Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Activity 3 Seawater! See Water and Salts!: Temperature ( C) at Start of Boiling
Caricato da
Isle R. AlvarezTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Activity 3 Seawater! See Water and Salts!: Temperature ( C) at Start of Boiling
Caricato da
Isle R. AlvarezCopyright:
Formati disponibili
Name: Date:
Grade & Sec: Group#:
Activity 3
Seawater! See water and salts!
I. Objective
In this part, you should be able to collect distilled water and salts from seawater through the distillation and evaporation
processes.
II. Materials: (List the materials needed refer to figures 1 & 2.)
III. Data Gathering
1. Refer to figures 1 and 2 and answer the questions below. Write only the letter.
Fig. 1 Simple distillation set-up C Fig. 2 Evaporation set-up
______ hot bath ______ seawater _____ distilled water _____ white crystals (salt)
================================================================================================
Activity 4
Looks may be deceiving
I. Objectives: The students are expected to
1. describe the change in temperature of a substance during boiling;
2. describe the change in temperature of a mixture during boiling; and
3. differentiate between substances and mixtures based on how temperature changes during boiling.
II. Materials: List the materials used as shown in fig. 3 Boiling setup
III. Data gathering
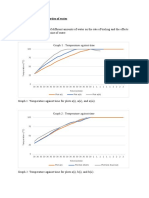
Using the data on the table, graph the temperature readings of the liquid samples and the time given to read. Use graphing paper,
red ink for distilled water and blue ink for sea water. Attach your graph.
Liquid Samples Distilled water Sea Water
Appearance/Odor
Temperature (˚C) at start of boiling 95˚C 100˚C
Temperature 30 seconds 98˚C 95˚C
(˚C) after 60 seconds 98˚C 98˚C
90 seconds 99˚C 110˚C
120 seconds 99˚C 105˚C
150 seconds 100˚C 115˚C
Q1. Refer to your graph, what do you notice about the temperature of distilled water and seawater during boiling?
Potrebbero piacerti anche
- Activity Sheet Maam AllynDocumento3 pagineActivity Sheet Maam AllynL-lynne NitramNessuna valutazione finora
- Weather Studies: The Commonwealth and International Library: Rural and Environmental Studies DivisionDa EverandWeather Studies: The Commonwealth and International Library: Rural and Environmental Studies DivisionNessuna valutazione finora
- Worksheet 1 Grade 7 (A, B, D)Documento2 pagineWorksheet 1 Grade 7 (A, B, D)Amal DhainyNessuna valutazione finora
- Activity Sheets in SolutionDocumento2 pagineActivity Sheets in SolutionAURORA () CARIAGANessuna valutazione finora
- TO1-Group-5 Soil Mechanics Lab Experiment No. 2Documento5 pagineTO1-Group-5 Soil Mechanics Lab Experiment No. 2Xam AcostaNessuna valutazione finora
- Emil Rahimov Report#3Documento10 pagineEmil Rahimov Report#3Emilll de CostaNessuna valutazione finora
- Freezing and Melting of Water: ExperimentDocumento8 pagineFreezing and Melting of Water: ExperimentagrikarthiNessuna valutazione finora
- Activity Sheet - Boiling WaterDocumento3 pagineActivity Sheet - Boiling WaterCrisanto LlorenteNessuna valutazione finora
- Donghan Max Wu - EoTTChangesofstateY7Documento12 pagineDonghan Max Wu - EoTTChangesofstateY7zenitsu9648Nessuna valutazione finora
- Mapúa University: Experiment No. - 2Documento15 pagineMapúa University: Experiment No. - 2Mark PulongbaritNessuna valutazione finora
- Boiling PointDocumento3 pagineBoiling PointaeneNessuna valutazione finora
- Temperature Practice Worksheet (Teacher)Documento1 paginaTemperature Practice Worksheet (Teacher)YingxiNessuna valutazione finora
- 18 Freeze Melt Water NspireDocumento3 pagine18 Freeze Melt Water NspireTha KantanaNessuna valutazione finora
- Exp 2 G7 1Documento13 pagineExp 2 G7 1Glenda Lizel BiceraNessuna valutazione finora
- Corrocher Patric Trending DataDocumento4 pagineCorrocher Patric Trending DataJackie McCarthyNessuna valutazione finora
- Experiment 2 - Separation of A MixtureDocumento4 pagineExperiment 2 - Separation of A MixtureSruthi MopuriNessuna valutazione finora
- Name of ExperimentDocumento7 pagineName of Experimentbayarali1993Nessuna valutazione finora
- IG2 Thermal Physics Practice TestDocumento5 pagineIG2 Thermal Physics Practice TestrehanNessuna valutazione finora
- Name of ExperimentDocumento7 pagineName of Experimentbayarali1993Nessuna valutazione finora
- "Heat (Q) and Change of Temperature (T) ": Student WorksheetDocumento4 pagine"Heat (Q) and Change of Temperature (T) ": Student WorksheetErna RisfaulaNessuna valutazione finora
- "Heat (Q) and Change of Temperature (T) ": Student WorksheetDocumento4 pagine"Heat (Q) and Change of Temperature (T) ": Student WorksheetErna RisfaulaNessuna valutazione finora
- Grade-3 Q1 W4 D1 IT-MATTERS Edited PDFDocumento10 pagineGrade-3 Q1 W4 D1 IT-MATTERS Edited PDFAPRILYN LIMOSNERONessuna valutazione finora
- Science 9: Activity 3 Soil and WaterDocumento1 paginaScience 9: Activity 3 Soil and Waterteleganne21Nessuna valutazione finora
- Lab 12 (SBA MM)Documento3 pagineLab 12 (SBA MM)intrinsicalinkyNessuna valutazione finora
- Grade 8 Laboratory Manual PDFDocumento25 pagineGrade 8 Laboratory Manual PDFSachi SummersNessuna valutazione finora
- Chemistry: Important Questions Before Half Yearly ExamDocumento14 pagineChemistry: Important Questions Before Half Yearly ExamSanjay GuptaNessuna valutazione finora
- Script C: Cambridge IGCSEDocumento14 pagineScript C: Cambridge IGCSEVanithalakshmi KumaresanNessuna valutazione finora
- Universidad Tecnologica Centroamericana: Cambios de FaseDocumento14 pagineUniversidad Tecnologica Centroamericana: Cambios de FaseAndrea SortoNessuna valutazione finora
- Soil Mechanics: "Grain Size Analysis: Sieve Test and Hydrometer Test"Documento11 pagineSoil Mechanics: "Grain Size Analysis: Sieve Test and Hydrometer Test"Andrea MagtutoNessuna valutazione finora
- Temperature To Heat Transfer (Pear) Set 1 (Question)Documento9 pagineTemperature To Heat Transfer (Pear) Set 1 (Question)6C09 FUNG CHI LAMNessuna valutazione finora
- C1501 Experiment 1 - Basic Laboratory TechniquesDocumento6 pagineC1501 Experiment 1 - Basic Laboratory TechniquesThuto SmithNessuna valutazione finora
- Calor I MertDocumento10 pagineCalor I MertUnknownNessuna valutazione finora
- What Happens When Ice MeltsDocumento2 pagineWhat Happens When Ice Meltsliagiba_abbyNessuna valutazione finora
- Physical Properties of Matter: ExperimentDocumento4 paginePhysical Properties of Matter: Experimentch chNessuna valutazione finora
- Experiment 1 - Mini Ice PlantDocumento7 pagineExperiment 1 - Mini Ice PlantJoren HuernoNessuna valutazione finora
- DateDocumento7 pagineDatebayarali1993Nessuna valutazione finora
- Score Correct Answer:3, 0: Remedial Test 1 - MatterDocumento5 pagineScore Correct Answer:3, 0: Remedial Test 1 - MatterRentika SiahaanNessuna valutazione finora
- Lab 15 Specific Heat of An Unknown MetalDocumento12 pagineLab 15 Specific Heat of An Unknown Metaljohn linNessuna valutazione finora
- The Difference Between Distilled Water and Salt Water Solution in Terms of Boiling Point.Documento6 pagineThe Difference Between Distilled Water and Salt Water Solution in Terms of Boiling Point.David Lancelot PiadNessuna valutazione finora
- Chem Lab ManualDocumento6 pagineChem Lab ManualAlan RoyNessuna valutazione finora
- Experiment No. 1 CalorimetryDocumento7 pagineExperiment No. 1 CalorimetryElah Mae Evangelista QuintilaNessuna valutazione finora
- 0625 Thermal Properties and Temperature - P1 - QPDocumento6 pagine0625 Thermal Properties and Temperature - P1 - QPapdrenlNessuna valutazione finora
- Metal Cup With Cotton Wool Layer: Table ADocumento2 pagineMetal Cup With Cotton Wool Layer: Table AhahaNessuna valutazione finora
- Physical Properties of WaterDocumento4 paginePhysical Properties of WaterLuk Ee RenNessuna valutazione finora
- Chem Exp 7 HydratesDocumento6 pagineChem Exp 7 HydratesAika AlmasovaNessuna valutazione finora
- Experiment 1-Student's Version - Edited2Documento8 pagineExperiment 1-Student's Version - Edited2214297Nessuna valutazione finora
- Calorimetry LabDocumento5 pagineCalorimetry LabUnknownNessuna valutazione finora
- Exp. (3) Comparing Specific Heat of Water and Vegetable Oil: Year Mr. Mohammed MahmoudDocumento17 pagineExp. (3) Comparing Specific Heat of Water and Vegetable Oil: Year Mr. Mohammed Mahmoudجیهاد عبدالكريم فارس0% (1)
- Exp - 1 Melting Point of IceDocumento2 pagineExp - 1 Melting Point of IceharishNessuna valutazione finora
- Experiment No. 1:: CalorimetryDocumento7 pagineExperiment No. 1:: CalorimetryEye ShieldNessuna valutazione finora
- Structure Question 1:: Solid and Liquid Liquid and Gas Gas OnlyDocumento7 pagineStructure Question 1:: Solid and Liquid Liquid and Gas Gas OnlyjirongNessuna valutazione finora
- The Melting and Freezing Points of NapthaleneDocumento17 pagineThe Melting and Freezing Points of NapthaleneIsaac AndrewNessuna valutazione finora
- Heat of Fusion of WaterDocumento6 pagineHeat of Fusion of WaterAishaNessuna valutazione finora
- Cambridge IGCSE: BIOLOGY 0610/63Documento12 pagineCambridge IGCSE: BIOLOGY 0610/631254 mai mohammed mohammed CenterNessuna valutazione finora
- Experiment 2 CHM 420Documento6 pagineExperiment 2 CHM 420bellaamin100% (2)
- ChemistryDocumento6 pagineChemistryAin SyakilahNessuna valutazione finora
- Enthalpy of Solution and ReactionDocumento5 pagineEnthalpy of Solution and ReactionCarmen GoguNessuna valutazione finora
- Experiment 7Documento22 pagineExperiment 7Hanifah Kurnia MuchtarNessuna valutazione finora
- Specific Heat: Driving QuestionsDocumento12 pagineSpecific Heat: Driving QuestionsGaille CastroNessuna valutazione finora
- High Voltage Testing LaboratoryDocumento6 pagineHigh Voltage Testing LaboratoryMohd Izham IdrisNessuna valutazione finora
- Bmsee Ii Math Challenge!Documento32 pagineBmsee Ii Math Challenge!Venus Cadenas AscarezNessuna valutazione finora
- Diffusion in Metals: The Flux of History: A. A. HoweDocumento7 pagineDiffusion in Metals: The Flux of History: A. A. HoweDaniel OrdoricaNessuna valutazione finora
- Molecules 18 02328Documento48 pagineMolecules 18 02328Yamid OrtizNessuna valutazione finora
- RMA4 Users Guide 09-27-2011Documento187 pagineRMA4 Users Guide 09-27-2011Athanasius Kurniawan Prasetyo AdiNessuna valutazione finora
- Schematic and Quill CreateDocumento18 pagineSchematic and Quill CreateDudika 5704Nessuna valutazione finora
- AccesoDocumento4 pagineAccesoerazorafaelNessuna valutazione finora
- Sem - MTMM (David A. Kenny)Documento8 pagineSem - MTMM (David A. Kenny)AJayNessuna valutazione finora
- Sucrose SolutionDocumento2 pagineSucrose Solutionrosangela_darcNessuna valutazione finora
- Desen Tehnic Note de Curs Si Aplicatii - Macarie Si Olaru - 2007Documento432 pagineDesen Tehnic Note de Curs Si Aplicatii - Macarie Si Olaru - 2007alexNessuna valutazione finora
- Chaos in The Hodgkin-Huxley Model: John Guckenheimer Ricardo A. OlivaDocumento10 pagineChaos in The Hodgkin-Huxley Model: John Guckenheimer Ricardo A. OlivaaldoNessuna valutazione finora
- Rock Proof: Liquid Water Integral WaterproofDocumento2 pagineRock Proof: Liquid Water Integral Waterproofimran jamalNessuna valutazione finora
- Begin As Follows: To Compute An MPN and The 95% Confidence Limits On The Log of The MPN: Enter The Inoculum/tube in Column ADocumento1 paginaBegin As Follows: To Compute An MPN and The 95% Confidence Limits On The Log of The MPN: Enter The Inoculum/tube in Column AlailinayahNessuna valutazione finora
- Presented By:: ASQ Section 0700 ASQ Section 0701Documento35 paginePresented By:: ASQ Section 0700 ASQ Section 0701fennyNessuna valutazione finora
- Pivot Master Floating Roof Drain SystemDocumento8 paginePivot Master Floating Roof Drain Systemnaveenbaskaran1989Nessuna valutazione finora
- Zeljkovic CedomirDocumento6 pagineZeljkovic CedomirPatriceEmNessuna valutazione finora
- Prototype To Help The Improvement of E-Toll PaymentDocumento14 paginePrototype To Help The Improvement of E-Toll Paymentfarhan satriyaNessuna valutazione finora
- 049-Itp For Lighting and Small Power (Building) PDFDocumento15 pagine049-Itp For Lighting and Small Power (Building) PDFKöksal Patan100% (1)
- AUC SheetDocumento26 pagineAUC SheetTanmay SagarNessuna valutazione finora
- GeomaticaDocumento5 pagineGeomaticaAlcantaraSusyNessuna valutazione finora
- Determination of Elastic Constants of Additive Manufactured Inconel 625 Specimens Using An Ultrasonic TechniqueDocumento11 pagineDetermination of Elastic Constants of Additive Manufactured Inconel 625 Specimens Using An Ultrasonic TechniqueHamidreza JavidradNessuna valutazione finora
- Resident Physics Lectures: Mammography X-Ray SystemDocumento42 pagineResident Physics Lectures: Mammography X-Ray SystemLajja Parikh PatelNessuna valutazione finora
- Practice Problem Sheet On FM-I Group ADocumento1 paginaPractice Problem Sheet On FM-I Group ASWAGATAM BAZNessuna valutazione finora
- Sri Chaitanya Physics Integer Type Question BANKkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkDocumento74 pagineSri Chaitanya Physics Integer Type Question BANKkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkkSai GokulNessuna valutazione finora
- Chapter 5 QS015 2017 18 Nota Pelajar 1Documento220 pagineChapter 5 QS015 2017 18 Nota Pelajar 1Ahmad MutqmaNessuna valutazione finora
- Pak 37 17 18Documento39 paginePak 37 17 18Lift carry LapsittingNessuna valutazione finora
- Gel ElectrophoresisDocumento13 pagineGel ElectrophoresisVishnu Reddy Vardhan PulimiNessuna valutazione finora
- Industrial HygieneDocumento29 pagineIndustrial Hygieneghoshna jyotiNessuna valutazione finora
- Evaluation of Michaelis-Menten ParametersDocumento109 pagineEvaluation of Michaelis-Menten ParametersAnonymous 0zrCNQ100% (1)
- CH 3 - AE Analysis and Design of Two-Way SlabsDocumento111 pagineCH 3 - AE Analysis and Design of Two-Way Slabsephrem100% (3)