Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Hypnopompic Seizures: Original Article
Caricato da
Jovan IlićDescrizione originale:
Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Hypnopompic Seizures: Original Article
Caricato da
Jovan IlićCopyright:
Formati disponibili
Original article
Epileptic Disord 2010; 12 (4): 270-4
Hypnopompic seizures
Amer M. Awad, Hans O. Lüders
Neurological Institute, Case Western Reserve University,
University Hospitals Case Medical Center, Cleveland, Ohio, USA
Received May 17, 2010; Accepted September 22, 2010
ABSTRACT – The relationship between epilepsy and sleep is complex and
bidirectional. Ictal awakening is probably a common and well-described phe-
nomenon. In this small observational study we describe arousal from sleep as
the only, or at least main, manifestation of some epileptic seizures. We coin the
term “hypnopompic seizures” to describe this entity. Five patients with intrac-
table epilepsy were monitored by continuous video-electroencephalogram.
Four of them had left temporal lobe epilepsy and one patient had generalised
epilepsy. Hypnopompic seizures accounted for 30-100% of their seizure types
captured during monitoring. All the seizures occurred during stage II sleep and
were brief. Hypnopompic seizures are extremely subtle and may be underdiag-
nosed and underreported. Future larger studies are needed to shed some light on
this unique entity and its neuropathophysiology. Epileptologists should be aware
of this type of seizure and careful review of electroencephalograms during
the transition from sleep to arousal is imperative to capture these seizures.
Physicians, patients and families also need to be aware of such a subtle manifes-
tation of seizures. Improved awareness of hypnopompic seizures and subtle
seizures, in general, help guide accurate and early diagnosis, thorough monitor-
ing and appropriate management.
Key words: hypnopompic, seizures, arousal, sleep, ictal arousal
The relationship between arousal from Patients
sleep and epileptic seizures has been
described and evaluated (Janz, 1953; The clinical and electrographic fea-
Janz, 1974; Shouse, 1987; Wieser, tures of the patients are summarised
1991; Manni et al., 1997; Malow in table 1.
et al., 2000; Dasheiff and Kofke,
2003). The term “awakening epilepsy” Patient 1
(Aufwach-Epilepsien) was first coined
by Janz in 1953 (Janz, 1953). He The patient is a 31-year-old right-
referred to the awakening as a trigger- handed man with history of lesional
ing mechanism for epileptic seizures. left temporal lobe epilepsy secondary
In this study, we have explored the to left temporal oligodendroglioma
opposite phenomenon of awakening status post resection. He was admitted
as the result, rather than the cause, of to the epilepsy monitoring unit (EMU)
the seizure. To our knowledge, the for evaluation of new onset seizures.
phenomenon of awakening as the The seizures were described by the
doi: 10.1684/epd.2010.0336
only or the most prominent manifesta- patient as abdominal auras followed
tion of an epileptic seizure has not by inability to talk. The video-EEG
Correspondence: been previously described or evalu- (VEEG) evaluation revealed a normal
A.M. Awad, MD
11100 Euclid Ave.,
ated. We coin the term “hypnopompic interictal recording. During his eight
Cleveland, OH 44106, USA (arousing) seizures” to describe this days stay in the EMU, the patient
<ameraldo@gmail.com> phenomenon. had only one epileptic seizure.
270 Epileptic Disord Vol. 12, No. 4, December 2010
Hypnopompic seizures
Table 1. The clinical and electrographic features of the five patients.
Patient Age Gender Epileptogenic Aetiology Number Number EEG seizure pattern
Zone of seizures of hypnopompic of hypnopompic
recorded seizures seizure
1 31 Male Left temporal Oligodendroglioma 1 1 (100%) Rhythmic theta
2 56 Female Left temporal Mesotemporal 3 1 (30%) Rhythmic theta
sclerosis
3 48 Male Left temporal Unknown 17 5 (45%) Rhythmic theta
4 28 Female Generalised Unknown 90 30 (30%) Burst of polyspikes
5 55 Female Left temporal Unknown 3 2 (75%) Rhythmic theta
On scalp EEG, the electrographic seizure was characterized inal aura followed by aphasia with left temporal seizure
by evolving rhythmic delta activity. The EEG onset onset, another manifested by olfactory aura with left
occurred over the left temporal region. The seizure lasted temporal seizure onset and a third characterized by a
for 30 seconds. The only clinical manifestation of the 65-second-long EEG seizure pattern consisting of
seizure was arousal from sleep. Post-ictally, the patient repetitive sharp and rhythmic theta activity over the left
was confused, amnesic and dysphasic. The patient denied temporal area (figure 1). The only clinical symptomato-
having aura or any breathing problem. On careful observa- logy of the seizure was waking up from sleep. After the
tion, specific sensory stimuli, such as light or sound that may event, the patient was awake and alert but was amnesic
have woken the patient up were not observed. On exami- of the event. She denied having aura or breathing difficul-
nation, there was no tongue biting or urinary incontinence. ties. On examination, there was no urinary incontinence
and no evidence of tongue biting. The patient denied
being woken up by a loud noise or light.
Patient 2
The patient is a 56-year-old right-handed woman with
Patient 3
history of lesional left temporal lobe epilepsy secondary to
left mesotemporal sclerosis status post left temporal lobec- The patient is a 48-year-old right-handed man with history
tomy. The patient was admitted to the EMU for evaluation of non-lesional left temporal lobe epilepsy since the age
of breakthrough seizures after the surgery. She described of nine. The patient was admitted for evaluation of intrac-
her seizures as olfactory aura or abdominal aura followed table seizures, described by the mother as blank staring
by inability to communicate. The inter-ictal EEG showed and automatism followed by asymmetric tonic posturing
left temporal slowing and sharp waves. While in the EMU, of both arms. The patient’s inter-ictal EEG revealed left
three events were recorded: one characterized by abdom- temporal slowing with no epileptiform discharges. While
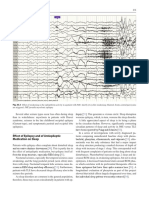
Figure 1. Longitudinal bipolar montage showing an EEG seizure pattern characterized by rhythmic theta activity over the left temporal region
associated with arousal.
Epileptic Disord Vol. 12, No. 4, December 2010 271
A.M. Awad, H.O. Lüders
in the EMU, the patient had 17 seizures with left temporal Patient 5
onset characterized by rhythmic theta activity. At least
The patient is a 55-year-old right handed woman with
five of the 17 seizures lasted for approximately 40 seconds
intractable non-lesional left temporal lobe epilepsy.
and the only clinical manifestation was awakening from
Historically, her seizures consisted of abdominal aura
sleep with the patient being amnesic to the event, denying
followed by aphasia, unresponsiveness and/or vomiting.
aura or breathing problems as well as being woken up by
Her inter-ictal EEG showed left temporal sharp waves.
light or sound. There was no evidence of other seizure
The patient had three seizures while being monitored in
manifestations like tongue biting or urinary incontinence
the EMU. Of the three seizures, two occurred during
on physical examination.
sleep. The only symptom of the nocturnal seizures was
awakening from sleep. The patient denied aura and
Patient 4 breathing problems. The EEG showed a prolonged ictal
The patient is a 28-year-old right-handed woman with discharge lasting for one minute and 40 seconds, which
autism and intractable generalised epilepsy. The patient started as rhythmic theta activity over the left temporal
underwent leg surgery during which she did not receive region.
her antiepileptic medications. Postoperatively, she started
having clusters of tonic seizures every five minutes.
A bedside VEEG was initiated to evaluate possible status Discussion
epilepticus. Her EEG showed very frequent (every 5-10
seconds) 2-2.5 Hz generalised spike-and-slow wave com- The propensity of seizures to wake up the patient from
plexes and generalised polyspikes. The patient was asleep sleep has only been studied in very few clinical trials in
throughout the whole recording. During the first six hours humans. The main studies to address this question include
of recording, more than 60 tonic seizures were recorded, those by Manni et al. (1997), Malow et al. (2000) and
characterized clinically by tonic posturing of both upper Dasheiff and Kofke (2003).
extremities lasting for 20-30 seconds and electrographi- Manni and co-workers evaluated 20 patients with focal
cally by electrodecremental response with superimposed epilepsy by ambulatory EEG. They analyzed 49 seizures
20-25 Hz activity, followed by beta activity then alpha of which 39 occurred during sleep. The investigators
activity then spike-and-slow wave complexes. While found that 72% of focal seizures, which occurred during
observing the VEEG, we noted that every 2-5 minutes sleep, were followed by awakening. The authors did
the EEG showed a burst of polyspikes lasting for about not provide details on the clinical manifestations of the
six seconds, followed by generalised delta activity (hyper- nocturnal seizures. Malow et al. (2000) studied 14 patients
synchrony) and subsequently sudden arousal (figure 2). with a total number of 67 mesial temporal lobe seizures
About 30 bursts were counted in the first six hours. The recorded by intracranial electrodes and found the electro-
only, or at least the most prominent, clinical manifestation graphic seizure preceded arousal from sleep in
of these bursts was sudden arousal from sleep. 13 patients. Dasheiff and Kofke (2003) retrospectively
Figure 2. Longitudinal bipolar montage showing an EEG seizure pattern characterized by generalized polyspikes followed by delta activity
and arousal.
272 Epileptic Disord Vol. 12, No. 4, December 2010
Hypnopompic seizures
reviewed intracranial EEG recordings in 172 patients with auras of which the patient is amnesic, etc) could also
a total number of 7571 clinical seizures and 8017 electro- explain awakening. Finally, the fact that the patients
graphic seizures. In their study, 22 patients had 308 sei- were amnesic does not exclude aura or other seizure
zures during sleep. They noticed that patients woke up phenomena since non-rapid eye movement (NREM)
after the electrographic seizure onset. For four events, sleep is not conducive to storing memory.
spontaneous awakening was followed by a clinical sei- Despite the uncertainty of the underlying mechanism, this
zure. The authors noted that electrographic seizures report shows that seizures, in which the only detectable or
could only wake the patient if the discharges were gener- the most prominent phenomenon is an unexplained
alised rather than focal. awakening, do exist. This entity is clinically important
In our series of four patients with focal epilepsy and one due to its subtlety and we suggest that clinically-driven
patient with generalised epilepsy, we observed that the seizure classification should take this phenomenon
electrographic seizure preceded the awakening and that into consideration. Health care providers may include
awakening was the only manifestation of the seizure, or at “sudden unexplained awakening” as a possible seizure
least the most prominent manifestation. In contrast to pattern in their inquiry about seizures. Families need to
Dasheiff and Kofke’s findings, not only generalised but be aware of this entity and seek medical attention. During
also focal EEG seizure patterns were capable of arousing video-EEG monitoring, epileptologists and co-workers
the patient from sleep. In addition, temporal lobe seizures may need to pay special attention to periods of sudden
have not been previously associated with arousal as the unexplained arousals and examine the patient thoroughly
predominant manifestation. after the arousal.
The mechanism by which a seizure can wake up the
patient is largely unknown but there are several theories
that could explain this phenomenon. Three inter-related Conclusion
subthalamocortical systems mediate the switching
between wakefulness and sleep. These systems include: Hypnopompic seizures are seizures that solely or pre-
the ascending reticular activating system (ARAS), the dominantly present with awakening from sleep. The
ventral preoptic (VLPO) area and the orexin/hypocretin exact mechanism(s) and frequency of this type of seizures
system (Saper, 2000; Nestler et al., 2001; Marcus et al., remain unknown. Carefully conducted studies should
2001; Posner et al., 2007; Sakurai, 2007). shed some light on this intriguing phenomenon that is
Despite the fact that the arousal systems are classically probably underdiagnosed. Awareness of this entity will
viewed as “ascending”, there is a “descending” cortical help improve the yield and accuracy of VEEG
feedback system that has to be active continuously to monitoring. □
maintain consciousness (Afifi and Bergman, 2005;
Blumfield, 2002). This could explain the mechanism of Disclosure.
None of the authors has any conflict of interest or financial support to
hypnopompic seizures; a seizure may spread downstream disclose.
to the reticular activating system and arouse the patient
before any other symptomatology. Moreover, a connec-
tion between mesial temporal lobe and midbrain raphe References
nuclei is also well-described (Giovacchini et al., 2005).
This hypothesis could be tested in animals by implanting Afifi AK, Bergman RA. Functional Neuroanatomy. In: New York:
intracranial electrodes in the brain stem and/or alterna- The McGraw Hill Companies Inc, 2005: 402-3.
tively by stimulating cortical intracranial electrodes while Blumfield H. Neuroanatomy through Clinical Cases. Sunderland:
the animal is sleeping. In a study that was conducted on Massachussets: Sinauer Associates Inc, 2002: 590-1.
cats by Shouse (1987), thalamic hyperexcitability was sug- Dasheiff RM, Kofke WA. Primarily generalized seizures are more
gested to be a potential mechanism of epileptic awakening. effective than partial seizures in arousing patients from sleep.
Another possibility to explain hypnopompic seizures Neurol Res 2003; 25: 63-7.
includes a surge in excitatory neurotransmitters which Giovacchini G, Toczek MT, Bonwetsch R, et al. 5-HT 1A recep-
could, in turn, stimulate the reticular activating system. tors are reduced in temporal lobe epilepsy after partial-volume
In addition, secondary factors may also cause arousal. correction. J Nucl Med 2005; 46: 1128-35.
These secondary causes include, but are not limited to: Janz D. Epilepsy and the sleeping–waking cycle. In: Magnus O,
autonomic and respiratory changes previously mentioned Lorentz de Hass, AM. The Epilepsies, Ch 26. Handbook of
by Dasheiff and Kofke (2003) and reactionary phenomena Clinical Neurology, Vol 15. New York: Elsevier, 1974.
to the ictal event, such as pain or discomfort. In addition, Janz D. Aufwach-Epilepsien. Arch Psychiatr Nervenkr 1953; 191:
other subtle seizure phenomena which can pass unno- 73-98.
ticed by the patient and the observers (i.e. very brief loss Malow BA, Bowes RJ, Ross D. Relationship of temporal lobe
of awareness, subtle muscle tension which could only be seizures to sleep and arousal: A combined scalp–intracranial
detected by additional electromyography (EMG) leads, electrode study. Sleep 2000; 23: 231-4.
Epileptic Disord Vol. 12, No. 4, December 2010 273
A.M. Awad, H.O. Lüders
Manni R, Galimberti CA, Sartori I, Politini L, Murelli R, Tartara A. Sakurai T. Discovery of orexin. Nippon Yakurigaku Zasshi 2007;
Nocturnal partial seizures and arousals/awakenings from sleep: 130: 19-22.
an ambulatory EEG study. Funct Neurol 1997; 12: 107-11.
Saper CB. Brainstem modulation of sensation, movement and
Marcus JN, Aschkenasi CJ, Lee CE, et al. Differential expression of consciousness. In: Kandel ER, Schwartz JH, Jessell TM, eds.
orexin receptors 1 and 2 in the rat brain. J Comp Neurol 2001; Principles of Neural Science. New York: McGraw-Hill, 2000:
435: 6-25. 889-909.
Nestler EJ, Hyman SE, Malenka RC. Molecular
Neuropharmacology. A Foundation for Clinical Neuroscience. Shouse MN. Thalamocortical mechanisms of state-dependent
In: New York: McGraw-Hill Companies, 2001: 289-313. seizures during amygdala kindling and systemic penicillin
epilepsy in cats. Brain Res 1987; 425: 198-203.
Posner JB, Saper CB, Schiff ND, Plum F. Plum and Plosner’s
diagnosis of stupor and coma. In: New York: Oxford University Wieser HG. Temporal lobe epilepsy, sleep and arousal:
Press, 2007: 328. stereo-EEG findings. Epilepsy Res 1991; 2 (Suppl.): 97-119.
274 Epileptic Disord Vol. 12, No. 4, December 2010
Potrebbero piacerti anche
- Assessment of Adult ADHDDocumento24 pagineAssessment of Adult ADHDPsydoc100% (4)
- Manly P. Hall - Spinal Column & The KundaliniDocumento48 pagineManly P. Hall - Spinal Column & The KundaliniBret Trismegistus100% (31)
- Your Body Speaks Your MindDocumento54 pagineYour Body Speaks Your MindSatinder Bhalla100% (2)
- Phi RythmDocumento6 paginePhi RythmStanley IgweNessuna valutazione finora
- Boundless: Upgrade Your Brain, Optimize Your Body & Defy Aging - ReferenceDocumento6 pagineBoundless: Upgrade Your Brain, Optimize Your Body & Defy Aging - Referencexumaxedo11% (9)
- The Psychology of Hysteria - A Selection of Classic Articles on the Analysis and Symptoms of HysteriaDa EverandThe Psychology of Hysteria - A Selection of Classic Articles on the Analysis and Symptoms of HysteriaNessuna valutazione finora
- Nursing Management of Seizures and EpilepsyDocumento36 pagineNursing Management of Seizures and EpilepsyMatthew Ryan100% (6)
- Quantum Physics in Neuroscience and Psychology - A New Theory With Respect To Mind - Brain Interacti PDFDocumento72 pagineQuantum Physics in Neuroscience and Psychology - A New Theory With Respect To Mind - Brain Interacti PDFAlina Petrut100% (1)
- Modified Emdr Resource Development & Installation ProtocolDocumento5 pagineModified Emdr Resource Development & Installation ProtocolYanina Andrea Fernandez PintoNessuna valutazione finora
- 4th Emotions Feelings and Moral Decision Making M3L1Documento7 pagine4th Emotions Feelings and Moral Decision Making M3L1JamNessuna valutazione finora
- Santrock Section 3 InfancyDocumento21 pagineSantrock Section 3 InfancyAssumpta Minette Burgos100% (5)
- The Central Nervous System - Per Brodal, MD, PHDDocumento1.368 pagineThe Central Nervous System - Per Brodal, MD, PHDHenryk BazalarNessuna valutazione finora
- Exploring Dynamic Experience in Psychology, The Arts, Psychotherapy, and DevelopmentDocumento178 pagineExploring Dynamic Experience in Psychology, The Arts, Psychotherapy, and DevelopmentMitra BhatiaNessuna valutazione finora
- Pediatric Epilepsy in Indonesia: With 4 Dimensions Epilepsy ClassificationDocumento64 paginePediatric Epilepsy in Indonesia: With 4 Dimensions Epilepsy ClassificationYahya Taqiuddin RobbaniNessuna valutazione finora
- Glutamate: Neurotransmitters Disturbed Increased Sympathetic Stimulation Increased Autonomic StimulationDocumento3 pagineGlutamate: Neurotransmitters Disturbed Increased Sympathetic Stimulation Increased Autonomic StimulationCM NajitoNessuna valutazione finora
- Sleep and Epilepsy: Sejal V. Jain, MD, and Sanjeev V. Kothare, MDDocumento7 pagineSleep and Epilepsy: Sejal V. Jain, MD, and Sanjeev V. Kothare, MDRezky 'kiki' Oktarianti SyahputriNessuna valutazione finora
- Curs 1.1 CURS Epilepsii 1 CraiuDocumento97 pagineCurs 1.1 CURS Epilepsii 1 CraiuBogdan Urichianu100% (1)
- Biology Investigatory Parkinson's PDFDocumento16 pagineBiology Investigatory Parkinson's PDFaneesh100% (2)
- CURS EpilepsieDocumento97 pagineCURS EpilepsieMateescu CristianNessuna valutazione finora
- Epilepsia CursivaDocumento3 pagineEpilepsia Cursiva---Nessuna valutazione finora
- 1 s2.0 S1389945717302496 Main PDFDocumento3 pagine1 s2.0 S1389945717302496 Main PDFvalentinaNessuna valutazione finora
- 1era Crisis Epileptic ADocumento6 pagine1era Crisis Epileptic Aalvarorm1Nessuna valutazione finora
- Epilepsi: Dr. Chyntia M. Sahetapi SP.SDocumento20 pagineEpilepsi: Dr. Chyntia M. Sahetapi SP.SvickNessuna valutazione finora
- Sleep and Epilepsy Syndromes: Bernhard SchmittDocumento9 pagineSleep and Epilepsy Syndromes: Bernhard Schmittmarcoteixa22Nessuna valutazione finora
- Gelastic and Cursive Seizures - Neurology - 1973Documento11 pagineGelastic and Cursive Seizures - Neurology - 1973---Nessuna valutazione finora
- Re Ex Myoclonic Epilepsy of Infancy: Seizures Induced by Tactile StimulationDocumento8 pagineRe Ex Myoclonic Epilepsy of Infancy: Seizures Induced by Tactile StimulationOziNessuna valutazione finora
- Ictal Urinary Urge Indicates Seizure Onset in The Nondominant Temporal LobeDocumento5 pagineIctal Urinary Urge Indicates Seizure Onset in The Nondominant Temporal LobejerejerejereNessuna valutazione finora
- EpilepsyDocumento107 pagineEpilepsyamsabavanNessuna valutazione finora
- Bmjcred00647 0019Documento3 pagineBmjcred00647 0019Vignesh SelvamNessuna valutazione finora
- Seizure PathophysiologyDocumento26 pagineSeizure PathophysiologyNarianne Mae Solis Bedoy100% (1)
- Folleto de Servicio Marketing y Creatividad Ilustrado Doodle Azul y AmarilloDocumento2 pagineFolleto de Servicio Marketing y Creatividad Ilustrado Doodle Azul y Amarillomateopadillita0529Nessuna valutazione finora
- Epi Gral 2018 1Documento92 pagineEpi Gral 2018 1Anonimo DesconocidoNessuna valutazione finora
- Sleep Medicine 2015 (304 529)Documento226 pagineSleep Medicine 2015 (304 529)Guillermo MoranteNessuna valutazione finora
- Epilepsia Mioclónica Benigna Refleja de La Infancia. A Propósito de Una Nueva ObservaciónDocumento4 pagineEpilepsia Mioclónica Benigna Refleja de La Infancia. A Propósito de Una Nueva ObservaciónKathia AguilarNessuna valutazione finora
- Diagnosis Dan Klasifikasi EpilepsiDocumento33 pagineDiagnosis Dan Klasifikasi EpilepsiVhera MoksinNessuna valutazione finora
- EpilepsyDocumento77 pagineEpilepsymirabel IvanaliNessuna valutazione finora
- Absans, MiklonikDocumento11 pagineAbsans, MiklonikrendyNessuna valutazione finora
- Management of Psychogenic Nonepileptic SeizuresDocumento3 pagineManagement of Psychogenic Nonepileptic SeizuresRudolph MuliawanNessuna valutazione finora
- Seizure and Epilepsy July 2014Documento28 pagineSeizure and Epilepsy July 2014ashuNessuna valutazione finora
- Bab 1Documento31 pagineBab 1PandeNessuna valutazione finora
- Veterinary Internal Medicne - 2017 - WielaenderDocumento5 pagineVeterinary Internal Medicne - 2017 - WielaenderGonzalo VeraNessuna valutazione finora
- Epilepsy Update Diagnosis, Classification and ManagementDocumento7 pagineEpilepsy Update Diagnosis, Classification and ManagementfelipeNessuna valutazione finora
- Fahrs Disease and Aspergers Syndrome inDocumento27 pagineFahrs Disease and Aspergers Syndrome inSaran RalucaNessuna valutazione finora
- Hypsarrythmia in IsDocumento20 pagineHypsarrythmia in IsAndrew SantosoNessuna valutazione finora
- Ictal Vomiting LocalisationDocumento4 pagineIctal Vomiting LocalisationmayankNessuna valutazione finora
- The Neurobiology of EpilepsyDocumento11 pagineThe Neurobiology of EpilepsyCharpapathNessuna valutazione finora
- Medicina: Cognitive Status Epilepticus: Two Case ReportsDocumento5 pagineMedicina: Cognitive Status Epilepticus: Two Case ReportsErick SolisNessuna valutazione finora
- Myoclonus and Myoclonic Seizures: Hirokazu OGUNIDocumento2 pagineMyoclonus and Myoclonic Seizures: Hirokazu OGUNIPeby M. RamdhanNessuna valutazione finora
- Sleep-Related Epilepsy: Mar Carren O, MD, PHD Santiago Ferna Ndez, MDDocumento17 pagineSleep-Related Epilepsy: Mar Carren O, MD, PHD Santiago Ferna Ndez, MDMônica ScattolinNessuna valutazione finora
- Volvular EpilepsyDocumento4 pagineVolvular Epilepsyzudan2013Nessuna valutazione finora
- EpilepsyDocumento43 pagineEpilepsyMpt SportsNessuna valutazione finora
- What Is Lennox-Gastaut Syndrome in The Modern Era?: Hirokazu OguniDocumento2 pagineWhat Is Lennox-Gastaut Syndrome in The Modern Era?: Hirokazu OguniWinsen HaryonoNessuna valutazione finora
- Of Of: The Role The System in Phenomena Temporal Lobe EpilepsyDocumento16 pagineOf Of: The Role The System in Phenomena Temporal Lobe EpilepsyBONA VICTOR SIMANJUNTAK MDNessuna valutazione finora
- Head Atonic Attacks. D August 21, 2012Documento5 pagineHead Atonic Attacks. D August 21, 2012memoaranedaNessuna valutazione finora
- TPNEDocumento8 pagineTPNENelson AlvarengaNessuna valutazione finora
- Epilepsy: Diagnosis, Classi Fication and Management: Key PointsDocumento7 pagineEpilepsy: Diagnosis, Classi Fication and Management: Key PointsDavid FlorianNessuna valutazione finora
- The Diagnosis and Management of Epilepsy: Journal of Tropical Pediatrics January 2002Documento9 pagineThe Diagnosis and Management of Epilepsy: Journal of Tropical Pediatrics January 2002Roy MarechaNessuna valutazione finora
- Hypnosis 0001 PDFDocumento11 pagineHypnosis 0001 PDFalanNessuna valutazione finora
- Myoclonus and Myoclonic Seizures: Hirokazu OGUNIDocumento2 pagineMyoclonus and Myoclonic Seizures: Hirokazu OGUNIPeby M. RamdhanNessuna valutazione finora
- Recurrent Absence Status Epilepticus: Clinical and EEG CharacteristicsDocumento10 pagineRecurrent Absence Status Epilepticus: Clinical and EEG CharacteristicsLidwina ApyakaNessuna valutazione finora
- Tony Ro Et Al - Feeling Sounds After A Thalamic LesionDocumento9 pagineTony Ro Et Al - Feeling Sounds After A Thalamic LesionDsgmmmmNessuna valutazione finora
- Sag Nier 2015Documento5 pagineSag Nier 2015Marina AlexopoulouNessuna valutazione finora
- Encefalitis LimbicaDocumento11 pagineEncefalitis LimbicaRandy UlloaNessuna valutazione finora
- Epilepsy: Bassel F. Shneker, MD, and Nathan B. Fountain, MDDocumento53 pagineEpilepsy: Bassel F. Shneker, MD, and Nathan B. Fountain, MDDiane Mx100% (1)
- Acute Unilateral Vestibulopathy Strupp & Magnusson (2015)Documento17 pagineAcute Unilateral Vestibulopathy Strupp & Magnusson (2015)J WNessuna valutazione finora
- Sleep and Epilepsy Strange Bedfellows No MoreDocumento26 pagineSleep and Epilepsy Strange Bedfellows No Morerifki irsyadNessuna valutazione finora
- EMG Findings of Facial Muscles in ALS PDFDocumento3 pagineEMG Findings of Facial Muscles in ALS PDFemilio9fernandez9gatNessuna valutazione finora
- Anticonvulsant Part 1Documento42 pagineAnticonvulsant Part 1Rameez ShamounNessuna valutazione finora
- Epilepsi: Prof - Dr.Dr.H.Ruslan Muhyi, Spa (K) Devisi Saraf Anak Bag Ika FK Unlam/Rsud Ulin BanjarmasinDocumento14 pagineEpilepsi: Prof - Dr.Dr.H.Ruslan Muhyi, Spa (K) Devisi Saraf Anak Bag Ika FK Unlam/Rsud Ulin BanjarmasinWiresa RenaltaNessuna valutazione finora
- Evaluation of Anti-Epileptic Activity of Ethanolic Extract On Swiss Albino Male MiceDocumento36 pagineEvaluation of Anti-Epileptic Activity of Ethanolic Extract On Swiss Albino Male MiceNitinNessuna valutazione finora
- Lesson 4: Seizure: The Underlying Cause Is AnDocumento6 pagineLesson 4: Seizure: The Underlying Cause Is AnRocelyn CristobalNessuna valutazione finora
- Arsitektur Dan BauDocumento3 pagineArsitektur Dan BauEvan DylanNessuna valutazione finora
- Storyboard and Script SampleDocumento5 pagineStoryboard and Script Sampleapi-208376609100% (1)
- Neurotransmitter - Prof. RondangDocumento35 pagineNeurotransmitter - Prof. RondangYoga WitularNessuna valutazione finora
- Psychology Perspectives ChartDocumento2 paginePsychology Perspectives ChartmNessuna valutazione finora
- 013 TolmanDocumento11 pagine013 Tolmanronaaustero28Nessuna valutazione finora
- An Introduction To The Philosophy of Psychology (Daniel Weiskopf Fred Adams) (Z-Library)Documento168 pagineAn Introduction To The Philosophy of Psychology (Daniel Weiskopf Fred Adams) (Z-Library)Salomon BarbosaNessuna valutazione finora
- LAB - Taste - SmellDocumento2 pagineLAB - Taste - SmellIris AnnNessuna valutazione finora
- Neural Control and Coordination Grade 11Documento30 pagineNeural Control and Coordination Grade 11Dr. Remya RanjithNessuna valutazione finora
- Cerebral DominanceDocumento49 pagineCerebral DominanceDevika VijayanNessuna valutazione finora
- Attention and PerceptionDocumento10 pagineAttention and PerceptionSalami ibrahimNessuna valutazione finora
- Nervous SystemDocumento2 pagineNervous SystemsheilamaemaglaquiNessuna valutazione finora
- 2............. Human Anatomy and Physiology MCQDocumento7 pagine2............. Human Anatomy and Physiology MCQshahida hassanNessuna valutazione finora
- Human Brain: Presented by Ayesha KhanDocumento20 pagineHuman Brain: Presented by Ayesha KhanDilshad JanNessuna valutazione finora
- Radiology Report 2300985Documento4 pagineRadiology Report 2300985JyotiNessuna valutazione finora
- CNS Structural Functional Organization 2020Documento24 pagineCNS Structural Functional Organization 2020FatimaNessuna valutazione finora
- Ericsson 2G&3G Engineering Parameter-15rd DEC V12Documento1.019 pagineEricsson 2G&3G Engineering Parameter-15rd DEC V12Nino BongoyNessuna valutazione finora
- Lesson-5: How The Human Brain WorksDocumento14 pagineLesson-5: How The Human Brain Worksprecious maningasNessuna valutazione finora
- Bobath & Brunnstrom ApproachesDocumento40 pagineBobath & Brunnstrom ApproachesManiu EmeseNessuna valutazione finora
- Lesson 2 The Measurement of Motor Performance v2Documento11 pagineLesson 2 The Measurement of Motor Performance v2Drift AlvinNessuna valutazione finora