Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Chem26.1 Appendix Exp11 21718
Caricato da
Alexander Gordon InesTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Chem26.1 Appendix Exp11 21718
Caricato da
Alexander Gordon InesCopyright:
Formati disponibili
J. A. Ines/Chemistry 26.
1 (2018) P a g e |3
`APPENDIX
I. Concentration of Standard Cu (II), ppm
M1V1 = M2V2
1. 2.00 mL working standard solution: 3. 6.00 mL working standard solution:
2500 ppm × 0.002 L 2500 ppm × 0.006 L
= 100 ppm Cu (II) = 300 ppm Cu (II)
0.050 L 0.050 L
2. 4.00 mL working standard solution: 4. 8.00 mL working standard solution:
2500 ppm × 0.004 L 2500 ppm × 0.008 L
= 200 ppm Cu (II) = 400 ppm Cu (II)
0.050 L 0.050 L
5. 10.00 mL working standard solution:
2500 ppm × 0.010 L
= 500 ppm Cu (II)
0.050 L
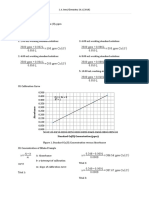
III. Calibration Curve
0.500
0.450
0.400
0.350 y = 0.0009x + 1E-04
Absorbance
0.300 R² = 0.9997
0.250
0.200
0.150
0.100
0.050
0.000
0.00 100.00 200.00 300.00 400.00 500.00 600.00
Standard Cu(II) Concentration (ppm)
Figure 1. Standard Cu(II) Concentration versus Absorbance
III. Concentration of Diluted Sample
x=
A−b
A- Absorbance Trial 2:
m
b- y-intercept of calibration x=
0.349−0.0001
= 387.67 ppm Cu (II)
curve 0.0009
m- slope of calibration curve Trial 3:
Trial 1: 0.348−0.0001
x= = 386.56 ppm Cu (II)
0.0009
0.348−0.0001
x= = 386.56 ppm Cu (II)
0.0009
J. A. Ines/Chemistry 26.1 (2018) P a g e |4
Potrebbero piacerti anche
- Chem26.1 Appendix Exp11 21718Documento3 pagineChem26.1 Appendix Exp11 21718Alexander Gordon InesNessuna valutazione finora
- Chem 26.1 Experiment 11Documento5 pagineChem 26.1 Experiment 11Iza SalvadorNessuna valutazione finora
- Supplementary MaterialDocumento7 pagineSupplementary MaterialInigo JohnsonNessuna valutazione finora
- Lab AnalysisDocumento4 pagineLab AnalysisErnestasBlaževičNessuna valutazione finora
- Chemy 310 Experiment 5Documento9 pagineChemy 310 Experiment 5Faisal MumtazNessuna valutazione finora
- Kurva Hubungan Konsentrasi Dan AbsorbansiDocumento2 pagineKurva Hubungan Konsentrasi Dan Absorbansidsw27Nessuna valutazione finora
- AbsorptionDocumento7 pagineAbsorptionIrvan DwikiNessuna valutazione finora
- Kurva Kalibrasi NH3 Air: X - X - N y - X - Xy - N BDocumento3 pagineKurva Kalibrasi NH3 Air: X - X - N y - X - Xy - N BFenisa SainyakitNessuna valutazione finora
- Aas ManualDocumento2 pagineAas ManualCharitra Prakash ChourasiaNessuna valutazione finora
- Figures and TablesDocumento3 pagineFigures and TablesAngeline RabuyoNessuna valutazione finora
- Analysis of Nitrite Ion Contents in Meat Sample by UV Vis Spectroscopy Method Results and DiscussionDocumento2 pagineAnalysis of Nitrite Ion Contents in Meat Sample by UV Vis Spectroscopy Method Results and DiscussionANH NGUYỄN HUỲNH MINHNessuna valutazione finora
- Penentuan Uji Kelarutan KTI ParacetamolDocumento6 paginePenentuan Uji Kelarutan KTI ParacetamolMinul1412Nessuna valutazione finora
- Chemical Kinetics: Chapter 14.1-2Documento22 pagineChemical Kinetics: Chapter 14.1-2Sagar GurowNessuna valutazione finora
- Perhitungan Laprak Kai VisibleDocumento7 paginePerhitungan Laprak Kai VisibleWanda SariNessuna valutazione finora
- Lab 1Documento6 pagineLab 1Tiyah TimothyNessuna valutazione finora
- Exercicis UVvis - SoluciãDocumento17 pagineExercicis UVvis - SoluciãclerrosseNessuna valutazione finora
- Flume AnalysisDocumento11 pagineFlume AnalysisLarizza TesicoNessuna valutazione finora
- Abs Vs Concentration of MN: 1.2 F (X) 0.0099552x + 0.0265 R 0.9913511755Documento2 pagineAbs Vs Concentration of MN: 1.2 F (X) 0.0099552x + 0.0265 R 0.9913511755ashNessuna valutazione finora
- Seminar 1 PDFDocumento29 pagineSeminar 1 PDFDiana Andrade LarrañagaNessuna valutazione finora
- GraphsDocumento1 paginaGraphsbikeseth10Nessuna valutazione finora
- Problem GaoDocumento3 pagineProblem Gaom.a.saeedNessuna valutazione finora
- Chem 26.1 - Lab Report 8Documento5 pagineChem 26.1 - Lab Report 8Gio Angelo IdosNessuna valutazione finora
- KFA Derivatif 5-6 ADocumento11 pagineKFA Derivatif 5-6 AElvina Iskandar TanurahardjaNessuna valutazione finora
- Bab Iv Hasil Dan Pembahasan 4.1 Data PengamatanDocumento9 pagineBab Iv Hasil Dan Pembahasan 4.1 Data PengamatanSazgiaMaudaraNessuna valutazione finora
- Curva Standard: Concentra Absorb BK 0 - 0.001 P2 0.2 0.002 P4 0.4 0.005Documento2 pagineCurva Standard: Concentra Absorb BK 0 - 0.001 P2 0.2 0.002 P4 0.4 0.005pamelaNessuna valutazione finora
- Accuracy Percision LabDocumento2 pagineAccuracy Percision LabAlexaNessuna valutazione finora
- PHY 2042 Experiment #10Documento2 paginePHY 2042 Experiment #10Kelsey WNessuna valutazione finora
- Lembar Perhitungan RiakDocumento10 pagineLembar Perhitungan RiakDiora PurbaNessuna valutazione finora
- 1264 - 1219 - PIC Prak 1 2018Documento9 pagine1264 - 1219 - PIC Prak 1 2018RafaelNessuna valutazione finora
- Calculos AAIDocumento3 pagineCalculos AAIPatty RubyNessuna valutazione finora
- kp2 Responsi-FiltrationDocumento10 paginekp2 Responsi-FiltrationNoer FaizinNessuna valutazione finora
- Atomic Absorption Spectroscopy: CHEMY 313 Analytical ChemistryDocumento7 pagineAtomic Absorption Spectroscopy: CHEMY 313 Analytical ChemistryJassim123 SabtNessuna valutazione finora
- Bab 2Documento3 pagineBab 2ArifinCoyNessuna valutazione finora
- Perhitungan Dan Daftar PustakaDocumento7 paginePerhitungan Dan Daftar PustakaAlya SholikhatulNessuna valutazione finora
- Kurva Kalibrasi: Konsentrasi (PPM)Documento2 pagineKurva Kalibrasi: Konsentrasi (PPM)Shavira AriyanNessuna valutazione finora
- Assignment 5Documento8 pagineAssignment 5Adam FaisalNessuna valutazione finora
- Experiment 5 - Data TreatmentDocumento6 pagineExperiment 5 - Data TreatmentShawn Ann SilanNessuna valutazione finora
- Visible Spectroscopy of FeDocumento1 paginaVisible Spectroscopy of FeAnnaReyesNessuna valutazione finora
- Question & Answer Set-6 PDFDocumento3 pagineQuestion & Answer Set-6 PDFhp2020Nessuna valutazione finora
- Exercises - EnzymesDocumento9 pagineExercises - EnzymesBug AphidNessuna valutazione finora
- Absorban Ammonium: Absorban Konsentrasi (MG/L)Documento2 pagineAbsorban Ammonium: Absorban Konsentrasi (MG/L)sisiNessuna valutazione finora
- Experiment.4 Uv/Vis Spectrophotometry: Department of Chemistry University of Bahrain CHEMY310Documento6 pagineExperiment.4 Uv/Vis Spectrophotometry: Department of Chemistry University of Bahrain CHEMY310Zahra Al-BasriNessuna valutazione finora
- T (Jam) Kadar Obat (MG/L) 0.5 213.00 2.33 1 186.00 2.27 1.5 163.00 2.21 3 128.00 2.11 6 79.00 1.90 9 47.00 1.67 12 29.00 1.46 Log Kadar Obat (MG/L)Documento4 pagineT (Jam) Kadar Obat (MG/L) 0.5 213.00 2.33 1 186.00 2.27 1.5 163.00 2.21 3 128.00 2.11 6 79.00 1.90 9 47.00 1.67 12 29.00 1.46 Log Kadar Obat (MG/L)Annisa Fauzia UlhaqNessuna valutazione finora
- TEST 1 SolutionDocumento25 pagineTEST 1 Solutionsgupta_192494Nessuna valutazione finora
- Chemlab2full Report Exp11Documento11 pagineChemlab2full Report Exp11Kirthinee JegatheesanNessuna valutazione finora
- 500 Analisa Kolam Renang 2Documento16 pagine500 Analisa Kolam Renang 2ENG INKNessuna valutazione finora
- Foster Cole 101230199 Malaïka Zarrouki 2021-01-29Documento7 pagineFoster Cole 101230199 Malaïka Zarrouki 2021-01-29Cole FosterNessuna valutazione finora
- Haloalkanes WorksheetDocumento6 pagineHaloalkanes WorksheetraginieraNessuna valutazione finora
- Hasil Adsorben KontrolDocumento5 pagineHasil Adsorben KontrolDwi NoviantiNessuna valutazione finora
- Unsteady NumericalsDocumento6 pagineUnsteady NumericalsYadnyesh TaNessuna valutazione finora
- Data TurbineDocumento3 pagineData TurbineMuhamad IzzanNessuna valutazione finora
- Benzene 2Documento8 pagineBenzene 2VishwarajNessuna valutazione finora
- Inductively Coupled Plasma Emission SpectrosDocumento6 pagineInductively Coupled Plasma Emission SpectrosOm PhileNessuna valutazione finora
- Flas K# Mass of Charcoa L (G) Initial Concentrati On of Acoh (M) Volume of Naoh Used (ML) Run 1 Run 2Documento2 pagineFlas K# Mass of Charcoa L (G) Initial Concentrati On of Acoh (M) Volume of Naoh Used (ML) Run 1 Run 2shaiNessuna valutazione finora
- Input: Units of Measurement Foundation Information LoadingDocumento4 pagineInput: Units of Measurement Foundation Information LoadingMayuresh KudveNessuna valutazione finora
- Experiment 1: The Visible Spectra of Soft Drinks: A. Pre-Laboratory QuestionsDocumento5 pagineExperiment 1: The Visible Spectra of Soft Drinks: A. Pre-Laboratory QuestionsMuhd Mirza HizamiNessuna valutazione finora
- Lembar Perhitungan Haris p1Documento6 pagineLembar Perhitungan Haris p1Haris IfroniNessuna valutazione finora
- ChE 35L 1adsorptionDocumento12 pagineChE 35L 1adsorptionMichael Alex Sison MabaoNessuna valutazione finora
- Quantitative Determination of Soda Ash Composition by Double Indicator TitrationDocumento2 pagineQuantitative Determination of Soda Ash Composition by Double Indicator TitrationAlexander Gordon InesNessuna valutazione finora
- Chem26.1 ATQ Exp11 21718Documento2 pagineChem26.1 ATQ Exp11 21718Alexander Gordon InesNessuna valutazione finora
- Chem26.1 FR Exp7 21718Documento7 pagineChem26.1 FR Exp7 21718Alexander Gordon InesNessuna valutazione finora
- Appendix: J. A. Ines/Chemistry 26.1 (2018) - 3Documento6 pagineAppendix: J. A. Ines/Chemistry 26.1 (2018) - 3Alexander Gordon InesNessuna valutazione finora
- Lecture Notes - Chem 16 LE3Documento8 pagineLecture Notes - Chem 16 LE3Alexander Gordon InesNessuna valutazione finora
- Philippine Literature During The Spanish Colonial PeriodDocumento3 paginePhilippine Literature During The Spanish Colonial PeriodAlexander Gordon InesNessuna valutazione finora
- Lecture 1 - Temperature and Thermal EquilibriumDocumento21 pagineLecture 1 - Temperature and Thermal EquilibriumAlexander Gordon InesNessuna valutazione finora
- Chem 26.1 - Mock Formal ReportDocumento6 pagineChem 26.1 - Mock Formal ReportAlexander Gordon InesNessuna valutazione finora
- Vatican Railway SystemDocumento54 pagineVatican Railway SystemAlexander Gordon InesNessuna valutazione finora
- Lecture Notes - Chem 16 LE3Documento8 pagineLecture Notes - Chem 16 LE3Alexander Gordon InesNessuna valutazione finora
- Application of Statistical Concepts in The Determination of Weight Variation in SamplesDocumento2 pagineApplication of Statistical Concepts in The Determination of Weight Variation in SamplesAlexander Gordon InesNessuna valutazione finora
- Chem26.1 ATQ6 Double Indicator TitrationDocumento2 pagineChem26.1 ATQ6 Double Indicator TitrationAlexander Gordon InesNessuna valutazione finora
- Chem26.1 - ATQ6 - Double Indicator TitrationDocumento2 pagineChem26.1 - ATQ6 - Double Indicator TitrationAlexander Gordon InesNessuna valutazione finora
- Physics 72.1 - Electromagenetic Induction PrelabDocumento1 paginaPhysics 72.1 - Electromagenetic Induction PrelabAlexander Gordon InesNessuna valutazione finora
- BestPractices PDFDocumento14 pagineBestPractices PDFAnonymous tChrzngvNessuna valutazione finora
- IRDM Assignment-I PDFDocumento4 pagineIRDM Assignment-I PDFPiyush AggarwalNessuna valutazione finora
- 08 BQ - PADSB - Elect - P2 - R2 (Subcon Empty BQ)Documento89 pagine08 BQ - PADSB - Elect - P2 - R2 (Subcon Empty BQ)Middle EastNessuna valutazione finora
- Assembling Your Antenna SystemDocumento27 pagineAssembling Your Antenna SystemKam MusNessuna valutazione finora
- The Messenger 190Documento76 pagineThe Messenger 190European Southern ObservatoryNessuna valutazione finora
- 2.fundamentals of MappingDocumento5 pagine2.fundamentals of MappingB S Praveen BspNessuna valutazione finora
- IOTA Observers Manual All PagesDocumento382 pagineIOTA Observers Manual All PagesMarcelo MartinsNessuna valutazione finora
- Consider Typical Robots Consider Typical RobotsDocumento16 pagineConsider Typical Robots Consider Typical RobotsOthers ATBP.Nessuna valutazione finora
- Management of Odontogenic Infection of Primary Teeth in Child That Extends To The Submandibular and Submental Space Case ReportDocumento5 pagineManagement of Odontogenic Infection of Primary Teeth in Child That Extends To The Submandibular and Submental Space Case ReportMel FANessuna valutazione finora
- Ventilation WorksheetDocumento1 paginaVentilation WorksheetIskandar 'muda' AdeNessuna valutazione finora
- Frankenstein ExtractDocumento1 paginaFrankenstein ExtractAnneNessuna valutazione finora
- D'Shawn M. Haines: 423 East Fox Trail, Williamstown, NJ 08094 (856) 366-7049Documento2 pagineD'Shawn M. Haines: 423 East Fox Trail, Williamstown, NJ 08094 (856) 366-7049dshawnNessuna valutazione finora
- EWC 662 English Writing Critical Group Work Portfolio: Submitted ToDocumento31 pagineEWC 662 English Writing Critical Group Work Portfolio: Submitted ToNurul Nadia MuhamadNessuna valutazione finora
- How To Deliver A Good PresentationDocumento9 pagineHow To Deliver A Good PresentationGhozi Fawwaz Imtiyaazi LabiibaNessuna valutazione finora
- Thermo Exam QuestionsDocumento4 pagineThermo Exam QuestionssiskieoNessuna valutazione finora
- Cell Signaling - The ComponentsDocumento7 pagineCell Signaling - The Componentsk10 Lớp Dinh DưỡngNessuna valutazione finora
- SFA TRAINING MODULE Week 1Documento14 pagineSFA TRAINING MODULE Week 1Ivan Perez100% (1)
- Annual Premium Statement: Bhupesh GuptaDocumento1 paginaAnnual Premium Statement: Bhupesh GuptaBhupesh GuptaNessuna valutazione finora
- Amazing RaceDocumento2 pagineAmazing Racegena sanchez BernardinoNessuna valutazione finora
- Shock Cat 2009Documento191 pagineShock Cat 2009gersonplovasNessuna valutazione finora
- Research InstrumentsDocumento28 pagineResearch InstrumentsAnjeneatte Amarille AlforqueNessuna valutazione finora
- Eimco Elecon Initiating Coverage 04072016Documento19 pagineEimco Elecon Initiating Coverage 04072016greyistariNessuna valutazione finora
- Balfour Castle EstateDocumento20 pagineBalfour Castle EstatejahumphriesNessuna valutazione finora
- Unit One Mathematical EconomicsDocumento15 pagineUnit One Mathematical EconomicsSitra AbduNessuna valutazione finora
- Cognitive InfocommunicationsDocumento229 pagineCognitive Infocommunicationsradhakodirekka8732Nessuna valutazione finora
- Jibachha's Textbook of Animal Health Volume-IIDocumento16 pagineJibachha's Textbook of Animal Health Volume-IIjibachha sahNessuna valutazione finora
- PESTEL AnalysisDocumento2 paginePESTEL AnalysisSayantan NandyNessuna valutazione finora
- Executing and Releasing Value (V4.0.4.1) - A4Documento27 pagineExecuting and Releasing Value (V4.0.4.1) - A4V100% (1)
- Book Review: Cancy Mcarn Issues in Teacher Education, Spring 2009Documento4 pagineBook Review: Cancy Mcarn Issues in Teacher Education, Spring 2009juan_carlos0733Nessuna valutazione finora
- Ocular Trauma - BantaDocumento211 pagineOcular Trauma - BantaLuisa Fernanda Arboleda100% (1)