Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
2 Grand Test 4
Caricato da
Manya SinghDescrizione originale:
Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
2 Grand Test 4
Caricato da
Manya SinghCopyright:
Formati disponibili
Dr. Sangeeta Khanna Ph.
D 1 CHEMISTRY COACHING CIRCLE
D:\Important Data\2017\+2\Physical\Grand Test\+2 Grand Test -4 2.7.2017\+2 Grand test-4.doc
Dr. Sangeeta Khanna Ph.D
Grand Test – 4 (2.7.2017) Test -6
Topic: Complete Physical Chemistry
READ INSTRUCTIONS CAREFULLY
1. The test is of 2 hour 30 minutes duration.
2. The maximum marks are 420.
3. This test consists of 98 questions.
4. Keep your mobiles switched off during Test in the Halls.
Section – A (Single Answer Type) Negative Marking [-1]
This Section contains 50 multiple choice questions. Each question has four choices A), B), C) and D) out
of which ONLY ONE is correct. Marks: 50 × 4 = 200
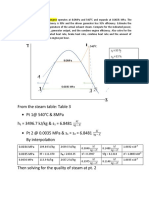
1. For the first order reaction, 3A B concentration varies
with time as shown in the adjacent graph. The half-life of
the reaction would be
Conc.
M
B
a. 2 minute
b. 4 minute A
c. 8 minute 2 4 6 8
d. 16 minute Time (min)
A
Sol. From graph it follows that at time 4 minute from the start of the reaction, [A] = [B]
3A B
t=0 a
t = 4 min. a–x x/3
x 3a 3a a
a-x x a-x a
3 4 4 4
i.e. in 4 minutes concentration of A has reduced to 25% of the initial value therefore, t3/4 = 4 min. As t3/4
= 2t1/2 for a first order reaction, so half-life of this reaction = 2 min.
2. A radioactive sample has initial activity of 28 dpm. half hour later its activity is 14 dpm. How many
atoms of nuclide were present initially?
a. 200 b. 400 c. 600 d. 1216
D
Sol. Activity = × number of atoms present at a given time
0.693
28 = × number of atoms present = Number of atoms present
t1 / 2
1
t1 / 2 hrs = 30 minutes
2
28 30
Number of atoms present = . = 1216
0.693
3. The axial angles in triclinic crystal system are
(a) = = = 90° (b) = = 90°, 90°
(c) 90° (d) = = 90°
C
Dr. Sangeeta Khanna Ph.D 2 CHEMISTRY COACHING CIRCLE
D:\Important Data\2017\+2\Physical\Grand Test\+2 Grand Test -4 2.7.2017\+2 Grand test-4.doc
Dr. Sangeeta Khanna Ph.D
4. The ionic radii of Rb+ and I– are 1.46 and 2.16 Å, respectively. The most probable type of structure
exhibited by it is
(a) CsCl type (b) NaCl type (c) ZnS type (d) CaF2 type
B
5. An ionic crystalline solid, MX3, has a cubic unit cell. Which of the following arrangement of the ions is
consistent with the stoichiometry of the compound?
(a) M3+ ions of the corners and X– ions at the faces centres
(b) M3+ ions at the corners and X– ions at the body centres
(c) X– ions at the corners and M3+ ions at the face centres
(d) X– ions at the corners and M3+ ions at the body centres
A
6. Give the correct order of initials T (true) of F (false) for following statements:
I. In an anti-flourite structure, anions form FCC and cations occupy all the tetrahedral voids.
II. If the radius of cation and anion is 20 and 95 pm, then the coordination number of cation in the
crystal is 4.
III. An atom or ion is transferred from a lattice site to an interstitial position in Frenkel defect.
IV. Density of crystal always increases due to substitutional impurity defect.
(a) TTTT (b) FFFF (c) FFTT (d) TFTF
D
30
Sol. II = 0.21 (triangular void)
85
7. A ferromagnetic substance becomes a permanent magnet when it is placed in a magnetic field
because
(a) all the domains get oriented in the direction of magnetic field
(b) all the domains get oriented in the direction opposite to the direction of magnetic field
(c) domains get oriented randomly
(d) domains are not affected by magnetic field
A
8. Van’t Hoff factor of 0.01 M CH3COOH solution is 1.04. What will be the ionisation constant and pH of
solution
Ka pH Ka Ph Ka pH Ka pH
a. 1.27× 10-5 2.9 b.1.57 × 103 5.2 c. 1.6 × 10-5 3.4 d.1.6×10-6 4.10
C
Sol. CH3COOH CH3COO + H+
i=1+
= 0.04
Ka = C2 i.e. = 0.01 × (0.04)2 = 16 × 10-6
+
H = C
= 0.01 × 0,04
= 4 × 10–4
9. A solution weighing ‘a’ g has molality b. The molecular mass of solute if the mass of solute is c g, will
be:
c 1000 b 1000 b 1000 c 1000
a. b. c. d.
b (a c ) a (a b ) c (a c ) b (b a)
A
w B 1000
Sol. Molality, m =
mB wA
c 1000
b =
m B (a c )
c 1000
mB =
b (a c )
Dr. Sangeeta Khanna Ph.D 3 CHEMISTRY COACHING CIRCLE
D:\Important Data\2017\+2\Physical\Grand Test\+2 Grand Test -4 2.7.2017\+2 Grand test-4.doc
Dr. Sangeeta Khanna Ph.D
10. Total vapour pressure of mixture of 1 mol A ( PA0 = 150 torr) and 2 mol B( PB0 = 240 torr) is 200 torr. In
this case:
a. there is positive deviation from Raoult’s law
b. there is negative deviation from Raoult’s law
c. there is no deviation from Raoult’s law
d. molecular masses of A and B are also required for calculating the deviation
B
1 2
Sol. XA = , XB =
3 3
P = PA0 X A PB0 XB
1 2
= 150 240 50 160 210 mm
3 3
Pexp. < Pcalculated
There is negative deviation from Raoult’s law.
11. Which of the following is correct order of elevation in Boiling point.
(I) 1 N NaCl (II) 1 N Na2SO4 (III) 1 N Na3PO4 (IV) 1 N urea
a. I < II < III < IV b. IV < I < III < II c. IV < III < II < I d. IV = III < I < II
C
Sol. Convert normality to molarity & then calculate (i × M):
1 1
(i) 1 × 2 = 2 (ii) 3 1.5 (iii) 4 1.33 (vi) 1
2 3
12. The solution containing 4.0 g of PVC in 1 L of dioxane was found to have osmotic pressure of 0.006
atm at 300 K. The molecular mass of the polymer PVC (in gm) is
(a) 16,420 (b) 1642 (c) 1,64,200 (d) 4105
A
W RT 4 0.082
Sol. O.P ; M 300 16420 gm
M V 0.006 1
13. Vapour pressure of the liquid
(a) increases with increase in temperature.
(b) decreases with increase in temperature
(c) is independent of temperature.
(d) either increases or decreases with the increase in temperature, depending on the nature of liquid.
A
14. For which of the following pair, the heat of mixing, Hmix, is approximately zero?
(a) CH3COOCH3 + CHCl3 (b) CH3COOH + H2O
(c) C2H5OH + CH3OH (d) CH3COCH3 + C6H6
C
Sol. For ideal solution
15. Pure water boils at 373 K and nitric acid at 359 K. The azeotropic mixture of water and nitric acid boils
at 393.5 K. On distillation of the azeotropic mixture,
(a) pure nitric acid will distil over first.
(b) pure water will distil over first
(c) one of them will distil over with small amount of the other.
(d) both of them will distil over in the same composition as they are in the mixture.
D
Dr. Sangeeta Khanna Ph.D 4 CHEMISTRY COACHING CIRCLE
D:\Important Data\2017\+2\Physical\Grand Test\+2 Grand Test -4 2.7.2017\+2 Grand test-4.doc
Dr. Sangeeta Khanna Ph.D
16. The ebullioscopic constant of a liquid solvent is the elevation of boiling point of
(a) one molar solution of non-volatile, non-electrolyte solute in it.
(b) one normal solution of non-volatile, non-electrolyte solute in it.
(c) one formal solution of non-volatile, non-electrolyte solute in it.
(d) one molal solution of non-volatile, non-electrolyte solute in it.
D
17. Phenol dimerizes in benzene. If the observed molecular mass of phenol in solution is 120, its degree of
dimerization is
(a) 0.600 (b) 0.433 (c) 0.277 (d) 0.866
B
94
Sol. i = 0.78 ; i = 1 - + ; = 0.44
12 2
18. Which one of the following pairs of solution can we expect to be isotonic at the same temperature?
(a) 0.1 M – urea and 0.1 M – NaCl (b) 0.1 M – urea and 0.1 M – MgCl2
(c) 0.1 M Na2SO4 and 0.1 M – NaCl (d) 0.1 M – Na2SO4 and 0.1 M – Ca(NO3)2
D
19. A 0.001 molal solution of a complex MA8 in water has the freezing point of -0.0054°C. Assuming 100%
ionization of the complex in water, which of the following is the correct representation of the complex?
(Kf of water = 1.86 Km–1)
(a) [MA8] (b) [MA7]A (c) [MA6]A2 (d) [MA5]A3
C
Sol. 0.0054 = i 1.86 × 0.001
i3
20. A pressure cooker reduces cooking time for food because
(a) Heat is more evenly distributed in the cooking space
(b) boiling point of water involved in cooking is increased
(c) the higher pressure inside the cooker crushes the food material
(d) cooking involves chemical changes helped by a rise in temperature
B
Sol. This is because the boiling point of water increases as the pressure increases
21. Two Equimolar solutions in the same solvent have
(a) Same boiling point but different freezing point
(b) Same freezing point but different boiling point
(c) Same boiling and same freezing points
(d) Different boiling and different freezing point
C
Sol. This is because colligative properties depend upon number of particles
22. The density (in g mL-1) of a 3.60 M sulphuric acid solution, that is, 29% H2SO4 (molar mass=98 g mol–1)
by mass will be
(a) 1.45 (b) 1.64 (c) 1.88 (d) 1.22
D
Sol. Let the density of the solution be . The molarity of the solution = 3.60 M (given). This means that 1 L
of solution contains 3.6 mol or (3.6 × 98) g of H2SO4 (98 g of mol–1 is the molecular mass of H2SO4.
Since, the solution is 29% by mass which means 29 g of H2SO4 is present in 100 g, therefore
100/ mL solution contains 29 g of H2SO4
3.6 × 98 g of H2SO4. Therefore,
100 29 1000 3.6 98 100 %• d 10
1.22 g L-1 or M
3.6 98 29 1000 M.Wt .
Dr. Sangeeta Khanna Ph.D 5 CHEMISTRY COACHING CIRCLE
D:\Important Data\2017\+2\Physical\Grand Test\+2 Grand Test -4 2.7.2017\+2 Grand test-4.doc
Dr. Sangeeta Khanna Ph.D
23. Maximum freezing point will be for 1 molal solution of assuming equal ionization in each case,
(a) [Fe(H2O)6]Cl3 (b) [Fe(H2O)5Cl]Cl2H2O
(c) Fe(H2O)4Cl2]Cl2H2O (d) [Fe(H2O)3Cl3]3H2O
D
1
Sol. f.pt
No. of particles
24. Intermolecular forces in liquid A are considerably large than intermolecular forces in liquid B. Which of
the following properties is NOT expected to be larger for A than B?
a. The vapour pressure at 20 °C
b. The temperature at which the vapour pressure is 100mm Hg
c. The critical temperature
d. The heat of vaporization (Hvap)
A
25. The colour of colloidal particles of gold obtained by different methods differ because of:
a. Variable valency of gold b. Different concentration of gold particles
c. Different types of impurities d. Different diameters of colloidal particles
D
26. When 1.2 g of sulphur is melted with 15 g of naphthalene, the solution freezes at 77.2°C. What is the
molar mass of this form of sulphur?
Data for naphthalene:
Melting point (m.pt.) 80°C
Freezing point depression constant Kf 6.80 K m–1
a. 180 g mol–1 b. 194 g mol–1 c. 260 g mol–1 d. 450 g mol–1
B
w 1000
Sol. Tf = Kf × B
mB w A
K w 1000
mB = f B
Tf wA
6.8 1.2 1000 8160
= 194 g mol -1
2.8 15 42
27. What is the molarity of HCl in a solution prepared by dissolving 5.5 g HCl in 200 g ethanol if the density
of the solution is 0.79 g mL–1?
a. 21 M b. 0.93 M c. 1.7 M d. 0.579 M
D
W(mass ) 205.5
Sol. V 260.12 m
d 0.79
w 1000 5.5 1000
M B = 0.579 M
mB V 36.5 260.12
28. CNS– ions give red colour with Fe3+ ions in aqueous solution as:
Fe (3aq ) 3CNS (aq) Fe (CNS ) 3 (aq)
red
If 0.1 M KCNS solution is separated from 0.1 M FeCl3 solution by means of semipermeable
membrane, red colour will appear on:
a. FeCl3 solution side b. KCN’s solution side c. both sides d. neither side
D
Sol. Only solvent molecules and not the solute molecules or ions can pass through the semipermeable
membrane. Hence, Fe3+ and CNS– ions do not come in contact on either side
Dr. Sangeeta Khanna Ph.D 6 CHEMISTRY COACHING CIRCLE
D:\Important Data\2017\+2\Physical\Grand Test\+2 Grand Test -4 2.7.2017\+2 Grand test-4.doc
Dr. Sangeeta Khanna Ph.D
29. Calculate the amount of ice that will separate out on cooling a solution containing 50 g ethylene glycol
in 200 g water to -9.3°C. (Kf = 1.86 K mol–1 kg)
a. 38.71 gm b. 45.23 gm c. 161.29 gm d. 50.00 gm
A
Sol. mglycol = 62
K w 1000 1.86 50 1000
T = f ; 9.3
m W 62 WWater
W water = 161.29 g
Wt. of ice separated = 200 – 161.29
30. The coagulation value in millimoles per litre of electrolytes used for the coagulation of As2S3 are as
below:
I. NaCl = 52 II. KCl = 51 III. BaCl2 = 0.69 IV. MgSO4 = 0.22
The correct order of their flocculating power is:
a. I > II > III > IV b. I > II > III = IV c. IV > III > II > I d. IV = III > II > I
C
Sol. Coagulation value is inversely proportional to their flocculating power.
31. The oxidation of oxalic acid by acidified KMnO4 becomes faster as the reaction progresses due to :
a. presence of MnO 4 b. presence of K+
c. presence of SO 24 d. auto catalysis by Mn2+
D
32. The degree of adsorption of solution on solid surface depends on concentration of solution:
x
K C1/n
m
In which of the conditions, we get following type of graph?
log x
m
log C
1
a. C = 0 b. 0 c. C = constant d. C = 2M
n
B
1
Sol. Horizontal straight line will be possible when slop is zero, i.e., =0
n
33. The stability of lyophillic sols can be described by
a. Brownian movement only b. like charges only
c. solvation only d. all the 3 factors contribute
D
34. Which of the following statements are FALSE?
(A) Gases with increase TC adsorb more
(B) Physical adsorption continuously decreases with increasing temperature
(C) Chemical adsorption continuously increases with increasing temperature
(D) Chemisorption is multilayered
a. A, B, C only b. B, C only c. C, D only d. only D is incorrect
C
Dr. Sangeeta Khanna Ph.D 7 CHEMISTRY COACHING CIRCLE
D:\Important Data\2017\+2\Physical\Grand Test\+2 Grand Test -4 2.7.2017\+2 Grand test-4.doc
Dr. Sangeeta Khanna Ph.D
35. When gels are allowed to stand, they give out small quantity of liquid (or water). This process is called
a. Coagulation of gel b. Syneresis
c. Thixotropy d. None of the above
B
36. It is necessary to remove CO when NH3 is obtained from Haber’s process because
a. CO will react with NH3 b. CO will be occupied in active site of catalyst
c. CO is poisonous d. CO is a good donor
B
37. Which of the following reaction is non-redox?
(a) 2NaNO3 2NaNO2 + O2 (b) CaO + SiO2 CaSiO3
(c) Fe + H2SO4 FeSO4 + H2 (d) 4Ag + 8CN– + O2 + 2H2O 4[Ag(CN)2]– + 4OH–
B
38. The ratio of coefficients of HNO3, Fe(NO3)2 and NH4NO3 in the following redox reaction:
Fe + HNO3 Fe(NO3)2 + NH4NO3 + H2O
are, respectively,
(a) 10 : 1 : 4 (b) 10 : 4 : 1 (c) 4 : 10 : 1 (d) 4 : 1 : 10
B
Sol. 4 × [Fe Fe+2 + 2e–
8e– + NO 3 NH 4
4Fe + NO 3 NH 4 4Fe 2
4Fe + HNO3 NH4NO3 + 4Fe(NO3)2
Add 10 NO 3 ions to Reactant side
4Fe + 10HNO3 NH4NO3 + 4Fe(NO3)2 + 3H2O
39. The vapour density of metal chloride is 77. If its equivalent weight is 3, its atomic mass will be
(a) 3 (b) 6 (c) 9 (d) 12
D
77 2
Sol. Valency 4; At. Wt. = 4 × 3 = 12
3 35.5
40. The standard reduction potentials of Pt|Cr2O72–, Cr+3; Pt|MnO4–, Mn+2; Pt|Ce+4, Ce+3 in the presence of
acid are 1.33 V, 1.51 V and 1.61 V, respectively, at 25°C. The decreasing order of oxidizing power is
(a) Cr2O72– > MnO4– > Ce+4 (b) MnO4– > Cr2O72– > Ce+4
–
+4
(c) Ce > MnO4 > Cr2O7 2–
(d) MnO4– > Ce+4 > Cr2O72–
C
41. From the following E° values for the half-cells:
(i) D D2+ + 2e–; E° = –1.5 V (ii) B+ + e– B; E° = – 0.5 V
– –
(iii) A A + e ; E° = 1.5 V
3– 2–
(iv) C + e C ; E° = +0.5 V
2+ +
Which combination of two half-cells would result in a cell with largest potential?
(a) i and iii (b) i and iv (c) iii and iv (d) ii and iv
A
42. A student made the following observations in the laboratory:
(I) Clean copper metal did not react with 1 M – Pb (NO3)2 solution.
(II) Clean lead metal dissolves in 1 M – AgNO3 solution and crystals of Ag metal appeared.
(III) Clean silver metal did not react with 1 M – Cu(NO3)2 solution.
The order of decreasing reducing character of the three metals is
(a) Cu > Pb > Ag (b) Cu > Ag > Pb (c) Pb > Cu > Ag (d) Pb > Ag > Cu
C
Dr. Sangeeta Khanna Ph.D 8 CHEMISTRY COACHING CIRCLE
D:\Important Data\2017\+2\Physical\Grand Test\+2 Grand Test -4 2.7.2017\+2 Grand test-4.doc
Dr. Sangeeta Khanna Ph.D
43. Which of the following graph truly represents the titration of CH3COOH solution against NaOH
solution?
(a) (b)
Cond. Cond.
VNaOH VNaOH
(c) (d)
Cond. Cond.
VNaOH VNaOH
C
44. The following reactions represent the reduction of IO 3 ion into I– ion in acidic and basic medium.
Predict in which medium IO 3 ion will act as a better oxidizing agent?
IO 3 6H 6e I 3H2O; E 0.907 V
IO 3 3H2O 6e I 6OH ; E 0.260 V
(a) Acid medium (b) Basic medium (c) Equally in both (d) Not predictable
A
45. A galvanic cell is set up from a zinc bar weighing 100 g and 1.0 L of 1.0 M copper sulphate solution.
How long would the cell run if it is assumed to deliver a steady current of 1.0 A? (Zn = 65.4)
(a) 53.6 h (b) 26.8 h (c) 81.97 h (d) 40.99 h
A
I t 1
Sol. Cu = 1 mole; n ; t = i ×2 × 96500 × 53.6 hr
xf 60 60
46. The current of 9.65 A flowing for 10 min deposits 3.0 g of a metal. The equivalent weight of the metal is
(a) 10 (b) 30 (c) 50 (d) 96.5
C
I t E.Wt 3 96500
Sol. W ; E.Wt 50
96500 9.65 10 60
47. In a conductivity cell, the two platinum electrodes, each of area 10 cm2 are fixed 1.5 cm apart. The cell
contained 0.05 N solution of a salt. If the two electrodes are just half dipped into the solution which has
a resistance of 50 , the equivalent conductance of the salt solution, in –1 cm2 eq–1, is
(a) 120 (b) 60 (c) 240 (d) 3000
A
1 1000
Sol. eq.
R a N
10
As solution is half dipped so a = = 5 cm2
2
1 1.5 1000
120
50 5 0.05
Dr. Sangeeta Khanna Ph.D 9 CHEMISTRY COACHING CIRCLE
D:\Important Data\2017\+2\Physical\Grand Test\+2 Grand Test -4 2.7.2017\+2 Grand test-4.doc
Dr. Sangeeta Khanna Ph.D
48. Resistance of 0.2 M solution of an electrolyte is 50 . The specific conductance of the solution is 1.4 S
m–1. The resistance of 0.5 M solution of the same electrolyte is 280 . The molar conductivity of 0.5 M
solution of the electrolyte (in S m2 mol–1 ) is
(a) 5 × 10–3 (b) 5 × 103 (c) 5 × 102 (d) 5 × 10–4
D
1
Sol. K
R a
1.4 × 50 =
a
70 m-1 =
a
Kx
M
1000 M
1 70
M = 5 × 10-4 -1 m-1 mol-1
280 1000 0.5
49. The conductivity of saturated solution of Ba3(PO4)2 is 1.2 × 10–5 –1 cm–1. The limiting equivalent
conductivities of BaCl2, K3PO4 and KCl are 160, 140 and 100 –1 cm2 eq–1, respectively. What is
solubility of Ba3(PO4)2 in mol/lit.
(a) 10–5 (b) 5 × 10–6 (c) 1.08 × 10–25 (d) 1.08 × 10–27
B
K 1000 1.2 10 -5 10 3
Sol. m 2400; M ; ; M = 5 × 10–6
M M
50. In a galvanic cell, the salt bridge
(a) does not participate chemically in the cell reaction
(b) stops the diffusion of ions from one electrode to another
(c) is necessary for the occurrence of the cell reaction
(d) ensures mixing of the two electrolytic solutions.
A
SECTION – B (ASSERTION & REASON) Negative Marking [-1]
This Section contains 20 multiple choice questions. Each question has four choices A), B), C) and
D) out of which ONLY ONE is correct. (20 × 4 = 80 Marks)
(A) Mark a if both A and R are correct and R is the correct reason of A.
(B) Mark b if both A and R are correct and R is not the correct reason of A.
(C) Mark c if A is correct and R is wrong.
(D) Mark d if A is wrong and R is correct.
1. Assertion: A colloidal sol of Al(OH)3 prepared by adding H2O in AlCl3 is more readily coagulated by
0.1 M NaCl than by 0.1 M Na2SO4.
Reason: The coagulating power of an electrolyte is related to the valency of the active ions.
(a) (A) (b) (B) (c) (C) (d) (D)
D
Sol. It will form positive colloids Al(OH)3.Al+3 so its effective ion is anion.
2. Assertion. Both soaps and detergents adsorb on the surface of dust & are called associated colloids.
Reason. They contain hydrophilic and hydrophobic moieties in their molecule and thus show
adsorption and association (micellization).
(a) (A) (b) (B) (c) (C) (d) (D)
A
Dr. Sangeeta Khanna Ph.D 10 CHEMISTRY COACHING CIRCLE
D:\Important Data\2017\+2\Physical\Grand Test\+2 Grand Test -4 2.7.2017\+2 Grand test-4.doc
Dr. Sangeeta Khanna Ph.D
3. Assertion: At CMC colligative properties of solution increases.
Reason: Bigger alkyl group in electrolyte decreases CMC.
(a) (A) (b) (B) (c) (C) (d) (D)
D
4. Assertion (A): The order of the following elementary reaction
2NO(g) + 2H2(g) 2H2O(g) + N2(g) is 3.
Reason (R): Order of reaction with respect to a given reactant is the power of the reactant’s
concentration in the elementary reaction.
(a) (A) (b) (B) (c) (C) (d) (D)
D
Sol. It is not an elementary reaction otherwise order will be 4
5. Assertion (A): Activity of 1 g pure U235 will be greater than the 1 gm of U3O8.
Reason (R): In the combined state, the activity of radioactive element decreases.
(a) (A) (b) (B) (c) (C) (d) (D)
C
Sol. U3O8 will have less number of uranium atoms
6. Assertion (A):- A catalyst provides an alternative path to the reaction in which conversion of reactants
into products takes place quickly.
Reason (R):- The catalyst forms an activated complex of lower potential energy with the reactant, so
more number of molecules are able to cross the barrier per unit time.
(a) (A) (b) (B) (c) (C) (d) (D)
A
7. Assertion (A): Distance between nearest lattice points in BCC is greater than in FCC having the same
edge length.
Reason (R): FCC has greater packing efficiency than BCC
(a) (A) (b) (B) (c) (C) (d) (D)
A
8. Assertion (A): KCl is more likely to show Schottky defect while LiI is more likely to show Frenkel
defect.
Reason (R): Schottky defect is more likely in ionic solids in which cations and anions are of
comparable size while Frenkel defect is more likely in ionic solids in which cations and anions have
large differences in their ionic sizes.
(a) (A) (b) (B) (c) (C) (d) (D)
A
9. Assertion (A): Conductivity of silicon increases by doping it with group 15 elements.
Reason (R): Doping means introduction of small amount of impurities like P, As or Bi into the pure
silicon crystal.
(a) (A) (b) (B) (c) (C) (d) (D)
B
10. Assertion (A): On heating ferromagnetic or ferrimagnetic substances, they become paramagentic
Reason (R): The electrons can change their spin on heating
(a) (A) (b) (B) (c) (C) (d) (D)
B
Sol. Paramagnetism is due to random orientation of magnetic dipole it is entropy driven.
11. Assertion (A): Desalination is the process of reverse osmosis, used to get the drinking water from
sea-water.
Reason (R): When sea-water is separated from normal water using semipermeable membrane and
high pressure is applied to the side of sea-water then water molecules pass towards normal water.
(a) (A) (b) (B) (c) (C) (d) (D)
Dr. Sangeeta Khanna Ph.D 11 CHEMISTRY COACHING CIRCLE
D:\Important Data\2017\+2\Physical\Grand Test\+2 Grand Test -4 2.7.2017\+2 Grand test-4.doc
Dr. Sangeeta Khanna Ph.D
A
Sol. Desalination is the process of reverse osmosis. On applying extra pressure to solution side, the
osmosis takes place in reverse direction.
12. Assertion (A): Observed colligative property of acetic acid dissolved in benzene is found greater than
calculated colligative property.
Reason (R): Acetic acid undergoes association to form dimmer, when dissolved in benzene
(a) (A) (b) (B) (c) (C) (d) (D)
D
Sol. When acetic acid is dissolved in benzene, it undergoes dimerisation thus colligative properties must
decrease.
13. Assertion (A): Rate of evaporation of water is less than that of ether under identical conditions.
Reason (R): Stronger is the intermolecular force, slower is the rate of evaporation and H2O has
stronger interparticle forces.
(a) (A) (b) (B) (c) (C) (d) (D)
A
14. Assertion (A): Ebullioscopy or cryoscopy cannot be used for the determination of molecular weight of
polymers.
Reason (R): High molecular weight solute leads to very low value of Tb or Tf.
(a) (A) (b) (B) (c) (C) (d) (D)
A
15. Assertion (A): Colloidal particles of As2S3 are negatively charged.
Reason (R): Gold sol can be prepared by Bredig’s arc method.
(a) (A) (b) (B) (c) (C) (d) (D)
B
16. Assertion (A): In lead storage battery, concentration of H2SO4 decreases during discharge.
Reason (R): It is due to formation of insoluble PbSO4.
(a) (A) (b) (B) (c) (C) (d) (D)
A
17. Assertion (A): In Galvanic cell, anode and cathode are, respectively, negative and positive electrode.
Reason (R): At anode, oxidation takes place and at cathode reduction takes place.
(a) (A) (b) (B) (c) (C) (d) (D)
B
18. Assertion (A): The conductivity of metals decreases while that of electrolytic solution increases with
increase in temperature.
Reason (R): Electrons in metals are very tightly held by the nucleus and are not free to move.
(a) (A) (b) (B) (c) (C) (d) (D)
C
19. Assertion (A): An electrochemical cell [galvanic cell] can be set up only when the redox reaction is
spontaneous.
Reason (R): A reaction is spontaneous if free energy change at constant temperature and pressure is
negative, which is converted into electrical energy.
(a) (A) (b) (B) (c) (C) (d) (D)
A
20. Assertion (A):: Electrode potential of any electrode will change on changing any of its intensive
properties.
Reason (R): Any intensive property of system changes on changing any of its properties, whether
intensive or extensive
(a) (A) (b) (B) (c) (C) (d) (D)
C
Dr. Sangeeta Khanna Ph.D 12 CHEMISTRY COACHING CIRCLE
D:\Important Data\2017\+2\Physical\Grand Test\+2 Grand Test -4 2.7.2017\+2 Grand test-4.doc
Dr. Sangeeta Khanna Ph.D
SECTION – C (Paragraph Type) Negative Marking [-1]
This Section contains 9 questions. Each of these questions has four choices A), B), C) and D) out of
which ONLY ONE is correct. 9 × 4 = 36 Marks
Comprehension – 1
Gold sol is metallic sol and it is negatively charged. It can be prepared by reduction method and
Bredig’s arc method. When NaCl solution is added to a gold sol, it results in coagulation. But there is
no coagulation of gold sol, when NaCl solution is added to a gold sol after adding gelatine. Protective
action is more at low temperature. Gold sol has very little or no affinity with its dispersion medium.
1. The stability of gelatine is
a. more than that of gold sol b. less than that of gold sol
c. equal to that of gold sol d. it can’t be compared
A
2. If the temperature of gold sol containing sodium chloride and gelatin increases to 70°C, then
a. protective action of gelatine will increase
b. protective action of gelatine will decrease
c. protective action do not get affected by increasing temperature
d. NaCl undergo more ionisation and adsorption of Na+ ions take place on surface of sol particle to
create zeta potential
B
3. When excess quantity of NaCl electrolyte is added to a pure gold colloid
a. it result in unstability of sol
b. it result in stabilization of sol
c. protection of gold sol takes place by NaCl electrolyte
d. it is not related to stability of sol
A
Comprehension 2
BaTiO3 crystallizes in the perovskite structure. This structure may be described as a cubic lattice with
barium ions occupying the corners of the unit cell, oxide ions occupying the face centres and titanium
ions occupying the centres of the unit cells.
4. If titanium is described as occupying holes in Ba-O lattice, what type of holes does it occupy?
(a) tetrahedral (b) octahedral (c) cubic (d) triangular
B
5. What fraction of the holes of this type does it occupy?
(a) 0.25 (b) 0.50 (c) 1.00 (d) 0.75
A
Sol. Out of four octahedral void per unit cell it occupies 1 hole
Paragraph – 3
The colligative properties of electrolytes require a slightly different approach than the one used for the
colligative properties of non-electrolytes. The electrolytes dissociate into ions in a solution. It is the
number of solute particles that determines the colligative properties of a solution. The electrolyte
solutions, therefore, show abnormal colligative properties. To account for this effect we define a
quantity called the van’t Hoff factor, given by
Dr. Sangeeta Khanna Ph.D 13 CHEMISTRY COACHING CIRCLE
D:\Important Data\2017\+2\Physical\Grand Test\+2 Grand Test -4 2.7.2017\+2 Grand test-4.doc
Dr. Sangeeta Khanna Ph.D
Actual number of particles in solution after dissociation
i
Number of formula units i nitially dissolved in solution
i = 1(for non-electrolytes);
i > 1 (for electrolytes, undergoing dissociation)
i < 1 (for solutes, undergoing association).
6. M.Wt. of Benzoic acid is found to be nearly double than actual M.Wt. by using colligative properties. It
is due to
a. Dissociation to form double No. of particles
b. Molecular state will not change
c. Due to association to form dimer via H-bonding
O
||
d. Due to trimerisation in benzene via C OH gp
C
7. A substance trimerises when dissolved in a solvent A. The van’t Hoff factor ‘i’ for the solution if = 100
a. 1 b. 1/3 c. 3 d. unpredictable
B
8. For a solution of a non-electrolyte in water, the van’t Hoff factor is:
a. always equal to 0 b. 1 c. always equal to 2 d. > 1 but < 2
B
9. 0.1 M K4[Fe(CN)6] is 60% ionized. What will be its van’t Hoff factor?
a. 1.4 b. 2.4 c. 3.4 d. 4.4
C
Sol. i = 1 + (5 – 1)
= 1 + 4 × 0.6 = 3.4
SECTION – D (More than One Answer Type) No Negative Marking
This Section contains 9 questions. Each question has four choices A), B), C) and D) out of which One
or More than one answer may be correct. 9 × 5 = 40 Marks
1. Which of the following statements are correct?
a. The small the gold number of a lyophilic colloid, larger will be its protective power
b. Lyophilic sols are easily coagulated by small amount of electrolyte
c. FeCl3 coagulates blood
d. The flocculation value of As2S3 sol is independent of the anion of coagulating electrolyte
A,C,D
Sol. (a) is correct because it will be most effective if it has lowest gold number.
(c) is correct because FeCl3 coagulates blood by neutralizing the charge.
(d) is correct because As2S3 is –vely charged colloid and coagulated by +ve ions (cations).
its floacculation is independent of charge on anion.
2. Which of the following statement(s) is/are incorrect regarding the defects in solids?
(a) AgBr crystal show both Schottky and Frenkel defect.
(b) Impurity defect by doping of Arsenic in Silicon results ‘n’-type semiconductor.
(c) Doping in crystal introduces dislocation defect.
(d) Metal deficient defect can occur with extra anion present in the interstitial voids.
C,D
Dr. Sangeeta Khanna Ph.D 14 CHEMISTRY COACHING CIRCLE
D:\Important Data\2017\+2\Physical\Grand Test\+2 Grand Test -4 2.7.2017\+2 Grand test-4.doc
Dr. Sangeeta Khanna Ph.D
3. Which of the following statement(s) is/are true regarding the electrical properties of solids?
(a) (Conductivity) metals << (Conductivity insulators < (Conductivity) semiconductors
(b) Depending upon temperature, TiO3 can behave as insulator or conductor.
(c) I2(s) is non-conducting
(d) n-type semiconductor will have conductivity less than pure semiconductor.
B,C
4. Which of the following processes can destroy the emulsion?
a. Freezing b. By electrophoresis at high potential
c. Addition of emulsifier d. By dilution
A,B
5. The given graphs / data I, II, III and IV represent general trends observed for different physisorption
and chemisorption processes under mild conditions of temperature and pressure. Which of the
following choice(s) about I, II, III and IV is (are) correct?
(I) (II)
P constant P constant
Amount of gas
Amount of gas
adsorbed
adsorbed
T
T
(III) (IV)
Potential Energy
Amount of gas
200 K
adsorbed
Eads
0
250 K Distance of molecule from the surface
Hads = 150 kJ mol
-1
a. I is physisorption and II is chemisorption b. I is physisorption and III is chemisorption
c. IV is chemisorption and II is chemisorption d. IV is chemisorption and III is chemisorption
A,C
6. Solution of two liquids A and B showing negative deivation from Raoult’s law, will show:
a. Hmix < 0 b. Vmix < 0 c. P < PA0 X A PB0 XB d. Smix < 0
A, B, C
7. Which of the following are correct about the catalyst?
a. They participate in the reaction but recovered at last
b. It does not affect G
c. It does not affect H
d. It alters the mechanism of reaction
A,B,C,D
Dr. Sangeeta Khanna Ph.D 15 CHEMISTRY COACHING CIRCLE
D:\Important Data\2017\+2\Physical\Grand Test\+2 Grand Test -4 2.7.2017\+2 Grand test-4.doc
Dr. Sangeeta Khanna Ph.D
8. Ionic conductance at infinite dilution of Al3+ and SO42– ions are 60 and 80 –1 cm2 eq–1, respectively.
The correct detail(s) regarding Al2(SO4)3 is/are
(a) the molar conductance is 140 –1 cm2 mol–1.
(b) the equivalent conductance is 140 –1 cm2 mol–1.
(c) the molar conductance is 840 –1 cm2 mol–1.
(d) the molar conductance is 23.33 –1 cm2 mol–1.
B,C
Sol. eq 60 80 140; M x eq 6 140 840
9. For the reduction of NO 3 ion in an aqueous solution, E° is +0.96 V. Values of E° for some metal ions
are given below:
V2+(aq) + 2e– V(s) E° = – 1.19 V
Fe3+(aq) + 3e– Fe(s) E° = – 0.04 V
–
Au (aq) + 3e Au(s)
3+
E° = +1.40 V
Hg2+(aq) + 2e– Hg(l) E° = +0.86 V
The pair(s) of metal that is(are) oxidized by NO3– in aqueous solution is(are)
(a) V and Hg (b) Hg and Fe (c) Fe and Au (d) Fe and V
A,B,D
Sol. AU cannot be oxidised by NO 3 as Reduction potential of gold is higher that NO 3
SECTION – E (Matrix Type) No Negative Marking
This Section contains 3 questions. Each question has four choices (A, B, C and D) given in Column I and
five statements (p, q, r, and s) in Column II. Any given statement in Column I can have correct matching
with one or more statement(s) given in Column II. 8 × 3 = 24 Marks
1. Match Column – I with Column – II. (One or More than One Match)
Column – I Column – II
(A) Spontaneous galvanic cell (P) AgCl AgBr
Pt , Ag ( s ) ( s) Ag
(0.1M KCl (0.1M KBr
(B) Non spontaneous Galvanic cell (Q) Pt H2 H H H2 (g), Pt
1 atm 1 M 1 M 2 atm
(C) Ecell > E 0cell (R) Pt, Ag AgCl, KCl H H2 (g), Pt
( s) 0.1 M 1 M 1 atm
(D) Ecell < E 0cell (S) Pt H2 HCl Cl 2 , Pt
or Pt , H2 HCl HCl Cl 2 , Pt
1 atm 0.1 M 1 atm 0.1M 0.1M
Pt Fe Fe Cu Cu , Pt
(T)
0.5 M 0.2 M 0.2 M 0.4 M
E0 Ag+ / Ag = 0.80 V, E0 Cl2 / Cl– = 1.36 V
E Fe / Fe = - 0.77 V, E0 Cu++/Cu+ = 0.16 V
0 ++ +++
Ksp AgCl = 10-10, Ksp AgBr = 10-12
Sol. (A) S; (B) P, Q, R, T; (C) R, S, T ; (D) P, Q
(P) Ag + Cl– e– + AgCl
AgBr + e– Ag + Br –
Dr. Sangeeta Khanna Ph.D 16 CHEMISTRY COACHING CIRCLE
D:\Important Data\2017\+2\Physical\Grand Test\+2 Grand Test -4 2.7.2017\+2 Grand test-4.doc
Dr. Sangeeta Khanna Ph.D
0.059 K sp AgCl
E = E° – log
1 K sp AgBr
E < E° non-spontaneous
(Q) For hydrogen electrode
E 0cell 0
0.0591 [H ] anode
2
(PH 2 )cathode
E cell 0 log
2 [H ] cathode (PH 2 )anode
0.0591 1 2
log
2 1 1
= -ve non-spontaneous
0.059 [ Ag ][Cl ]
(R) E = E° - log
[H ] P 2
1
1
H2
E° = E C E A
= 0 – 0.8 = -0.8 V
K sp AgCl
0.8 0.059 log
[H ]
= -0.8 – 0.059 log 10-10
= - 0.5 (non-spontaneous)
E > E°
(S) E0cell 1.36 0 1.36 V
H2 2H+ + 2e–
2e– + Cl2 2 Cl–
H2 + Cl2 2H+ + 2Cl–
PH2 PCl2 1atm
Ecell E0ce.ll 0.0591 log (H ) (Cl - )
E0cell 0.0591 log (10-1) (10-1)
E 0cell - 0.0591 (- 2)
E 0cell 0.0591 (2) V Spontaneous
Ecell > E 0cell
(T) E 0cell 0.16 0.77
= -0.61 V
[Fe ] [Cu ]
Ecell = E 0cell 0.0591 log
[Fe ] [Cu ]
0.2 0.4
E 0cell 0.0591log
0.5 0.2
8
= E0cell 0.06 log
10
0
E cell 0.06( 0.1)
E 0cell 0.006
E 0.61 0.006
E = - ve
E cell E 0cell non-spontaneous
Dr. Sangeeta Khanna Ph.D 17 CHEMISTRY COACHING CIRCLE
D:\Important Data\2017\+2\Physical\Grand Test\+2 Grand Test -4 2.7.2017\+2 Grand test-4.doc
Dr. Sangeeta Khanna Ph.D
2. Match the solids in column I with the properties in column II (Single Match)
Column I Column II
(A) Germanium (P) a non-conducting solid becoming good conductor on melting
(B) KCl (Q) a high melting solid involving covalent by bonded atoms
(C) Sodium (R) a solid melting far below room temperature and held together by Van
der Waal’s forces
(D) Solid methane (S) a solid having melting point about 373 K and very good conductor of
electricity
Sol. A Q; B P; C S; D R
3. Match the entries of column I with appropriate entries of column II. (One or More than one match)
Column – I Column – II
M.Wt
(A) Al2(Cr2O7)3 Cr+3 (p) eq. Wt. =
18
(B) H2O2 O2 (q) Reduction Reaction
M.Wt
(C) Ba(MnO4)2 BaMnO4 (r) eq. Mass =
2
(D) As2O5 As2O3 (s) Oxidation reaction
Sol. A p, q; B r, s; C q, r; D q
SECTION – F (Integer Type) No Negative Marking
This Section contains 7 questions. The answer to each question is a single digit integer ranging
from 0 to 9. 7 × 5 = 35 Marks
1. Relative decrease in vapour pressure of an aqueous solution containing 2 mol NaCl in 3 mol H2O is
0.6. On reaction with AgNO3, this solution will form ppt. of AgCl. How many moles of AgCl will be
formed.
p 2 p
Sol. Calculated value of xB 0.4 ; Observed value of 0.6
p 23 p
0.6 i 1 1.5 1
Van’t Hoff factor = 1.5 ; = 0.5 or 50%
0.4 2 1 1
Since NaCl is 50% ionized
2 50
No. of mol of Cl– = =1
100
Mol of AgCl formed = 1 mol.
2. K2[HgI4] is 50% ionized in aqueous solution. What will be its von’t Hoff’s factor.
Sol. i = 1 + 2
= 1 + 2 - 5 = 2
3. An aqueous solution containing ionic salt having molality equal to 0.1892 freezes at -0.704°C. The
van’t Hoff factor of the ionic salt is given by _______. (Kf = 1.86 Km–1)
Sol. 2
4. A complex is represented as CoCl3xNH3. Its 0.1 molal solution in aqueous solution shows Tf =
0.558°C. Kf for H2O is 1.86 K molal-1. Assuming 100% ionization of complex and coordination number
of Co as 6, find the value of x.
Sol. 5
5. The coagulation of 10 ml colloidal solution of gold is completely prevented by addition of 0.02 gm of a
substance X to it before addition of 1 ml of 10% NaCl solution. The gold number of X is 2 × 10n n is
Sol. 1
Dr. Sangeeta Khanna Ph.D 18 CHEMISTRY COACHING CIRCLE
D:\Important Data\2017\+2\Physical\Grand Test\+2 Grand Test -4 2.7.2017\+2 Grand test-4.doc
Dr. Sangeeta Khanna Ph.D
6. The mole fraction of a solute in a solution is 0.1. At 298 K, molarity of this solution is the same as its
molality. Density of this solution at 298 K is 2.0 g cm-3. The ratio of the molecular weights of the solute
MW solute
and solvent, , is
MW solv ent
Sol. 9
X solute 0.1 1
X solv ent 0.9 9
Wsolute Msolv ent 1
…(1)
Wsolv ent Msolute 9
Wsolute + W solvent = Wsolution = density × volume
Wsolute + Wsolvent = 2 × V …(2)
Molarity = molality
nsolute n
solute
Vsolution Wsolv ent
W Wsolv ent
Wsolv ent Vsolution solute
2
2W solvent = W solute + W solvent
Wsolute = W solvent …(3)
Using eq. (1) and (3), we get
Msolute
9
Msolv ent
7. For the following electrochemical cell at 298 K,
Pt(s)|H2(g, 1bar) | H+ (aq, 1 M) || M4+ (aq), M2+(aq) | Pt(s)
[M2 (aq)]
Ecell 0.092 V w hen 10 x .
4
[M (aq)]
RT
Given: E0 4 0.151 V; 2.303 0.059 V
M / M 2 F
The value of x is
Sol. 2
Anode: H2(s) 2H+ + 2e–
Cathode: Mn+4 + 2e– Mn+2
Mn+4 + H2 Mn+2 + 2H+
[Mn 2 ] [H ] 2
log10
0.059
E E
2 [Mn 4 ] Ph
2
0.059
0.092 0.151 log10 (10 x )
2
0.059
0.092 0.151 x x=2
2
Dr. Sangeeta Khanna Ph.D 19 CHEMISTRY COACHING CIRCLE
D:\Important Data\2017\+2\Physical\Grand Test\+2 Grand Test -4 2.7.2017\+2 Grand test-4.doc
Potrebbero piacerti anche
- Addis Ababa City Government Education BureauDocumento11 pagineAddis Ababa City Government Education BureauErmias100% (1)
- Class 12 Chemistry Ch-3.Chemical KineticsDocumento41 pagineClass 12 Chemistry Ch-3.Chemical Kineticskarnan karupiah0% (1)
- Steam Turbine: Bearings Wheels and DiaphragmsDocumento128 pagineSteam Turbine: Bearings Wheels and DiaphragmsJeeEianYann100% (1)
- Trigonometric Ratios to Transformations (Trigonometry) Mathematics E-Book For Public ExamsDa EverandTrigonometric Ratios to Transformations (Trigonometry) Mathematics E-Book For Public ExamsValutazione: 5 su 5 stelle5/5 (1)
- Chem220 SpectrophotometryDocumento46 pagineChem220 SpectrophotometryButterlesstoastNessuna valutazione finora
- Control of Corrosion in Cooling WatersDocumento93 pagineControl of Corrosion in Cooling Waterssevero97Nessuna valutazione finora
- Theoretical Solid State Physics: International Series in Natural Philosophy, Volume 1Da EverandTheoretical Solid State Physics: International Series in Natural Philosophy, Volume 1Valutazione: 1 su 5 stelle1/5 (1)
- O Level Biology Practice Questions And Answers EnzymesDa EverandO Level Biology Practice Questions And Answers EnzymesValutazione: 5 su 5 stelle5/5 (1)
- Edec Standard: CoretronicDocumento14 pagineEdec Standard: Coretronic彭以和Nessuna valutazione finora
- Chemical Kinetics TestDocumento5 pagineChemical Kinetics Testrajneesh kumarNessuna valutazione finora
- 03 Ball Mill EDMDocumento47 pagine03 Ball Mill EDMrecai100% (1)
- NaphthaleneDocumento3 pagineNaphthaleneNur Hafeza75% (4)
- KAS Physics Prelims 2010Documento20 pagineKAS Physics Prelims 2010Amjid AliNessuna valutazione finora
- Class Test SolutionDocumento10 pagineClass Test SolutionAkash GoelNessuna valutazione finora
- Chemistry 3rd May Part TestDocumento8 pagineChemistry 3rd May Part TestJaswant Singh BistNessuna valutazione finora
- Chemical Kinetics - DPP-03 (Of Lec-04) - Integrated Rate Laws - DPP-3Documento3 pagineChemical Kinetics - DPP-03 (Of Lec-04) - Integrated Rate Laws - DPP-3ShanksNessuna valutazione finora
- CLIP - Chemical Kinetics PDFDocumento4 pagineCLIP - Chemical Kinetics PDFAman JaiswalNessuna valutazione finora
- Carries 1 Mark. No Negative Marks.: N O H S CL MN Na C Ag K Fe PBDocumento21 pagineCarries 1 Mark. No Negative Marks.: N O H S CL MN Na C Ag K Fe PBbharadwajnavneet599Nessuna valutazione finora
- JEE-Main 24 January (Memory Based) - Shift 1Documento27 pagineJEE-Main 24 January (Memory Based) - Shift 1The Daily PleasureNessuna valutazione finora
- Chemistry 102 Spring 2000 Lindahl's Sections Problem Set IiDocumento13 pagineChemistry 102 Spring 2000 Lindahl's Sections Problem Set Iimix shopNessuna valutazione finora
- EBS 336 - Tests 2 - Q&A - 2018 PDFDocumento4 pagineEBS 336 - Tests 2 - Q&A - 2018 PDFNurul Ain JabitNessuna valutazione finora
- DPPS-3 - Chemical KineticsDocumento2 pagineDPPS-3 - Chemical KineticsShrish PratapNessuna valutazione finora
- INOCHE2 Final Exam Reviewer 2T AY14-15 PDFDocumento8 pagineINOCHE2 Final Exam Reviewer 2T AY14-15 PDFroxy8marie8chanNessuna valutazione finora
- #21 UdaanNEETMiniTest PDFDocumento14 pagine#21 UdaanNEETMiniTest PDFKumar ElumalaiNessuna valutazione finora
- MCD4390 Week 10 Tutorial QuestionsDocumento5 pagineMCD4390 Week 10 Tutorial QuestionsGabbar100% (1)
- Chemical Kinetics JEE MAINS 2022 - 966591 - 2022 - 08 - 19 - 16 - 17Documento8 pagineChemical Kinetics JEE MAINS 2022 - 966591 - 2022 - 08 - 19 - 16 - 17AbhinavNessuna valutazione finora
- Gtse SolutionDocumento16 pagineGtse Solution1 AashuNessuna valutazione finora
- Physics (P-1) SolutionDocumento5 paginePhysics (P-1) SolutionAthang MalgundkarNessuna valutazione finora
- Cuet 2022 Chemistry 17 August Slot 1Documento47 pagineCuet 2022 Chemistry 17 August Slot 1NafeesNessuna valutazione finora
- Jee Mains Paper - Set-ADocumento35 pagineJee Mains Paper - Set-APravin PandeyNessuna valutazione finora
- Maharashtra Board Class 12 Chemistry Question Paper 2023Documento4 pagineMaharashtra Board Class 12 Chemistry Question Paper 2023johnhomelander04Nessuna valutazione finora
- 12th Chemistry EM Half Yearly Exam 2023 Question Paper Thenkasi District English Medium PDF DownloadDocumento2 pagine12th Chemistry EM Half Yearly Exam 2023 Question Paper Thenkasi District English Medium PDF DownloadSutha MaryNessuna valutazione finora
- Physics - 27 Jan Shift-2 JEE Main 2024 (Session 1)Documento10 paginePhysics - 27 Jan Shift-2 JEE Main 2024 (Session 1)sarthakraj8651Nessuna valutazione finora
- 1Documento6 pagine1Kuo Garol SarongNessuna valutazione finora
- Fiitjee - Jee (Main) : Physics, Chemistry & Mathematics Phase-4Documento13 pagineFiitjee - Jee (Main) : Physics, Chemistry & Mathematics Phase-4Abhinav GuptaNessuna valutazione finora
- Rise Target-JEE-14Documento12 pagineRise Target-JEE-14Lutfan LubaibNessuna valutazione finora
- The Canadian Chemistry Contest 2007: Substance ResponseDocumento4 pagineThe Canadian Chemistry Contest 2007: Substance ResponselylyNessuna valutazione finora
- Sample Paper Class 11Documento198 pagineSample Paper Class 11Kia Sharma100% (1)
- Sample Paper Class 11 PDFDocumento198 pagineSample Paper Class 11 PDFHashim M Kollam100% (1)
- Chemical KineticsDocumento19 pagineChemical KineticsEzhil MukilNessuna valutazione finora
- Trial Exam DCS Che 12 2022 FinalDocumento11 pagineTrial Exam DCS Che 12 2022 Finalnavin chhetriNessuna valutazione finora
- Physics Sample - 2 XII 2024Documento18 paginePhysics Sample - 2 XII 2024Kashif SiddiquiNessuna valutazione finora
- JEE-Main-31-01-2024 (Memory Based) (Evening Shift) PhysicsDocumento18 pagineJEE-Main-31-01-2024 (Memory Based) (Evening Shift) Physicschallapramodh1Nessuna valutazione finora
- WPT InorganicDocumento3 pagineWPT Inorganicpinnaacleclasses salemNessuna valutazione finora
- Turorial-3 - Cl302 Fogler Solution PDFDocumento4 pagineTurorial-3 - Cl302 Fogler Solution PDFshubhamNessuna valutazione finora
- 04.chemical Kinetics - 66-82 - PDFDocumento4 pagine04.chemical Kinetics - 66-82 - PDFWajid AliNessuna valutazione finora
- CHEMICAL KINETICS FinalDocumento6 pagineCHEMICAL KINETICS FinalBOTHRA CLASSESNessuna valutazione finora
- DPP (Chemistry) Chemical KineticsDocumento6 pagineDPP (Chemistry) Chemical KineticsNavinNessuna valutazione finora
- Class Xii Pre Board Question Paper ChemistryDocumento17 pagineClass Xii Pre Board Question Paper ChemistryJeremiah ShibuNessuna valutazione finora
- Mid Term General Chem II Fall 2001Documento6 pagineMid Term General Chem II Fall 2001dr.ibrahimsalemvpNessuna valutazione finora
- DPPS-2 - Chemical KineticsDocumento2 pagineDPPS-2 - Chemical KineticsShrish PratapNessuna valutazione finora
- Chemical Kinetics: (Physical Chemistry)Documento26 pagineChemical Kinetics: (Physical Chemistry)keshavNessuna valutazione finora
- JEE Main 2023 Chem 29th Jan Shift 1 QPDocumento10 pagineJEE Main 2023 Chem 29th Jan Shift 1 QPNidhi MishraNessuna valutazione finora
- ByjuDocumento13 pagineByjuRanjan ShuklaNessuna valutazione finora
- TUGAS KA 10 (Crismonia Rinta)Documento11 pagineTUGAS KA 10 (Crismonia Rinta)eko widodoNessuna valutazione finora
- Fiitjee JEE Main Phase IIDocumento15 pagineFiitjee JEE Main Phase IIPadamNessuna valutazione finora
- IIT JEE (Advanced) Mock - 7 (Paper - 2)Documento22 pagineIIT JEE (Advanced) Mock - 7 (Paper - 2)adityakatkade668Nessuna valutazione finora
- Himalaya Public School Chemistry Paper 2023Documento6 pagineHimalaya Public School Chemistry Paper 2023ImmortalNessuna valutazione finora
- Jee Main 2023 January 25 Shift 1 Question Paper With Solutions by VedantuDocumento23 pagineJee Main 2023 January 25 Shift 1 Question Paper With Solutions by VedantuAkshay guptha athmakuriNessuna valutazione finora
- Chemistry: Crash Course For JEE Main 2020Documento17 pagineChemistry: Crash Course For JEE Main 2020QSQFNessuna valutazione finora
- AIEEE ChemistryDocumento2 pagineAIEEE ChemistryRaja PramodNessuna valutazione finora
- JEE Main 2023 Chem 25th Jan Shift 1 QPDocumento13 pagineJEE Main 2023 Chem 25th Jan Shift 1 QPNitin KansalNessuna valutazione finora
- Zero and First Order (Chemical Kinetics) : Assingment # 1 SyllabusDocumento5 pagineZero and First Order (Chemical Kinetics) : Assingment # 1 Syllabusadityakumar krNessuna valutazione finora
- CUET Chemistry 2022 17 August-Slot-1Documento47 pagineCUET Chemistry 2022 17 August-Slot-1NafeesNessuna valutazione finora
- A K T A X: 4. Chemical KineticsDocumento4 pagineA K T A X: 4. Chemical KineticseamcetmaterialsNessuna valutazione finora
- Dbms Assignment 1Documento8 pagineDbms Assignment 1Manya SinghNessuna valutazione finora
- Chapter14 - Ionic EquilibriumDocumento57 pagineChapter14 - Ionic EquilibriumManya SinghNessuna valutazione finora
- Chemistry JEE-M 2016Documento10 pagineChemistry JEE-M 2016Manya SinghNessuna valutazione finora
- Chapter31 - Qualitative AnalysisDocumento22 pagineChapter31 - Qualitative AnalysisManya SinghNessuna valutazione finora
- Part - A (Physics) : Jee Main 2019 - 11 January - Morning Shift MathongoDocumento35 paginePart - A (Physics) : Jee Main 2019 - 11 January - Morning Shift MathongoManya SinghNessuna valutazione finora
- Paper - 2: Read The Instructions CarefullyDocumento30 paginePaper - 2: Read The Instructions CarefullyManya SinghNessuna valutazione finora
- Paper - 1: Read The Instructions CarefullyDocumento34 paginePaper - 1: Read The Instructions CarefullyManya SinghNessuna valutazione finora
- Fiitjee: Internal TestDocumento39 pagineFiitjee: Internal TestManya SinghNessuna valutazione finora
- PHYSICS (Theory) & Solution: Series GBM SET-2Documento21 paginePHYSICS (Theory) & Solution: Series GBM SET-2Manya SinghNessuna valutazione finora
- Checklist - Stress AnalysisDocumento2 pagineChecklist - Stress AnalysisRamalingam PrabhakaranNessuna valutazione finora
- MSC Oil & Gas Structural Engineering Course Descriptors 2015-2016Documento18 pagineMSC Oil & Gas Structural Engineering Course Descriptors 2015-2016Xabi BarrenetxeaNessuna valutazione finora
- Steelwork Design Guide To BS 5950-1-2000. Volume 2. Worked Examples. Part 2 - Example 2 Simply Supported Restrained Beam.Documento11 pagineSteelwork Design Guide To BS 5950-1-2000. Volume 2. Worked Examples. Part 2 - Example 2 Simply Supported Restrained Beam.Yilin ZuoNessuna valutazione finora
- Axial Piston Fixed Motor A10FM Axial Piston Plug-In Motor A10FE Series 52Documento38 pagineAxial Piston Fixed Motor A10FM Axial Piston Plug-In Motor A10FE Series 52Orlando AriasNessuna valutazione finora
- Todays Technician Automotive Heating and Air Conditioning Classroom Manual and Shop Manual Spiral Bound Version 6th Edition Schnubel Test BankDocumento11 pagineTodays Technician Automotive Heating and Air Conditioning Classroom Manual and Shop Manual Spiral Bound Version 6th Edition Schnubel Test Bankheulwenclarawnr100% (23)
- Orkot Marine Bearings Engineering Manual enDocumento32 pagineOrkot Marine Bearings Engineering Manual enVivekNessuna valutazione finora
- E201: Work, Energy and Power POLICIOUS, Mark Angelo FDocumento5 pagineE201: Work, Energy and Power POLICIOUS, Mark Angelo FMark Angelo PoliciosNessuna valutazione finora
- BallisticDocumento17 pagineBallisticMary Clair BarbadilloNessuna valutazione finora
- EEE 533: Semiconductor Device and Process Simulation: Spring 2001Documento9 pagineEEE 533: Semiconductor Device and Process Simulation: Spring 2001Garima BhatiaNessuna valutazione finora
- Chap 02Documento37 pagineChap 02Yuanjing Xu0% (1)
- X-Ray Diffraction: Dr. Mukesh KumarDocumento31 pagineX-Ray Diffraction: Dr. Mukesh Kumarhimanshu singhNessuna valutazione finora
- Atlas of Namibia - 03 ClimateDocumento36 pagineAtlas of Namibia - 03 ClimateCesar Ricardo Lopez ValerioNessuna valutazione finora
- Tarea 4Documento13 pagineTarea 4Isidro MedranoNessuna valutazione finora
- Soil Shear StrengthDocumento89 pagineSoil Shear Strengthmuhd nizamNessuna valutazione finora
- Variational Principle For A Particle in A BoxDocumento4 pagineVariational Principle For A Particle in A BoxajparnaibaNessuna valutazione finora
- C - 15 - 58 Project ProposalDocumento35 pagineC - 15 - 58 Project ProposalklklklNessuna valutazione finora
- Dew Point Calculation Chart: For Adhesive and Coating Applications Ambient Air Temperature in Degrees FahrenheitDocumento1 paginaDew Point Calculation Chart: For Adhesive and Coating Applications Ambient Air Temperature in Degrees FahrenheitBernathTurnipNessuna valutazione finora
- Timberoll BeltsDocumento4 pagineTimberoll Beltsplastena plastenaNessuna valutazione finora
- Thermal Stability and Performance Data For Sm-Co 2 17 High-TemperatureDocumento4 pagineThermal Stability and Performance Data For Sm-Co 2 17 High-TemperatureelectronenergyNessuna valutazione finora
- Surfadone LP: Specialty SurfactantsDocumento12 pagineSurfadone LP: Specialty SurfactantsSohaib BraziNessuna valutazione finora
- Ground Response Analysis, NoteDocumento56 pagineGround Response Analysis, NoteMuluNessuna valutazione finora
- sm1 014Documento2 paginesm1 014Paulo Henrique D. FavarettoNessuna valutazione finora
- From The Steam Table: Table 3 PT 1@ 540 C & 8mpa H 3496.7 KJ/KG & S 6.8481 PT 2 at 0.0035 Mpa & S S 6.8481 by InterpolationDocumento5 pagineFrom The Steam Table: Table 3 PT 1@ 540 C & 8mpa H 3496.7 KJ/KG & S 6.8481 PT 2 at 0.0035 Mpa & S S 6.8481 by InterpolationMark AgusNessuna valutazione finora
- DMFR 20140229-1Documento6 pagineDMFR 20140229-1aswad 0008Nessuna valutazione finora
- Photography - Depth of FieldDocumento3 paginePhotography - Depth of FieldmirnafarahatNessuna valutazione finora