Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Understanding The Difference: Surgical Mask N95 Respirator
Caricato da
WidarmaTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Understanding The Difference: Surgical Mask N95 Respirator
Caricato da
WidarmaCopyright:
Formati disponibili
2018
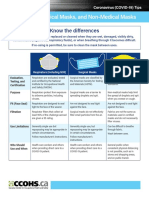
Understanding the Difference
! WARNING
Lorem ipsum dolor sit amet,
consectetur adipiscing elit. Nullam
scelerisque leo et eros convallis
condimentum. Phasellus tincidunt,
volutpat vitae.
Surgical Mask N95 Respirator
Testing and Cleared by the U.S. Food and Drug
Administration (FDA)
Evaluated, tested, and approved by
NIOSH as per the requirements in
Approval 42 CFR Part 84
Intended Use Fluid resistant and provides the wearer
protection against large droplets,
Reduces wearer’s exposure to particles
including small particle aerosols and
and Purpose splashes, or sprays of bodily or other large droplets (only non-oil aerosols).
hazardous fluids. Protects the patient
from the wearer’s respiratory emissions.
Face Seal Fit Loose-fitting Tight-fitting
Fit Testing No Yes
Requirement
User Seal Check No Yes. Required each time the respirator
is donned (put on)
Requirement
Filtration Does NOT provide the wearer with a
reliable level of protection from inhaling
Filters out at least 95% of airborne
particles including large and small
smaller airborne particles and is not particles
considered respiratory protection
Leakage Leakage occurs around the edge of the
mask when user inhales
When properly fitted and donned,
minimal leakage occurs around edges
of the respirator when user inhales
Use Limitations Disposable. Discard after each patient
encounter.
Ideally should be discarded after each
patient encounter and after aerosol-
generating procedures. It should
also be discarded when it becomes
damaged or deformed; no longer
forms an effective seal to the face;
becomes wet or visibly dirty; breathing
becomes difficult; or if it becomes
contaminated with blood, respiratory
or nasal secretions, or other bodily
fluids from patients.
Centers for Disease Control
and Prevention
National Institute for Occupational
Safety and Health
Potrebbero piacerti anche
- Technical Profile Life 1095-IntDocumento2 pagineTechnical Profile Life 1095-IntAlejandra VargasNessuna valutazione finora
- Technical Profile Life 1095-IntDocumento2 pagineTechnical Profile Life 1095-IntAlejandra VargasNessuna valutazione finora
- AHA Painting WorkDocumento3 pagineAHA Painting Workanilkumaranoop74Nessuna valutazione finora
- A Short Guide To Your RespiratorDocumento8 pagineA Short Guide To Your RespiratorkapilNessuna valutazione finora
- ADA COVID19 UnderstandingMasksDocumento1 paginaADA COVID19 UnderstandingMasksSergio Losada AmayaNessuna valutazione finora
- Eagle 20 EWDocumento11 pagineEagle 20 EWEliomar RivasNessuna valutazione finora
- Workplace Readiness 2nd Infographics 0Documento1 paginaWorkplace Readiness 2nd Infographics 0onahiatusNessuna valutazione finora
- Respirators, Surgical Masks, and Non-Medical Masks: Know The DifferencesDocumento2 pagineRespirators, Surgical Masks, and Non-Medical Masks: Know The DifferencesFahmi PratamaNessuna valutazione finora
- Group 2 - (PPE) Personal Protective EquipmentDocumento9 pagineGroup 2 - (PPE) Personal Protective EquipmentfrhhafizhaNessuna valutazione finora
- 3M Disposable Respirator 1860, 1860S, N95: Technical Data SheetDocumento2 pagine3M Disposable Respirator 1860, 1860S, N95: Technical Data SheetRoshidul AlomNessuna valutazione finora
- Herbicide: Only Use Herbicide With CONVISO SMART SeedsDocumento8 pagineHerbicide: Only Use Herbicide With CONVISO SMART SeedsDonny CosminNessuna valutazione finora
- Herbicide: Only Use Herbicide With CONVISO SMART SeedsDocumento8 pagineHerbicide: Only Use Herbicide With CONVISO SMART SeedsDonny CosminNessuna valutazione finora
- Ip 7 UpDocumento1 paginaIp 7 UpMilton-GenesisNessuna valutazione finora
- Caution: An Aqueous Suspension Biological Insecticide For Control of The Codling MothDocumento3 pagineCaution: An Aqueous Suspension Biological Insecticide For Control of The Codling MothHrafnaet SorgenNessuna valutazione finora
- Phy380 Chapter 1 Mind MapDocumento3 paginePhy380 Chapter 1 Mind Mapsabellaemellea16Nessuna valutazione finora
- CheckLists-5 2022Documento2 pagineCheckLists-5 2022Wikan Purwihantoro SudarmajiNessuna valutazione finora
- 3M 1860 Data SheetDocumento2 pagine3M 1860 Data SheetSoltic PeruNessuna valutazione finora
- Universal Mineral Seal Oil SDSDocumento6 pagineUniversal Mineral Seal Oil SDSRAFAEL MIERESNessuna valutazione finora
- Standard Precautions and Additional PrecautionsDocumento11 pagineStandard Precautions and Additional PrecautionsmiziezuraNessuna valutazione finora
- SDS eDocumento7 pagineSDS eHansamu TamaNessuna valutazione finora
- Safety Engineering Ce 1 D.......Documento8 pagineSafety Engineering Ce 1 D.......Hnut hinorNessuna valutazione finora
- 3M 8210 Data Sheet PDFDocumento8 pagine3M 8210 Data Sheet PDFkh88hmiNessuna valutazione finora
- 11.B Personnal HygieneDocumento10 pagine11.B Personnal HygieneGirgis AiadNessuna valutazione finora
- Multimedia PDFDocumento1 paginaMultimedia PDFBilal DinçelNessuna valutazione finora
- Check List Protection Respiratory - BesalcoDocumento5 pagineCheck List Protection Respiratory - BesalcoScribdTranslationsNessuna valutazione finora
- Isolation Precautions (CD Tech)Documento7 pagineIsolation Precautions (CD Tech)May Chelle ErazoNessuna valutazione finora
- 3M Disposable Respirator 1870+, N95: Technical Data SheetDocumento2 pagine3M Disposable Respirator 1870+, N95: Technical Data Sheetgeeko geekNessuna valutazione finora
- BDPL OCP 20 WI-01 Spill Kit Usage Guidelines-1Documento5 pagineBDPL OCP 20 WI-01 Spill Kit Usage Guidelines-1visamaintenance10Nessuna valutazione finora
- Infection Control - Personal Protective EquipmentDocumento8 pagineInfection Control - Personal Protective EquipmentmayyamaharaniNessuna valutazione finora
- Options Considerations in Selection GogglesDocumento1 paginaOptions Considerations in Selection GogglesPriskilla Grace TicoaluNessuna valutazione finora
- Biosafety in The LaboratoryDocumento1 paginaBiosafety in The LaboratoryALFARO CHAT CARMINA ROSARIONessuna valutazione finora
- MasksDocumento1 paginaMasksapi-460314063Nessuna valutazione finora
- Manual de Filtro de Aire 1028600Documento12 pagineManual de Filtro de Aire 1028600Ayme AlcazarNessuna valutazione finora
- Caution: Keep Out of Reach of ChildrenDocumento12 pagineCaution: Keep Out of Reach of ChildrenJosua CrystovelNessuna valutazione finora
- 8822 Tech Datasheet 2018Documento2 pagine8822 Tech Datasheet 2018vivek jayswalNessuna valutazione finora
- Product and Company Identification: Safety Data SheetDocumento6 pagineProduct and Company Identification: Safety Data Sheetjulianpellegrini860Nessuna valutazione finora
- Respiratory Protection For Producers: Respirator TypesDocumento5 pagineRespiratory Protection For Producers: Respirator TypesThyago Rodrigues de SouzaNessuna valutazione finora
- Physan - 20 Drum Epa 2010 2clDocumento1 paginaPhysan - 20 Drum Epa 2010 2clgafomo4046Nessuna valutazione finora
- Mame PraticalDocumento10 pagineMame PraticalDOUMBOUYA SIDIKINessuna valutazione finora
- HS - 4000 - Welding - (Niosh) - Survivair 4000 S - Series ManualDocumento7 pagineHS - 4000 - Welding - (Niosh) - Survivair 4000 S - Series ManualB V SUMANNessuna valutazione finora
- Instruction Manual Guide D'Utilisation Manual de InstruccionesDocumento15 pagineInstruction Manual Guide D'Utilisation Manual de InstruccionesJm KamachoNessuna valutazione finora
- Cleaning and Disinfectan TransducerDocumento17 pagineCleaning and Disinfectan Transducerlail ariftianiNessuna valutazione finora
- Owner's/Operator's Manual Manual de Funcionamiento para Usuarios y PropietariosDocumento32 pagineOwner's/Operator's Manual Manual de Funcionamiento para Usuarios y PropietariosCenema CenemaNessuna valutazione finora
- Slide-In Vacuum Tank Operator'S Manual: WarningDocumento32 pagineSlide-In Vacuum Tank Operator'S Manual: WarningJhon Edison CastroNessuna valutazione finora
- MSDS DM 648 PDFDocumento12 pagineMSDS DM 648 PDFArnoldo Sánchez DNessuna valutazione finora
- Hazardous Chemicals - Personal Protective Equipment (Ppe) : M.AishwaryaDocumento13 pagineHazardous Chemicals - Personal Protective Equipment (Ppe) : M.AishwaryaAishwarya mohanNessuna valutazione finora
- Abamectin015EC LABELDocumento3 pagineAbamectin015EC LABELAvheani Pamela MuladiNessuna valutazione finora
- Faggiolatti PumpsDocumento11 pagineFaggiolatti PumpsThairanil AbduljaleelNessuna valutazione finora
- 2012-PRT-0002 ProTone - Form 04-6472-R4Documento4 pagine2012-PRT-0002 ProTone - Form 04-6472-R4Fadhilah SurotoNessuna valutazione finora
- 16 COSHH Assessment For Kleiberit AdhesivesDocumento2 pagine16 COSHH Assessment For Kleiberit AdhesivesjaseerhsecentercoreNessuna valutazione finora
- AWS Foam Cleaner PISDocumento1 paginaAWS Foam Cleaner PISjack jansenNessuna valutazione finora
- Multimedia PDFDocumento2 pagineMultimedia PDFAndi MachoNessuna valutazione finora
- Care For Your Half-Mask Air-Purifying RespiratorDocumento1 paginaCare For Your Half-Mask Air-Purifying RespiratorQAQC SIEMNessuna valutazione finora
- Labsafety 2020Documento80 pagineLabsafety 2020amerNessuna valutazione finora
- JSA For DPTDocumento3 pagineJSA For DPTMohammed Minhaj100% (1)
- Magic Sulfur Dust: CautionDocumento2 pagineMagic Sulfur Dust: CautionCuriosityShopNessuna valutazione finora
- Frias Chrishalene SP400Documento12 pagineFrias Chrishalene SP400Ed Drexel LizardoNessuna valutazione finora
- Devilbiss Atomizers/Nebulizers/ Syringes/Powder Blowers Instruction GuideDocumento2 pagineDevilbiss Atomizers/Nebulizers/ Syringes/Powder Blowers Instruction GuideStacy WilsonNessuna valutazione finora
- Safety Data Sheet: 1. Identification of The Substance/Preparation and of The Company/UndertakingDocumento7 pagineSafety Data Sheet: 1. Identification of The Substance/Preparation and of The Company/UndertakingSergio Mendoza ZuñigaNessuna valutazione finora
- Data Bulletin Group Motor Installations:: Understanding National Electrical Code (NEC) 430.53 RequirementsDocumento8 pagineData Bulletin Group Motor Installations:: Understanding National Electrical Code (NEC) 430.53 RequirementsshoaibNessuna valutazione finora
- The Acceptability of Rubber Tree Sap (A As An Alternative Roof SealantDocumento7 pagineThe Acceptability of Rubber Tree Sap (A As An Alternative Roof SealantHannilyn Caldeo100% (2)
- Dual Shield 7100 Ultra: Typical Tensile PropertiesDocumento3 pagineDual Shield 7100 Ultra: Typical Tensile PropertiesDino Paul Castro HidalgoNessuna valutazione finora
- Electricity NotesDocumento35 pagineElectricity Notesapi-277818647Nessuna valutazione finora
- Cecilia-Puff-Tee-Final-OUSM-Designs-12 MESES A TALLA 8Documento19 pagineCecilia-Puff-Tee-Final-OUSM-Designs-12 MESES A TALLA 8Jose SanchezNessuna valutazione finora
- Hydrodynamic Calculation Butterfly Valve (Double Disc)Documento31 pagineHydrodynamic Calculation Butterfly Valve (Double Disc)met-calcNessuna valutazione finora
- OurCatholicFaith PowerPoint Chapter1Documento21 pagineOurCatholicFaith PowerPoint Chapter1VinNessuna valutazione finora
- 07ercoskun 05 01 PDFDocumento23 pagine07ercoskun 05 01 PDFjagmadridNessuna valutazione finora
- LG250CDocumento2 pagineLG250CCarlosNessuna valutazione finora
- Poly 103Documento20 paginePoly 103Sharifah Zulaikha BenYahyaNessuna valutazione finora
- Effects of Climate ChangeDocumento3 pagineEffects of Climate Changejiofjij100% (1)
- 12.5 Collision Theory - ChemistryDocumento15 pagine12.5 Collision Theory - ChemistryAri CleciusNessuna valutazione finora
- From Input To Affordance: Social-Interactive Learning From An Ecological Perspective Leo Van Lier Monterey Institute Oflntemational StudiesDocumento15 pagineFrom Input To Affordance: Social-Interactive Learning From An Ecological Perspective Leo Van Lier Monterey Institute Oflntemational StudiesKayra MoslemNessuna valutazione finora
- Innerwear Industry Pitch PresentationDocumento19 pagineInnerwear Industry Pitch PresentationRupeshKumarNessuna valutazione finora
- PIX4D Simply PowerfulDocumento43 paginePIX4D Simply PowerfulJUAN BAQUERONessuna valutazione finora
- AssessmentDocumento9 pagineAssessmentJuan Miguel Sapad AlpañoNessuna valutazione finora
- Kindergarten Math Problem of The Day December ActivityDocumento5 pagineKindergarten Math Problem of The Day December ActivityiammikemillsNessuna valutazione finora
- Plastics and Polymer EngineeringDocumento4 paginePlastics and Polymer Engineeringsuranjana26Nessuna valutazione finora
- Shandong Baoshida Cable Co, LTD.: Technical ParameterDocumento3 pagineShandong Baoshida Cable Co, LTD.: Technical ParameterkmiqdNessuna valutazione finora
- Cross Talk Details and RoutingDocumento29 pagineCross Talk Details and RoutingRohith RajNessuna valutazione finora
- Indor Lighting DesignDocumento33 pagineIndor Lighting DesignRajesh MalikNessuna valutazione finora
- PEH Q3 Long QuizDocumento1 paginaPEH Q3 Long QuizBenedict LumagueNessuna valutazione finora
- Straight LineDocumento15 pagineStraight LineAyanNessuna valutazione finora
- Someone Who Believes in YouDocumento1 paginaSomeone Who Believes in YouMANOLO C. LUCENECIONessuna valutazione finora
- TreesDocumento69 pagineTreesADITYA GEHLAWATNessuna valutazione finora
- Recipes From The Perfect Scoop by David LebovitzDocumento10 pagineRecipes From The Perfect Scoop by David LebovitzThe Recipe Club100% (7)
- Biophoton RevolutionDocumento3 pagineBiophoton RevolutionVyavasayaha Anita BusicNessuna valutazione finora
- Potassium Fixation As Affected by Alternate Wetting and Drying in Some Soil Series of JharkhandDocumento4 paginePotassium Fixation As Affected by Alternate Wetting and Drying in Some Soil Series of JharkhandDr Amrit Kumar JhaNessuna valutazione finora
- World's Standard Model G6A!: Low Signal RelayDocumento9 pagineWorld's Standard Model G6A!: Low Signal RelayEgiNessuna valutazione finora
- Esteem 1999 2000 1.3L 1.6LDocumento45 pagineEsteem 1999 2000 1.3L 1.6LArnold Hernández CarvajalNessuna valutazione finora