstonNessuna valutazione finora
- CPT Lecture19 Monsanto ProcessDocumento27 pagineCPT Lecture19 Monsanto ProcessRajeev MisraNessuna valutazione finora
- AJChE - Zaky Al Fatony - Final ProofingDocumento12 pagineAJChE - Zaky Al Fatony - Final ProofingZakyAlFatonyNessuna valutazione finora
- Lecture 2 Population & Water Demand ForecastingDocumento49 pagineLecture 2 Population & Water Demand ForecastingDoodh MakhanNessuna valutazione finora
- Hydrogen From Biomass - Catalytic Reforming of PyrolysisvaporsDocumento4 pagineHydrogen From Biomass - Catalytic Reforming of PyrolysisvaporsVentrue LeongNessuna valutazione finora
- Unit II - Engine Emission Control and 3 Way Catalytic ConverterDocumento28 pagineUnit II - Engine Emission Control and 3 Way Catalytic ConverterdrkbalaNessuna valutazione finora
- Environmentally Benign Approaches for Pulp BleachingDa EverandEnvironmentally Benign Approaches for Pulp BleachingNessuna valutazione finora
- Fire Water ProtectionDocumento68 pagineFire Water ProtectionZakyAlFatony100% (1)
- FT 00.raw: 2theta (Coupled Twotheta/Theta) WL 1.54060Documento3 pagineFT 00.raw: 2theta (Coupled Twotheta/Theta) WL 1.54060ZakyAlFatonyNessuna valutazione finora
- Safe Chart Kinanti Field Rev 0Documento2 pagineSafe Chart Kinanti Field Rev 0ZakyAlFatonyNessuna valutazione finora
- Pump Calculation - HydraulicDocumento42 paginePump Calculation - HydraulicZakyAlFatonyNessuna valutazione finora
- FT X0, DB ViewDocumento6 pagineFT X0, DB ViewZakyAlFatonyNessuna valutazione finora
- FT 00, DB ViewDocumento6 pagineFT 00, DB ViewZakyAlFatonyNessuna valutazione finora
- FT 01, DB ViewDocumento6 pagineFT 01, DB ViewZakyAlFatonyNessuna valutazione finora
- Zaky Al-Fatony Department of Chemistry, Faculty of Mathematics and Natural Sciences, Gadjah Mada UniversityDocumento1 paginaZaky Al-Fatony Department of Chemistry, Faculty of Mathematics and Natural Sciences, Gadjah Mada UniversityZakyAlFatonyNessuna valutazione finora
- Perhitungan Friction Head Atau Friction LoosesDocumento2 paginePerhitungan Friction Head Atau Friction LoosesZakyAlFatonyNessuna valutazione finora
- Daftar PustakaDocumento9 pagineDaftar PustakaZakyAlFatonyNessuna valutazione finora
- Steam Tables: Saturated Steam: TEMPERATURE TableDocumento16 pagineSteam Tables: Saturated Steam: TEMPERATURE TableZakyAlFatonyNessuna valutazione finora
- Safety Data Sheet: 1. Identification of The Substance/Preparation and of The Company Decant OilDocumento11 pagineSafety Data Sheet: 1. Identification of The Substance/Preparation and of The Company Decant OilZakyAlFatonyNessuna valutazione finora
- James J. Carberry - Chemical and Catalytic Reaction Engineering (2001, Dover Publications)Documento334 pagineJames J. Carberry - Chemical and Catalytic Reaction Engineering (2001, Dover Publications)ZakyAlFatonyNessuna valutazione finora
- Fischer-Tropsch Synthesis For Greener Fuel Oil Production: Pertamina UniversityDocumento14 pagineFischer-Tropsch Synthesis For Greener Fuel Oil Production: Pertamina UniversityZakyAlFatonyNessuna valutazione finora
- Zaky Al-FatonyDocumento16 pagineZaky Al-FatonyZakyAlFatonyNessuna valutazione finora
- Application of Computational Chemistry Method To Study The Antioxidant Activity of CurcuminDocumento12 pagineApplication of Computational Chemistry Method To Study The Antioxidant Activity of CurcuminZakyAlFatonyNessuna valutazione finora
- Application of Computational Chemistry Method To Study The Relative Antioxidant Efficiency of A Large Series of CarotenoidsDocumento15 pagineApplication of Computational Chemistry Method To Study The Relative Antioxidant Efficiency of A Large Series of CarotenoidsZakyAlFatonyNessuna valutazione finora
- AJChE - Zaky Al Fatony - Final ProofingDocumento12 pagineAJChE - Zaky Al Fatony - Final ProofingZakyAlFatonyNessuna valutazione finora
- K Factor or Watson FactorDocumento6 pagineK Factor or Watson FactorZakyAlFatonyNessuna valutazione finora
- Site Analysis Report - Mr. Reimond SilvestreDocumento13 pagineSite Analysis Report - Mr. Reimond Silvestrechristian reyesNessuna valutazione finora
- The Mine Drainage Quality Prediction of Surface Coal Mine Rock Samples With Humidity Column Test-3-10Documento8 pagineThe Mine Drainage Quality Prediction of Surface Coal Mine Rock Samples With Humidity Column Test-3-10Fanteri Aji DharmaNessuna valutazione finora
- NA14631A - CristopiaDocumento3 pagineNA14631A - CristopiaVietHienNessuna valutazione finora
- General Circulation of The Atmosphere Geography Notes For UPSCDocumento2 pagineGeneral Circulation of The Atmosphere Geography Notes For UPSCmayank tripathiNessuna valutazione finora
- 8 Heat and TemperatureDocumento3 pagine8 Heat and TemperatureMelanie TrinidadNessuna valutazione finora
- Contamination of Rivers and Water Reservoirs in and Around Addisababa City and Actions To Combat ItDocumento12 pagineContamination of Rivers and Water Reservoirs in and Around Addisababa City and Actions To Combat ItEphremZelekeNessuna valutazione finora
- Introduction To ChemistryDocumento22 pagineIntroduction To ChemistryCharles MintahNessuna valutazione finora
- Thermal Properties & Temperature 10 MSDocumento4 pagineThermal Properties & Temperature 10 MStapceNessuna valutazione finora
- DLP Science 6Documento6 pagineDLP Science 6Tim Lopez IINessuna valutazione finora
- Environmental Change and Security Program Report 3Documento240 pagineEnvironmental Change and Security Program Report 3The Wilson CenterNessuna valutazione finora
- Thesis - Maisarah Binti Zaharudin - KH17059Documento52 pagineThesis - Maisarah Binti Zaharudin - KH17059LogamalarNessuna valutazione finora
- Other Relevant Laws Codes and Standards in The Energy and Power IndustryDocumento69 pagineOther Relevant Laws Codes and Standards in The Energy and Power IndustryVanvan BitonNessuna valutazione finora
- Datasheet HANERSUN HN21 PERCDocumento2 pagineDatasheet HANERSUN HN21 PERCHelison MedinaNessuna valutazione finora
- Earthquakes and VolcanoesDocumento16 pagineEarthquakes and VolcanoesPitchaiahTelagathotiNessuna valutazione finora
- Ieep 109Documento3 pagineIeep 109ShubhamNessuna valutazione finora
- Ulleberg Paper Usar PDFDocumento13 pagineUlleberg Paper Usar PDFoscarNessuna valutazione finora
- PWRSLMDocumento3 paginePWRSLMGANESH MURUGANNessuna valutazione finora
- Hydrologic CycleDocumento5 pagineHydrologic CyclesupermaneditNessuna valutazione finora
- Science RevisionDocumento7 pagineScience RevisionRoséNessuna valutazione finora
- Manufacturing Cost EstimationDocumento7 pagineManufacturing Cost EstimationMadiha ZaimuriNessuna valutazione finora
- Power Sector in Haryana: An IntroductionDocumento26 paginePower Sector in Haryana: An IntroductionTharki BhuddhaNessuna valutazione finora
- Thermodynamic Properties of Superheated and Supercritical Steam PDFDocumento7 pagineThermodynamic Properties of Superheated and Supercritical Steam PDFRoberto ErazoNessuna valutazione finora
- The Environment Rephrasing KeyDocumento2 pagineThe Environment Rephrasing KeyPatricia CapaceNessuna valutazione finora
- One-Dimensional Flow of Water Through Soils: ImportanceDocumento26 pagineOne-Dimensional Flow of Water Through Soils: ImportanceAngieNessuna valutazione finora
- Chemical Engineering Thermodynamics BasicsDocumento46 pagineChemical Engineering Thermodynamics Basicsdppriya1984Nessuna valutazione finora
- Aluminium Chemical PropertieDocumento9 pagineAluminium Chemical PropertieShreenivas ThakurNessuna valutazione finora
- Primary PetrolDocumento2 paginePrimary PetrolHarikesa KalasilvanNessuna valutazione finora
- Sba 1Documento9 pagineSba 1Regina BrooksNessuna valutazione finora
- FBC Boilers - Question Answer 1Documento8 pagineFBC Boilers - Question Answer 1Balaji PillaiNessuna valutazione finora