Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Govind AmbiGen Poster
Caricato da
Yolanda MCopyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Govind AmbiGen Poster
Caricato da
Yolanda MCopyright:
Formati disponibili
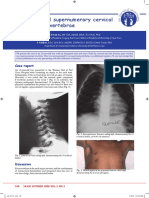
Practical Approach To Imaging Children With Ambiguous Genitalia
Govind B. Chavhan MD, Dimitri Parra MD, Kamaldine Oudjhane MD, Stephen Miller MD, Paul Babyn MD
Department of Diagnostic Imaging, The Hospital For Sick Children, University of Toronto, Canada
INTRODUCTION IMAGING OF AMBIGUOUS GENITALIA
Mixed gonadal dysgenesis
Correct and appropriate gender assignment in patients with ambiguous genitalia is necessary for the child’s healthy physical and ULTRASOUND:
psychological development. Workup of these patients is best accomplished via a coordinated approach by pediatric endocrinologist, Ü Primary modality, quick, and should include inguinal, perineal, renal and adrenal regions
geneticist, urologist and radiologist to arrive at timely diagnosis and proper management. Imaging has an important role to play in Ü Establishes presence or absence of gonads and Mullerian derivatives
accurately demonstrating anatomy and possible effects on other organs (1). Ü Easy to find uterus and ovaries in neonatal period as they are prominent under influence of maternal hormones
In this review we discuss currently used classifications of confusing conditions causing ambiguous genitalia, and the role of imaging Ü Identification of only one ovary in 40% and neither ovary in 16% cases has been seen in normal patients (5)
modalities like ultrasound, genitogram and MRI. Finally we provide a simplified approach towards workup and discuss risk of
development of cancer in these children.
Female pseudohermaphrodite:
EMBRYOLOGY a b c
The embryology of sexual differentiation is quite complex. Even though the chromosomal basis for sex is determined at conception, a b c
Figure 7: Normal right ovary and uterus on axial T2-weighted image (A) and normal uterus on sagittal T2-weighted image (B). Another axial T2-w
internal structures are undifferentiated up to six weeks of gestational age. Three important precursor components of genital system are image (C) shows ectopic ovaries (arrows) over ileopsoas muscles bilaterally in this case of mullerian agenesis
germ cells, genital ridge and two sets of internal sex ducts: the Mullerian/paramesonephric ducts and Wolffian/mesonephric ducts (2). At
approximately six week fetal age, the genital ridge becomes either gonad: ovary or testis. Germ cells populate undifferentiated gonads.
Testicular development is guided by testes determining factor/substance (TDF/TDS), which is encoded by SRY located on short arm of
Y-chromosome. Under influence of TDF germ cells in the genital ridge differentiate into Sertoli cells (which secrete Mullerian inhibiting DIAGNOSTIC APROACH
substance/factor (MIS/MIF)) and Leydig cells (which produce testosterone). MIF causes complete regression of Mullerian ducts while
a The aims of evaluating a child with ambiguous genitalia are: 1. establish genetic sex, 2. determine the hormonal profile,
testosterone promotes maturation of spermatogonia and regulates development of the male phenotype by paracrine and endocrine b c 3. evaluate the anatomy of internal and external genitalia and gonads, and 4. in older children, assess the phenotypic and
actions. By paracrine action, the Wolffian duct develops into epidydimis, vas deferens, ejaculatory duct and seminal vesicles.
Figure 1: Female Pseudohermaphrodite. This infant had ambiguous genitalia. Genotype was 46XX. Normal uterus and both ovaries were seen in the psychological sex (17).
In the absence of the Y chromosome gonads differentiate into ovaries at around 11-13 weeks. Ovarian hormones are thought to play no Chart II displays a simplified approach for understanding concepts. Actual workup may turn complex and tedious, requiring
pelvis. Longitudinal (A) and transverse (B) images of right adrenal gland show enlargement of the gland, however, corticomedullary differentiation
role in female phenotype differentiation. Absence of MIF leads to persistence of Mullerian structures, which develop into Fallopian tubes, multiple tests such as hormonal assay, chromosomal study, laparoscopy and biopsy, and genitogram.
is maintained. Similarly left gland (C) is also enlarged. There is also ‘cerebriform appearance’ to the glands. This was a case of congenital adrenal
uterus, cervix and upper vagina. Due to absence of testosterone, Wolffian ducts involute. d e f
hyperplasia resulting into virilization of external genitalia. 17-OH-Progesterone was elevated.
Undifferentiated external genital structures consist of the urogenital tubercle, urogenital swelling and urogenital folds. These structures Chart II: Ambiguous Genitalia
develop into the glans penis, scrotum and the shaft of penis in male respectively. In female they develop into the clitoris, labia majora and Ü Enlarged adrenal glands showing normal corticomedullary differentiation with measurement of single limb length >20mm and width Figure 4: Mixed Gonadal Dysgenesis. This child presented with ambiguous genitalia with perineal hypospadias and labial fusion. Normal infantile
minora respectively (2). >4mm is suggestive of congenital adrenal hyperplasia (1) uterus was seen (A). Morphologically appearing testis was seen anterolateral to the urinary bladder on the right side (B). Morphologically appearing
Ü Normal sized adrenal glands does not exclude the diagnosis of CAH (6, 7) ovary with cystic areas was seen on left side (C). There was another gonadal tissue on the left side anterolateral to the urinary bladder (D). This tissue Palpable Gonads No palpable Gonad
Overview of normal reproductive system development
Chart I: Ü ‘Cerebriform appearance’ is reportedly specific for CAH (8) had a cystic area within it. There was a long phallus buried under the skin (E). Genitogram (F) showed normal vagina with uterus (arrow) on top of it. Confirmed on US
Reproductive system precursors Biopsy of the right gonad revealed testicular tissue, left sided morphologically ovarian gonad showed fallopian tube and epidydimis without any as Testes
ovarian tissue, and left gonadal tissue anterolateral to the bladder showed dysgenetic gonads with primitive sex cord components.
Testes seen Intrabdominally No Testicular Tissue
Genital ridge Germ cells Sex ducts Ü At least a rudimentary uterus and Fallopian tube can be seen on the side of streak gonad.
No Uterus / Ovaries Uterus seen
Ü On the side of testes local MIF diffusion prevents development of Fallopian tube (10)
Uterus & Ovaries Only uterus seen
TDF Mullerian Wolffian ducts Extra gonad seen seen
Male pseudohermaphrodite: FLUOROSCOPY/GENITOGRAM: Male pseudo-
In absence of TDF from ducts hermaphrodite Search for gonads by
Ova
Y-chromosome Y-chromosome Ü Demonstrates male or female type of urethral configuration, any fistulous communication with vagina or rectum MR, Venography,
Ü Shows presence or absence of vagina, its relationship with urethra and specially the level of external sphincter, cervical No ovary or other Streak Gonad Ovary or Laparoscopy &
Uterus Epidydimis Female
impression gonad Ovotestes confirmed by biopsy
Fallopian tubes Vas deferens Pseudohermaphordite
Sertoli cells Leydig cells Seminal vesicles
Ü Is important to examine all perineal orifices and insert catheter for short distance into orifice to preserve morphological Ovaries
Ovary Testes Upper vagina Mixed Gonadal
Ejaculatory duct
appearance Persistent Mullerian
Dysgenesis
Ü Presence of hydrocolpos/hydrometrocolpos with ambiguous genitalia with two perineal orifices (one of which is anus) duct Syndrome Uterus +
confirm presence of a urogenital sinus malformation as a consequence of virilisation of the fetus (1) Streak Gonads
MIS Testosterone True Hermaphrodite streak gonads
Figure 5: Genitogram in complex urogenital sinus anomaly. This child had genital
ambiguity. Contrast injection through an orifice just below the phallus opacified a Pure Gonadal Dysgenesis
a b c tract (arrows), presumably urethra and a triangular pouch. Contrast then refluxed into
urethra and bladder anteriorly and vagina posteriorly from the triangular pouch. There * Structure sonographically appearing like testes or ovary on imaging may be dysgenetic. Structure appearing like ovary
Figure 2: Male Pseudohermaphrodite. This teenage phenotypic female presented with amenorrhoea and prominent labioscrotal folds giving some was another tract coming from the triangular area superior to the cannulated one and may represent Fallopian tube, epidydimis or combination of both. Biopsy confirmation may be required.
degree of genital ambiguity. Uterus and ovaries were not present in the pelvis (A). Right (B) and left (C) testes were seen in the inguinal canals. opening at the tip of phallus (arrowheads).
CLASSIFICATION Genotype was 46XY. This was a case of incomplete androgen insensitivity (Reifenstein syndrome) with some degree of ambiguity to genitals.
Complete androgen insensitivity is called Morris syndrome where child has female external genitals from birth.
The conditions causing ambiguous genitalia can be classified on pathophysiological basis as disorders of chromosomal, gonadal
Ü If not seen on US, testicular tissue should be searched for by MRI and testicular venography in view of increased risk of malignancy
RISK OF NEOPLASM
and phenotypic sex origin. On the basis of gonadal histology the disorders can be classified broadly into four groups- female
Ü Abnormality in MIF production in otherwise normal testes results in male phenotype/genotype with persistent Mullerian structures. Ü As 20-30% of children with XY pure gonadal dysgenesis and 15-20% with mixed gonadal dysgenesis develop a gonadal
pseudohermaphrodite, male pseudohermaphrodite, true hermaphrodite and gonadal dysgenesis (1). The salient features are discussed in
Table 1 and important imaging features are highlighted. This is called ‘persistent Mullerian duct syndrome’ (9). neoplasm within the first or second decade, streak gonads should be removed (16)
MRI Ü Presence of the H-Y antigen, a gene product of Y-chromosome, is implicated for development of neoplasms
Table 1.
Ü Multiplanar capability and superior tissue characterization by means of T1 and T2-weighted sequences can provide Ü Gonadoblastomas are the most commonly seen tumors and arise commonly from dysgenetic intra-abdominal gonads
Female Male True Gonadal dysgenesis Ü Presence of an echogenic focus associated with pelvic organs or in ectopic gonadal tissue in inguinal canals or labioscrotal
Pseudohermaphrodite Pseudohermaphrodite Hermaphrodite detailed anatomical information, however, not widely used yet
Mixed Pure True Hermaphrodite: Ü MRI useful in evaluation of ambiguous genitalia, with MR depiction of uterus possible in 93%, vagina in 95%, penis in folds, should be regarded with suspicion as gonadoblastomas often calcify
1. Genotype/ Karyotype 46XX, SRY/TDF gene 46XY 46XX (60-70%), 46XY, 45XY 45XO Mosaic Variable 46XX, 46XY 45XO 100%, testes in 88%, and ovary in 74% cases (11) Ü Other germ cell tumors seen are dysgerminomas, teratomas, teratocarcinoma, yolk sac tumor, embryonal carcinoma and
negative 46XX/XY- mosaic
Ü For evaluation of intrapelvic structures, MR and US are considered equally sensitive. For evaluation of gonads, MR is more choriocarcinoma (1)
2. Gonads Ovary only Testes only Both ovarian and testicular Testes + streak gonad (Testes Bilateral streak gonads
tissues, Ovary+testes, with Sertoli and Leydig cells, sensitive than US (12) Ü Increased risk of developing Wilm’s tumor, particularly when XYgonadal dysgenesis is associated with glomerulopathy in
Ovary+ovotestes, Bilat No germinal cells) Drash syndrome
ovotestes, Ovostestes+ testes. Ü Ectopic gonads, both testes and non-cystic immature ovaries, display medium signal intensity on T1-weighted and high
Uterus is almost always present signal intensity with an outer rim of medium signal on T2-weighted images (13) Ü Average age of development of Wilms tumor in Drash syndrome is 3 years
3. Phenotype Ambiguous, from mild clitoral Variable degree of Variable. Female with Variable Ambiguous or female Female. Sexual infantilism Ü Screening in the form of annual renal US is indicated in children with dysgenetic gonads up to school age for Wilms tumor
enlargement to complete feminization, Ambiguous clitoromegaly to male with and primary amenorrhoea at Ü Streak gonads are difficult to find and are seen as low-signal-intensity stripes on T2-weighted images (14)
virilization genitalia, small phallus, hypospadias, bifid scrotum puberty Ü High signal intensity in streak gonads could represent neoplastic change (13)
variable labio-scrotal fusion
4. Causes 1. Congenital adrenal 1.Inborn error of testosterone Genetic Genetic Genetic Ü Hypertrophied clitoris can be differentiated from penis on MRI by absent or poorly developed supporting structures of
hyperplasia.
2. Transplacental androgen
biosynthesis
2. Leydig cell aplasia/
penis such as bulbospongiosus and posteriorly located transverse perinei muscles in the female pseudohermaphrodite References
exposure hypoplasia
(15)
3. 5 alpha- reductase deficiency Ü Renal and adrenal evaluation can be performed in same MR examination with extended field of view 1. Wright NB, Smith C, Rickwood AMK, Carty HML. Imaging children with ambiguous genitalia Mullerian duct syndrome.Pediatric Radiol 1993; 23: 55-56.
a b c d
4. Androgen insensitivity and intersex states. Clinical Radiology 1995; 50:823-829. 10. Saenger P. Abnormal sex differentiation. Journal of Pediatrics 1984; 104: 1-17.
syndrome(AIS)
5. Isolated MIF activity Figure 3: True Hermaphrodite. This child had ambiguous external genitals. Normal uterus was seen in the pelvis on US (A). A gonadal tissue was seen 2. Kucinskas L, Just W. Human male sex determination and sexual differentiation: pathways, 11. Secaf E, Hricak H, Gooding CA et al. Role of MRI in the evaluation of ambiguous genitalia.
deficiency in the right inguinal canal (B) that was sonographically appeared a testis. Another gonad was seen in the left iliac fossa (C) that also morphologically molecular interaction and genetic disorders. Medicina (Kaunas) 2005; 41(8): 633-40. Pediatric Radiol 1994; 24: 231-35.
5. Types Six types of CAH. First 4 are AIH can be -- -- 1. 46XX, 46XY presents with
virilizing primary amenorrhoea and
appeared to be a testis. No follicles were seen in either of these gonads. Genitogram (D) shows normal vagina with reflux of contrast in the cervix 3. Joshi RR, Rao S, Desai M. Etiology and clinical profile of ambiguous genitalia- an overview of 12. Biswas K, Kapoor A, Karak AK et al. Imaging in intersex disorders. J Pediatr Endocrinol Metab
1. complete (Morris),
2. incomplete (Reifenstein) or
delayed secondary sexual (arrow). Urethral configuration was that of unusual female type or severe hypospadias. 10 years experience. Indian Pediatric 2006; 43: 974-79. 2004; 17: 841-45.
characters 4. Jha A. www.bui.ac.uk/Tutorials/Intersex.htm. 13. Gambino J, Caldwell B, Dietrich R, Walot I, Kangarloo H. Congenital disorders of sexual
3. mild (Kennedy)
2. 45XO with with typical
Biopsy of the right gonad showed immature testicular tissue and that of left gonad showed ovotestes. Cytogenetic analysis in this child showed
genotype of 46XY in both gonads. 5. Cohen HL, Shapiro MA, Mandel FS, Shapiro ML. Normal ovaries in neonates and infants: A differentiation: MR findings. AJR 1992; 158: 363-67.
Turner syndrome appearance
6. Diagnostic Features Presence of virilised external Normal/raised testosterone, Presence of both ovarian and Testes one side & streak gonad Streak gonads with sonographic study of 77 patients one day to 24 month old. AJR 1993; 14. Hricak H, Chang YCF, Thurnher S. Vagina: evaluation with MR imaging. Part 1. Normal
genitalia, non-palpable gonads good testosterone response to testicular tissue on other with ambiguous underdeveloped mullerian Ü Ovotestis is seen as a structure with some testicular echotexture as well as follicles. Gonads with normal ovarian and testicular
160:583-86. anatomy and congenital anomalies. Radiology 1988; 169: 169-74.
and presence of Mullerian HCG injection and absence genitalia derivatives appearance may prove ovotestes on histology.
structures on imaging and of mullerian structures on 6. Sivit CJ, Hung W, Taylor GA, Catena LM, Brown-Jones C, Kushner DC. Sonography in neonatal 15. Hricak H, Marotti M, Gilbert TJ et al. Normal penile anatomy and abnormal penile conditions:
raised 17-OH -Progesterone imaging congenital adrenal hyperplasia. AJR 1991; 156: 141-43. evaluation with MR imaging. Radiology 1988; 169: 683-90.
7. Usual Female Complete AIS- female According to anatomical Female No sexual ambiguity a b
Gender assignment Incomplete AIS- depending findings and genetic make up neonatally. 7. Bryan PJ, Caldamone AA, Morrison SC, Yulish BS, Owens R. Ultrasound findings in 16. Coran AG, Polley TZ. Surgical management of ambiguous genitalia in the infant and child.
on degree of virilisation but Contradictory gonad removed Female
Pure gonadal dysgenesis adrenogenital syndrome. Jornal of Ultrasound in Medicine 1988; 7:675-79. Journal of Pediatric Surgery 1991; 26:812.
usually female Ü Confused with Congenital Androgen Insensitivity Syndrome (CAIS) as they present at puberty with failure of menarche in a normal
8.Others features 60-70% of neonatal cases of Most diverse and difficult to Rare accounting for less than High cancer risk High cancer risk
Figure 6: Normal testes (arrows) are seen as hyperintense oval structures with hypointense rim around it on T2-weighted images (A) and displays 8. Avni EF, Rypens E, Smet MH, Galetty E. Sonographic demonstration of congenital adrenal 17. Bidarkar S, Hutson J. Evaluation and management of the abnormal gonad. Semin Pediatr
ambiguous genitalia diagnose group 10% cases
female phenotype. They usually will have normal or hypoplastic Mullerian derivatives while in CAIS there are no Mullerian derivatives. isointense signal on T1-weighted images. T2-weighted axial image of the pelvis (B) shows normal two corpora cavernosa (arrows) sorrounding hyperplasia in neonate: the cerebriform pattern. Pediatric Radiol 1993; 23: 88-90. Surgery 2005;14: 118-123.
Data from references- 1, 3, and 4 corpus spongiosus (arrowheads). 9. Adamsbaum C, Rolland Y, Josso N, Kalifa G. Radiological findings in three cases of persistent
JD Graphics Solutions
Potrebbero piacerti anche
- Poster Emma AccorsiDocumento1 paginaPoster Emma Accorsisagun maharjanNessuna valutazione finora
- Assessment and Interpretation of Vitamin and Trace.34Documento9 pagineAssessment and Interpretation of Vitamin and Trace.34ntnquynhproNessuna valutazione finora
- 2021 The Use of Fecal Calprotectin Testing in PaediatricDocumento24 pagine2021 The Use of Fecal Calprotectin Testing in PaediatricKhadijah Rizky SumitroNessuna valutazione finora
- Medicine: Congenital Absence of The Penis (Aphallia)Documento4 pagineMedicine: Congenital Absence of The Penis (Aphallia)Magic_OverNessuna valutazione finora
- Beaudoin 2003Documento6 pagineBeaudoin 2003Eduardo Alexander GuevaraNessuna valutazione finora
- 1 s2.0 S0169260722002759 MainDocumento12 pagine1 s2.0 S0169260722002759 MainMartaNessuna valutazione finora
- TutorialDocumento3 pagineTutorialAfifNessuna valutazione finora
- Better Late Than Never Clinical Value of Day7 Blastocysts 1672902759Documento5 pagineBetter Late Than Never Clinical Value of Day7 Blastocysts 16729027594ycxyd7fjqNessuna valutazione finora
- 73 1189 1 PBDocumento2 pagine73 1189 1 PBNguyễn Thị Kim TrọngNessuna valutazione finora
- Ambiguous Genitalia 3Documento4 pagineAmbiguous Genitalia 3syarifah salmaNessuna valutazione finora
- Cell Cycle and Mitosis Notes H Name I. The Cell CycleDocumento3 pagineCell Cycle and Mitosis Notes H Name I. The Cell CycleNickNessuna valutazione finora
- 2021 OrvietoDocumento5 pagine2021 Orvieto99ornelaNessuna valutazione finora
- Biometrical Threshold of Biparietal Diameter For Certain Fetal Sex Assignment by UltrasoundDocumento4 pagineBiometrical Threshold of Biparietal Diameter For Certain Fetal Sex Assignment by UltrasoundPraveena NagarajanNessuna valutazione finora
- 2020 Ferrick Hum RepDocumento13 pagine2020 Ferrick Hum RepPhuong NguyenNessuna valutazione finora
- Preterm BirthDocumento12 paginePreterm BirthCynthia Torres-GonzálezNessuna valutazione finora
- Reproductive HealthDocumento12 pagineReproductive Healthmd.ali.sharukhnawazNessuna valutazione finora
- Pnas 2115248119Documento7 paginePnas 2115248119Adolfo Mora SanchezNessuna valutazione finora
- E-Poster Enterics For Global Health Shigella Surveillance StudyDocumento1 paginaE-Poster Enterics For Global Health Shigella Surveillance StudyNeyama AlladinNessuna valutazione finora
- Ultrasound in Obstet Gyne - 2002 - Mazza - Biometrical Threshold of Biparietal Diameter For Certain Fetal Sex AssignmentDocumento4 pagineUltrasound in Obstet Gyne - 2002 - Mazza - Biometrical Threshold of Biparietal Diameter For Certain Fetal Sex AssignmentKartheka ThirumugamNessuna valutazione finora
- Best Position and Depth of Anaesthesia For.6Documento7 pagineBest Position and Depth of Anaesthesia For.6dmandatari7327Nessuna valutazione finora
- Embryogenesis in Higher Plants: An Overview: Marilyn A. L. West and John J HaradaDocumento9 pagineEmbryogenesis in Higher Plants: An Overview: Marilyn A. L. West and John J HaradaFahmi PasaribuNessuna valutazione finora
- Goldberg-HIV PosterDocumento1 paginaGoldberg-HIV PosterMicroposterNessuna valutazione finora
- Biology Science For Life With Physiology 5Th Edition Belk Solutions Manual Full Chapter PDFDocumento35 pagineBiology Science For Life With Physiology 5Th Edition Belk Solutions Manual Full Chapter PDFCassieYangiosx100% (12)
- Big Picture: Genes, Genomes and HealthDocumento16 pagineBig Picture: Genes, Genomes and HealthWellcome Trust100% (2)
- Pap ADocumento9 paginePap AlandabureNessuna valutazione finora
- Cerebelo, Periodo Sensitivo e AutismoDocumento15 pagineCerebelo, Periodo Sensitivo e Autismofhenrique77Nessuna valutazione finora
- Binder 3 (Natural Lens and Cataract) - RemovedDocumento20 pagineBinder 3 (Natural Lens and Cataract) - RemovedAyouvNessuna valutazione finora
- 57100998691 (1)Documento3 pagine57100998691 (1)SIMI DHARNessuna valutazione finora
- Live Birth After Transfer of A Single Euploid Vitrified-Warmed Blastocyst According To Standard Timing vs. Timing As Recommended by Endometrial Receptivity AnalysisDocumento8 pagineLive Birth After Transfer of A Single Euploid Vitrified-Warmed Blastocyst According To Standard Timing vs. Timing As Recommended by Endometrial Receptivity AnalysisAnh Vũ Hồ NgọcNessuna valutazione finora
- 157 FullDocumento3 pagine157 Fullnazanin zahra rezaeiNessuna valutazione finora
- The Tendency of Stunting Among Children Under Five in The North - 2023 - JornalDocumento8 pagineThe Tendency of Stunting Among Children Under Five in The North - 2023 - JornalJuan ZilkyNessuna valutazione finora
- Reproduction Lesson PlanDocumento11 pagineReproduction Lesson PlanSHILPA AGARWALNessuna valutazione finora
- 4c Genetic BabyDocumento3 pagine4c Genetic Babyapi-442397432Nessuna valutazione finora
- Dangers of Direct To Consumer TestingDocumento3 pagineDangers of Direct To Consumer TestinghannaNessuna valutazione finora
- Dsjuog 2015 09 372Documento10 pagineDsjuog 2015 09 372Swaleh YusufNessuna valutazione finora
- MedicineDocumento10 pagineMedicineRicardo PonceNessuna valutazione finora
- 2020 Jingyi Li XeroxatDocumento4 pagine2020 Jingyi Li Xeroxat99ornelaNessuna valutazione finora
- SETTLING THE VIRUS DEBATE SourceDocumento2 pagineSETTLING THE VIRUS DEBATE SourceNina VictorNessuna valutazione finora
- Usg 4DDocumento11 pagineUsg 4DAlharis FirmanNessuna valutazione finora
- September 17-21, 2018 Lesson PlanDocumento10 pagineSeptember 17-21, 2018 Lesson PlanSheryl Lou AngelesNessuna valutazione finora
- Babygram PDFDocumento22 pagineBabygram PDFArtikelWanitaDanKehamilanNessuna valutazione finora
- PHYSIOFemaleReproMARQUINO DUMLAODocumento8 paginePHYSIOFemaleReproMARQUINO DUMLAOGabby ElardoNessuna valutazione finora
- 2020 Brouillet-XeroxatDocumento18 pagine2020 Brouillet-Xeroxat99ornelaNessuna valutazione finora
- Part Two CH 8aDocumento16 paginePart Two CH 8a20200020895Nessuna valutazione finora
- New Debate Human Embryo: A Biological DefinitionDocumento7 pagineNew Debate Human Embryo: A Biological DefinitionPetru CernatNessuna valutazione finora
- What Is The Diagnostic Value of The Babygram?: Poster No.: Congress: Type: Authors: KeywordsDocumento20 pagineWhat Is The Diagnostic Value of The Babygram?: Poster No.: Congress: Type: Authors: KeywordskrisnoNessuna valutazione finora
- Urogenital System Embryology (ANA204) OFRDocumento8 pagineUrogenital System Embryology (ANA204) OFROloruntomi AdesinaNessuna valutazione finora
- Multifetal PintDocumento51 pagineMultifetal PintLeikkaNessuna valutazione finora
- Brain Tumours: Classification and Genes: V P CollinsDocumento10 pagineBrain Tumours: Classification and Genes: V P CollinsStevenNessuna valutazione finora
- Thy Box Timo Munoz-Chapuli2015Documento6 pagineThy Box Timo Munoz-Chapuli2015PABLO ALBERTO SANCHEZ BARRERANessuna valutazione finora
- Ambiguous Genitalia: Comparative Role of Pelvic Ultrasonography and GenitographyDocumento6 pagineAmbiguous Genitalia: Comparative Role of Pelvic Ultrasonography and GenitographyEni Maria SiscaNessuna valutazione finora
- Bioinformatics For High SchoolDocumento28 pagineBioinformatics For High SchoolHilman TaufiqNessuna valutazione finora
- First Trimester Screening For Preeclampsia An.6Documento8 pagineFirst Trimester Screening For Preeclampsia An.6ronaNessuna valutazione finora
- Biometrija Korpus KalozumaDocumento8 pagineBiometrija Korpus KalozumaBoroNessuna valutazione finora
- IDoR2015 Paediatric-Imaging-Book FINAL PDFDocumento129 pagineIDoR2015 Paediatric-Imaging-Book FINAL PDFMelii LujanNessuna valutazione finora
- 2019 LeaverDocumento27 pagine2019 Leaver99ornelaNessuna valutazione finora
- Shahd CVDocumento2 pagineShahd CVshoshoNessuna valutazione finora
- Human Embryo A Biological DefinitionDocumento7 pagineHuman Embryo A Biological Definitionjuan jose velascoNessuna valutazione finora
- Ex Part 1 Blueprinting Grid NewDocumento1 paginaEx Part 1 Blueprinting Grid NewKaushika KalaiNessuna valutazione finora
- Fundamentals of Human Embryology: Student Manual (second edition)Da EverandFundamentals of Human Embryology: Student Manual (second edition)Valutazione: 3 su 5 stelle3/5 (3)
- 6 - Tamplate For Incident - Accident - Near Miss ReportDocumento4 pagine6 - Tamplate For Incident - Accident - Near Miss ReportImran razaNessuna valutazione finora
- Case Presentation: Hirsutism and OligomenorrheaDocumento20 pagineCase Presentation: Hirsutism and Oligomenorrheadidu91Nessuna valutazione finora
- Medical Supplies - English VocabularyDocumento4 pagineMedical Supplies - English Vocabularyfatimageraldinep2564Nessuna valutazione finora
- Office Memo - Drug Free WorkplaceDocumento2 pagineOffice Memo - Drug Free WorkplaceAnthony ElmaNessuna valutazione finora
- Biphasic and Polyphasic SleepDocumento6 pagineBiphasic and Polyphasic SleepalNessuna valutazione finora
- Trends in Diagnosed Chronic Hepatitis B in A US Health System Population, 2006-2015Documento8 pagineTrends in Diagnosed Chronic Hepatitis B in A US Health System Population, 2006-2015farid ahmadNessuna valutazione finora
- Genesis Platinum Manual 8090Documento23 pagineGenesis Platinum Manual 8090Huni BuniNessuna valutazione finora
- Amal ResumeDocumento2 pagineAmal Resumeapi-242600239Nessuna valutazione finora
- Technology Update No. 7Documento24 pagineTechnology Update No. 7aRiTo!Nessuna valutazione finora
- Welcome To World of Dream Team For Your SuccessDocumento49 pagineWelcome To World of Dream Team For Your SuccessUNKNOWN DREAMERNessuna valutazione finora
- Chapter 7 CapsulesDocumento87 pagineChapter 7 CapsulesTeresa Saylo92% (26)
- Swab Contact MethodDocumento7 pagineSwab Contact Methodaca suki. haiNessuna valutazione finora
- Case Digest (6 & 27)Documento4 pagineCase Digest (6 & 27)frankieNessuna valutazione finora
- B.inggris Weny LestariDocumento5 pagineB.inggris Weny LestariRatna SusantiNessuna valutazione finora
- Exam Table For Shoulder and ElbowDocumento17 pagineExam Table For Shoulder and ElbowLouie OkayNessuna valutazione finora
- Psychometric Properties of The Consensus Sleep Diary in Those With Insomnia DisorderDocumento19 paginePsychometric Properties of The Consensus Sleep Diary in Those With Insomnia DisorderMondlTNessuna valutazione finora
- UV 365 Disinfection of Drinking Water Tanks: A Product by Expert 365 Pty LTD.Documento5 pagineUV 365 Disinfection of Drinking Water Tanks: A Product by Expert 365 Pty LTD.Faisal Ridho SaktiNessuna valutazione finora
- Antibiotic Use in Food Animals Worldwide, With A Focus On Africa - Pluses and MinusesDocumento8 pagineAntibiotic Use in Food Animals Worldwide, With A Focus On Africa - Pluses and MinusesxubicctNessuna valutazione finora
- One-Compartment Open Model: Intravenous Bolus AdministrationDocumento8 pagineOne-Compartment Open Model: Intravenous Bolus AdministrationnursalNessuna valutazione finora
- Questionnare On Work CultureDocumento6 pagineQuestionnare On Work CultureManish RamnaniNessuna valutazione finora
- MalariaDocumento170 pagineMalariatummalapalli venkateswara rao100% (4)
- Barangay Peace and Order and Public Safety PlanDocumento3 pagineBarangay Peace and Order and Public Safety PlanPeter Fritz Boholst100% (1)
- Environmental Monitoring Incubation Conditions - JustificationDocumento4 pagineEnvironmental Monitoring Incubation Conditions - Justificationveerreddy_157808Nessuna valutazione finora
- Routes of Drug AdministrationDocumento24 pagineRoutes of Drug Administrationmftaganas100% (1)
- Vulture ConservationDocumento14 pagineVulture ConservationSaba Parvin Haque100% (2)
- MiesDocumento40 pagineMiessanthiyasandyNessuna valutazione finora
- Effectivity: SY 2016-2017 Document Code: QR - AAD - 001 Revision No.: 00 Issue No.: 01 Date Issued: March 2015Documento7 pagineEffectivity: SY 2016-2017 Document Code: QR - AAD - 001 Revision No.: 00 Issue No.: 01 Date Issued: March 2015Sandre WaldenNessuna valutazione finora
- VACCP Template Checklist - SafetyCultureDocumento7 pagineVACCP Template Checklist - SafetyCulturepattysaborio520Nessuna valutazione finora
- Evacuation Earthquake PlanDocumento9 pagineEvacuation Earthquake PlanShane Giacinth AmarilaNessuna valutazione finora