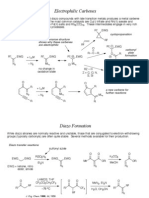
Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Handout1 Vaska Compound
Caricato da
Mior AfiqTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Handout1 Vaska Compound
Caricato da
Mior AfiqCopyright:
Formati disponibili
HANDOUT 1
Vaska's complex
From Wikipedia, the free encyclopedia.
Vaska's complex
General
Systematic name Carbonychlorolbis(triphenylphosphine)iridium(I)
Iridium(I)bis(triphenylphosphine)
carbonyl chloride
Other names
Vaska's complex
Vaska's compound
Molecular formula IrCl(CO)[P(C6H5)3]2.
Molar mass 780.25 g/mol
Appearance yellow crystals
CAS number [14871-41-1]
EINECS number 238-941-6
Properties
Density and phase ? g/cm3, ?
Solubility in water insol
Other solvents sparingly: benzene
Handout 1 page 1/5
Melting point 215 °C (decomp.)
Handout 1 page 2/5
Structure
Coordination
square planar
geometry
Crystal structure ?
Dipole moment ?D
Hazards
MSDS External MSDS
Main hazards none
NFPA 704
R: none
R/S statement
S: 22-24/25
RTECS number ?
Supplementary data page
Structure and
n, εr, etc.
properties
Thermodynamic Phase behaviour
data Solid, liquid, gas
Spectral data UV, IR, NMR, MS
Related compounds
Other anions IrI(CO)[P(C6H5)3]2
Other cations RhCl(CO)[P(C6H5)3]2
Related compounds Pd[P(C6H5)3]4
Except where noted otherwise, data are given for
materials in their standard state (at 25 °C, 100 kPa)
Infobox disclaimer and references
Vaska's complex is the trivial name for trans-chlorocarbonylbis(triphenylphosphine)-
iridium(I) with the formula IrCl(CO)[P(C6H5)3]2. This square planar diamagnetic
organometallic complex consists of a central iridium atom bound to two mutually trans
triphenylphosphine ligands as well as carbon monoxide, and chloride. The complex was
reported by Di Luzio and Vaska in 19611. Vaska's complex can undergo oxidative
additions and is notable for its ability to bind to O2 reversibly. It is a bright yellow
crystalline solid.
Handout 1 page 3/5
Preparation
The synthesis involves heating virtually any iridium chloride salt with P(C6H5)3 with a
CO source. The most popular method uses dimethylformamide (DMF) as a solvent.
Sometimes aniline is added to accelerate the reaction. Another popular solvent is 2-
methoxyethanol. The reaction typically is conducted under nitrogen. In the synthesis,
triphenylphosphine serves as both a ligand and a reductant, and the carbonyl ligand is
derived by decomposition of dimethylformamide probably via a deinsertion of an
intermediate Ir-C(O)H species. The following is a possible balanced equation for this
complicated reaction2.
H2IrCl6 + 3.5P(C6H5)3 + HCON(CH3)2 + 4C6H5NH2 + 1.5H2O → IrCl(CO)
[P(C6H5)3]2 + (CH3)2NH2+Cl- + 1.5 OP(C6H5)3
Typical sources of iridium used in this preparation are IrCl3.xH2O and H2IrCl6.
Reactions
Studies on Vaska's complex provided a conceptual framework for homogeneous catalysis.
Vaska's complex, with 16 valence electrons, is considered "unsaturated" and can thus
bind to one two-electron or two one-electron ligands to become electronically saturated
with 18 valence electrons. The addition of two one-electron ligands is called oxidative
addition. Upon oxidative addition, the oxidation state of the iridium increases from Ir(I)
to Ir(III). The four-coordinated square planar arrangement in the starting complex
converts to an octahedral, six-coordinate product. Vaska's complex undergoes oxidative
addition with conventional oxidants such as halogens, strong acids such as HCl, and other
molecules known to react as electrophiles, such as iodomethane (CH3I).
An interesting characteristic of Vaska's complex is that it binds O2 reversibly.
IrCl(CO)[P(C6H5)3]2 + O2 ↔ IrCl(CO)[P(C6H5)3]2O2
The dioxygen ligand is bonded to Ir(I) via both oxygen atoms, so-called side-on bonding.
In myoglobin and hemoglobin, O2 binds "end-on," attaching to the metal via only one of
the two oxygen atoms. The oxygenation reaction is carried out simply by bubbling O2
through a solution of Vaska's complex in toluene, which results in a colour change from
yellow to orange. The resulting dioxgen adduct reverts to the parent complex upon
heating in boiling benzene solution.
Handout 1 page 4/5
Spectroscopy
Infrared spectroscopy can be used to analyse the products of oxidative addition to Vaska's
complex because the reactions induce characteristic shifts of the stretching frequency of
the coordinated carbon monoxide.{fn|3}}. These shifts are dependent on the amount of π-
back bonding allowed from the newly associated ligands. The CO stretching frequencies
for Vaska's complex and oxidatively added ligands have been documented in literature4.
Vaska's Complex: 1967 cm-1
Vaska's + O2: 2015 cm-1
Vaska's + MeI: 2047 cm-1
Vaska's + I2: 2067 cm-1
Oxidative addition to give Ir(III) products reduces the π-bonding from Ir to C, which
causes the increase in the frequency of the carbonyl stretching band. The stretching
frequency changes depending on the ligands that have been added, but is always greater
than 2000 cm-1 for an Ir(III) complex.
References
Note 1: L. Vaska and J.W. DiLuzio "Carbonyl and Hydrido-Carbonyl Complexes
of Iridium by Reaction with Alcohols. Hydrido Complexes by Reaction with
Acid" J. Am. Chem. Soc., 83, 2784-5 (1961).DOI: 10.1021/ja01473a054
Note 2: Girolami, G.S.; Rauchfuss, T.B.; Angelici, R.J. Synthesis and Technique
in Inorganic Chemistry, Third ed.; University Science Books.: Sausalito, 1999,
pp190.
Note 3: L. Vaska and J.W. DiLuzio J. Am. Chem. Soc., 84, 679 (1962).
Note 4: Crabtree, R. The Organometallic Chemistry of the Transition Metals;
Third Ed.; John Wiley & Sons, Inc.: Canada, 2001, pp152.
Handout 1 page 5/5
Potrebbero piacerti anche
- CES - Management - Oil Tanker - Correct AnswersDocumento87 pagineCES - Management - Oil Tanker - Correct Answersboramir496793% (27)
- Plumbing Notes 1 PDFDocumento57 paginePlumbing Notes 1 PDFhoneyvie53% (15)
- Handout1 Vaska CompoundDocumento5 pagineHandout1 Vaska CompoundEustance JuanNessuna valutazione finora
- Handout1 Vaska CompoundDocumento4 pagineHandout1 Vaska CompoundMuhammad ShimaNessuna valutazione finora
- A Review of Organotin Compounds Chemistry and ApplDocumento9 pagineA Review of Organotin Compounds Chemistry and ApplJuan C HernandezNessuna valutazione finora
- NitrationDocumento27 pagineNitrationsubhashpithaniNessuna valutazione finora
- ThioureaDocumento8 pagineThioureaWidhy LestariNessuna valutazione finora
- Hydroformylation Reaction ProcessDocumento5 pagineHydroformylation Reaction ProcessJohann ChorenNessuna valutazione finora
- Phosgene: "Cocl2" Redirects Here. For The Compound Cocl, See - Not To Be Confused With, ,, orDocumento7 paginePhosgene: "Cocl2" Redirects Here. For The Compound Cocl, See - Not To Be Confused With, ,, orCarlos BustamanteNessuna valutazione finora
- Aldehydes and KetonsDocumento8 pagineAldehydes and KetonsnishaninishaNessuna valutazione finora
- Schwartz Reagent ReviewDocumento58 pagineSchwartz Reagent ReviewJackson PhiveNessuna valutazione finora
- Group 4Documento18 pagineGroup 4Rizwan SarwarNessuna valutazione finora
- Regioselective Hydromethoxycarbonylation of Terminal Alkynes Catalyzed by Palladium (II) Tetraphos ComplexesDocumento6 pagineRegioselective Hydromethoxycarbonylation of Terminal Alkynes Catalyzed by Palladium (II) Tetraphos ComplexesGreciel Egurrola SanchezNessuna valutazione finora
- Csir Ugc JRF Net Chemistry Paper 1 (Part B) Series - 1Documento22 pagineCsir Ugc JRF Net Chemistry Paper 1 (Part B) Series - 1polamrajuNessuna valutazione finora
- Pyridine - Wikipedia, The Free EncyclopediaDocumento15 paginePyridine - Wikipedia, The Free EncyclopediaAnkan Pal100% (1)
- Selective Oxidation of Aldehydes To Carboxylic Acids With Sodium Chlorite-Hydrogen PeroxideDocumento3 pagineSelective Oxidation of Aldehydes To Carboxylic Acids With Sodium Chlorite-Hydrogen PeroxidejavasoloNessuna valutazione finora
- Bahan NODocumento18 pagineBahan NOjujuNessuna valutazione finora
- Nitric Oxide: Jump To Navigation Jump To SearchDocumento86 pagineNitric Oxide: Jump To Navigation Jump To SearchChaeyoung SonNessuna valutazione finora
- Ald Keto Carboxylic AcidDocumento6 pagineAld Keto Carboxylic AcidCY Shivam SinghNessuna valutazione finora
- AJC Prelim 2008 Paper 1Documento14 pagineAJC Prelim 2008 Paper 1yuchao123Nessuna valutazione finora
- 1 s2.0 S1381116903002073 Main PDFDocumento8 pagine1 s2.0 S1381116903002073 Main PDFRafael SanchezNessuna valutazione finora
- Questions Answers Coordination PDFDocumento15 pagineQuestions Answers Coordination PDFlingarajugowdaNessuna valutazione finora
- A New Pyridine Bis N Heterocyclic Carbene Ligand and Its Coordination To RH Synthesis and CharacterizationDocumento5 pagineA New Pyridine Bis N Heterocyclic Carbene Ligand and Its Coordination To RH Synthesis and CharacterizationAbbas WshelNessuna valutazione finora
- JACS, Vol. 108, 1986, 452Documento10 pagineJACS, Vol. 108, 1986, 452rrgodboleNessuna valutazione finora
- Aldehydes and Ketones-DSVOLDocumento107 pagineAldehydes and Ketones-DSVOLMERCY ATUYANessuna valutazione finora
- 961 Efficient Method Going From OH To Cle3b0Documento3 pagine961 Efficient Method Going From OH To Cle3b0Wolmir NemitzNessuna valutazione finora
- Palladium Catalyzed Cross CouplingDocumento5 paginePalladium Catalyzed Cross Couplingshiro_heNessuna valutazione finora
- Chemistry HSSC-II SolutionDocumento12 pagineChemistry HSSC-II SolutionSAAD RIAZNessuna valutazione finora
- Tenkasi District Schools .Qu - KeyDocumento16 pagineTenkasi District Schools .Qu - Keydevilssworld143Nessuna valutazione finora
- 12 Chemistry Keypoints Revision Questions Chapter 12Documento20 pagine12 Chemistry Keypoints Revision Questions Chapter 12sangam patraNessuna valutazione finora
- Enamines and YlidesDocumento18 pagineEnamines and YlidesVijay Pradhan100% (1)
- Aldehydes & KetonesDocumento104 pagineAldehydes & KetonesCharin Kadian75% (4)
- Indium (III) - Catalyzed Reductive Bromination and Iodination of Carboxylic Acids To Alkyl Bromides and Iodides Scope, Mechanism, and One-Pot Transformation To Alkyl Halides and Amine DerivativesDocumento9 pagineIndium (III) - Catalyzed Reductive Bromination and Iodination of Carboxylic Acids To Alkyl Bromides and Iodides Scope, Mechanism, and One-Pot Transformation To Alkyl Halides and Amine DerivativesBekkahNessuna valutazione finora
- Coordination Compound CHEMHACKDocumento3 pagineCoordination Compound CHEMHACKakashhk1001Nessuna valutazione finora
- 1D Shebaldina2004Documento5 pagine1D Shebaldina2004Akshayan RNessuna valutazione finora
- CarbimazoleDocumento4 pagineCarbimazolevelangniNessuna valutazione finora
- c2 PDFDocumento40 paginec2 PDFSammie PingNessuna valutazione finora
- Carbenoids 2Documento15 pagineCarbenoids 2costea0028Nessuna valutazione finora
- ChelationDocumento12 pagineChelationAvinash PatilNessuna valutazione finora
- Synthesis of Potassium Tris (Oxalato) Ferrate (III)Documento7 pagineSynthesis of Potassium Tris (Oxalato) Ferrate (III)Timothy Tan83% (36)
- F Ac 18 1 2012 0510Documento36 pagineF Ac 18 1 2012 0510Handugan Quinlog NoelNessuna valutazione finora
- Nomenclature of Coordination Complexes With KeyDocumento7 pagineNomenclature of Coordination Complexes With KeyUmendra KhokharNessuna valutazione finora
- CoordinationDocumento15 pagineCoordinationkashyap100% (1)
- Inorganic Chemistry Volume 50 Issue 20 2011Documento12 pagineInorganic Chemistry Volume 50 Issue 20 2011Lee ToulouseNessuna valutazione finora
- Net Ionic Equations PacketDocumento9 pagineNet Ionic Equations PacketmountainchocolateNessuna valutazione finora
- Chemistry MSDocumento6 pagineChemistry MSUtkarsh SharmaNessuna valutazione finora
- Angewandte Chemie Brookhartetal 2000 Issue 9 Page 1679Documento5 pagineAngewandte Chemie Brookhartetal 2000 Issue 9 Page 1679Rosena PutriNessuna valutazione finora
- Synergistic Catalysis by Lewis Acid and Base Sites On Zro For Meerwein Ponndorf Verley ReductionDocumento7 pagineSynergistic Catalysis by Lewis Acid and Base Sites On Zro For Meerwein Ponndorf Verley ReductionRiza SaidNessuna valutazione finora
- Oxidation-Of-Olefins-By-Palladiumii 6pDocumento6 pagineOxidation-Of-Olefins-By-Palladiumii 6pLi HojunNessuna valutazione finora
- BT2 Revision Package 2013 - AnsDocumento70 pagineBT2 Revision Package 2013 - AnsSean Ng Jun JieNessuna valutazione finora
- New Microsoft Word DocumentDocumento42 pagineNew Microsoft Word DocumentSalamat AliNessuna valutazione finora
- Uv VisDocumento28 pagineUv VisVictor AristizabalNessuna valutazione finora
- Labuschagne Heat 2015Documento29 pagineLabuschagne Heat 2015EthanNessuna valutazione finora
- Revision:Edexcel Chemistry Unit 3B - Laboratory Techniques - Group 1 and 2 ReactionsDocumento4 pagineRevision:Edexcel Chemistry Unit 3B - Laboratory Techniques - Group 1 and 2 ReactionsAhmed ViaamNessuna valutazione finora
- Cadmium Coordination Compounds With Flexible Ligand 1,3-Bis (1,2,4-Triazol-1-Yl) Propane: Synthesis, Structure and Luminescent PropertiesDocumento8 pagineCadmium Coordination Compounds With Flexible Ligand 1,3-Bis (1,2,4-Triazol-1-Yl) Propane: Synthesis, Structure and Luminescent PropertiesSofi AmaliaNessuna valutazione finora
- Lanthanum OxideDocumento6 pagineLanthanum OxideEric MonsalveNessuna valutazione finora
- Handout6 Wilkinson's CatalystDocumento3 pagineHandout6 Wilkinson's CatalystMior AfiqNessuna valutazione finora
- Oxidation of Some Primary and Secondary Alcohols Using Pyridinium ChlorochromateDocumento5 pagineOxidation of Some Primary and Secondary Alcohols Using Pyridinium ChlorochromateAleja RodriguezNessuna valutazione finora
- Silicon in Organic Synthesis: Butterworths Monographs in Chemistry and Chemical EngineeringDa EverandSilicon in Organic Synthesis: Butterworths Monographs in Chemistry and Chemical EngineeringNessuna valutazione finora
- Coordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972Da EverandCoordination Chemistry—XIV: Plenary Lectures Presented at the XIVth International Conference on Coordination Chemistry Held at Toronto, Canada, 22—28 June 1972A. B. P. LeverNessuna valutazione finora
- The Organometallic Chemistry of N-heterocyclic CarbenesDa EverandThe Organometallic Chemistry of N-heterocyclic CarbenesNessuna valutazione finora
- Handout7 Wacker OxidationDocumento1 paginaHandout7 Wacker OxidationMior AfiqNessuna valutazione finora
- Organometallic Chemistry: Prof DR Hadariah Bahron Organometallic Chemistry March-July 2018Documento44 pagineOrganometallic Chemistry: Prof DR Hadariah Bahron Organometallic Chemistry March-July 2018Mior Afiq100% (1)
- Handout6 Wilkinson's CatalystDocumento3 pagineHandout6 Wilkinson's CatalystMior AfiqNessuna valutazione finora
- Handout 5 The Monsanto Acetic Acid Process: Insertion of Carbon MonoxideDocumento2 pagineHandout 5 The Monsanto Acetic Acid Process: Insertion of Carbon MonoxideMior AfiqNessuna valutazione finora
- Handout3 Schrock CarbeneDocumento3 pagineHandout3 Schrock CarbeneMior AfiqNessuna valutazione finora
- Organometallic Chemistry: Faculty of Applied Sciences Universiti Teknologi MaraDocumento1 paginaOrganometallic Chemistry: Faculty of Applied Sciences Universiti Teknologi MaraMior AfiqNessuna valutazione finora
- Handout4 Cyclopentadienyl LigandDocumento6 pagineHandout4 Cyclopentadienyl LigandMior AfiqNessuna valutazione finora
- Handout2 Fischer CarbeneDocumento5 pagineHandout2 Fischer CarbeneMuhammad ShimaNessuna valutazione finora
- Type of Chemical ReactionsDocumento13 pagineType of Chemical ReactionsSAHARAN ANANDNessuna valutazione finora
- Presenters Post16 Tcm18-118246Documento18 paginePresenters Post16 Tcm18-118246Kamariah IsmailNessuna valutazione finora
- Embedded Enzymatic Biomaterial Degradation: 6836 Macromolecules 2009, 42, 6836-6839Documento4 pagineEmbedded Enzymatic Biomaterial Degradation: 6836 Macromolecules 2009, 42, 6836-6839hhkkllNessuna valutazione finora
- Biomechanical Properties of A New Fiber-Reinforced CompositesDocumento10 pagineBiomechanical Properties of A New Fiber-Reinforced Compositesazam ahmedNessuna valutazione finora
- 7 - Casing DesignDocumento36 pagine7 - Casing Designام فاطمة البطاط100% (1)
- Mixture RequirementsDocumento11 pagineMixture Requirementsrajesh0% (1)
- Chocolate: Chocolate Is A Raw or Processed Food Produced From The Seed of The TropicalDocumento7 pagineChocolate: Chocolate Is A Raw or Processed Food Produced From The Seed of The TropicalNyimas Irina SilvaniNessuna valutazione finora
- CIO Vol.5 No.1-2 FinalDocumento115 pagineCIO Vol.5 No.1-2 FinalTrond ForeldrahNessuna valutazione finora
- Organic Chemistry PDFDocumento181 pagineOrganic Chemistry PDFShyam Yadav100% (1)
- Plate Fin Coil PDFDocumento2 paginePlate Fin Coil PDFHermawan LesmanaNessuna valutazione finora
- Gas Chromatography AmmoniaDocumento10 pagineGas Chromatography AmmoniacurlychemNessuna valutazione finora
- Hofmeister Series: Ions Franz Hofmeister ProteinsDocumento11 pagineHofmeister Series: Ions Franz Hofmeister ProteinsRajeshwari SridharanNessuna valutazione finora
- Tds Chemical Resistant CoatingDocumento3 pagineTds Chemical Resistant CoatingUtilities2Nessuna valutazione finora
- Cambridge International Examinations Cambridge International Advanced Subsidiary and Advanced LevelDocumento20 pagineCambridge International Examinations Cambridge International Advanced Subsidiary and Advanced LevelharshanauocNessuna valutazione finora
- Machinability of BS S132Documento2 pagineMachinability of BS S132goggerNessuna valutazione finora
- 810.00 MR-N, NC, NSDocumento110 pagine810.00 MR-N, NC, NSnqh2009100% (1)
- Maurice WilkinsDocumento15 pagineMaurice Wilkinsmenilanjan89nLNessuna valutazione finora
- Hyperbaric Oxygen TherapyDocumento7 pagineHyperbaric Oxygen Therapy18juni1995Nessuna valutazione finora
- Pneumatic Auto Feed Drilling Machine With Indexing Machine: SynopsisDocumento30 paginePneumatic Auto Feed Drilling Machine With Indexing Machine: SynopsisMaruthi JacsNessuna valutazione finora
- WW-WASG03 Electrical Wire Sizes-WEB 7-7-11 PDFDocumento1 paginaWW-WASG03 Electrical Wire Sizes-WEB 7-7-11 PDFSemion VirtudazoNessuna valutazione finora
- Born Oppenheimer ApproximationDocumento15 pagineBorn Oppenheimer ApproximationElizabeth HarrisonNessuna valutazione finora
- (T. R. Chouhan) Bhopal, The Inside Story - Carbide Workers Speak Out On The World's Worst Industrial DisasterDocumento214 pagine(T. R. Chouhan) Bhopal, The Inside Story - Carbide Workers Speak Out On The World's Worst Industrial DisasterANTENOR JOSE ESCUDERO GÓMEZNessuna valutazione finora
- Pharmacognostical and Preliminary Phytochemical Screening On Leaves of Trianthema Decandra Linn.Documento3 paginePharmacognostical and Preliminary Phytochemical Screening On Leaves of Trianthema Decandra Linn.anto_pharma7784Nessuna valutazione finora
- Zatamaru Cjenovnik PregledatiDocumento8 pagineZatamaru Cjenovnik PregledatiNemanja StrkicNessuna valutazione finora
- Gen Bio W3-5Documento9 pagineGen Bio W3-5Alyson EscuderoNessuna valutazione finora
- Chapter Four, Cycloalkanes (Part One - Monocyclic Alkane)Documento8 pagineChapter Four, Cycloalkanes (Part One - Monocyclic Alkane)Amin JamjahNessuna valutazione finora
- Fyup Chemistry SyllabusDocumento81 pagineFyup Chemistry SyllabusRaj KumarNessuna valutazione finora
- Model C-1 Electrostatic Airless Spray Gun: Customer Product Manual Part 104 326DDocumento50 pagineModel C-1 Electrostatic Airless Spray Gun: Customer Product Manual Part 104 326DUlpianoxx19920% (1)