Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
United States Patent Office: Motor Fuel Composition York, N.Y
Caricato da
Alex Kattamis0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
11 visualizzazioni4 pagineMotor Patent
Titolo originale
Us 3676089
Copyright
© © All Rights Reserved
Formati disponibili
PDF, TXT o leggi online da Scribd
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoMotor Patent
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PDF, TXT o leggi online su Scribd
0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
11 visualizzazioni4 pagineUnited States Patent Office: Motor Fuel Composition York, N.Y
Caricato da
Alex KattamisMotor Patent
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PDF, TXT o leggi online su Scribd
Sei sulla pagina 1di 4
United States Patent Office 3,676,089
Patented July 11, 1972
1. 2
down of deposits or continually burns and carries off any
3,676,089 potential deposits. However, this invention surprisingly
MOTOR FUEL COMPOSITION has caused a substantial and unexpected increase in ex
Herbert C. Morris, Wappingers Falls, and Peter Dorn, haust valve life for reasons not fully understood.
againgeville, N.Y., assignors to Texaco Inc., New
York, N.Y. OESCRIPTION OF THE PROR ARTAND RELATED
No Drawing. Filled Nov. 6, 1969, Ser. No. 874,700 COPENDING APPLICATION
Int. C. C10 1/18, 1/22
U.S. C. 44-62 6 Claims U.S. 3,131,150 discloses a class of reaction products of
N-substituted alkenyl succinimides and polyamines for
O use in a lubricating oil composition. The reaction products
ABSTRACT OF THE DISCLOSURE are prepared by reacting an alkenyl succinic anhydride in
Motor fuel composition comprising a mixture of hy which the alkenyl radical contains from 30 to about 200
drocarbons in the gasoline boiling range containing (a) carbon atoms with about an equal molar proportion of a
from 0.0004 to 0.1 weight percent based on said composi polyamine from the group consisting of tetraethylene
tion of an N-polyamine substituted alkenyl succinamic 5 pentamine and dimethylaminopropylamine. These reaction
acid or corresponding succinimide in combination with products are metal free or ashless detergents for lubricat
(b) from 0.003 to 0.20 volume percent of (1) a polymer ing oil compositions and are effective for dispersing the
of a C2 to Cs unsaturated hydrocarbon, (2) a copolymer precursors of deposits within the oil itself thereby inhibit
of a C2 to Cs unsaturated hydrocarbon, or (3) the corre ing deposits formation on the engine parts.
Sponding hydrogenated polymer or copolymer, said poly 20 U.S. 3,172,892 discloses the reaction product of high
ner or copolymer having a molecular weight in the range molecular weight succinic acids and succinic anhydride
from about 500 to 3500 and a method for operating an with an ethylene polyamine for use in a lubricating oil
internal combustion gasoline engine. composition. Specifically, a hydrocarbon substituted suc
cinic acid or succinic anhydride in which the substituted
25 hydrocarbon radical is a large, substantially aliphatic hy
BACKGROUND OF THE INVENTION drocarbon radical having at least about 50 carbon atoms
Field of invention is reacted with at least about one-half equivalent of an
ethylene amine and heated to effect acylation and removal
Internal combustion engines, particularly of the over of water formed thereby. These reaction products are ef
head valve design, are subject to a substantial build-up of 30 fective as sludge dispersants in crankcase lubricating oil
hard, tenacious deposits on the intake valves and ports of temperatures for internal combustion engines.
the engine. These deposits Seriously interfere with the op A copending patent application, now U.S. 3,502,451
eration of the engine. As the deposits level grOWs, the en discloses a motor fuel composition for a gasoline internal
gine exhibits loss of power, rough idling, and, occasionally, combustion engine containing a polymer, copolymer or
valve burning. When the deposits become excessive, por 35 hydrogenated polymer. Specifically, a polymer of a C2 to
tions break off and are drawn into the combustion cham Cs unsaturated hydrocarbon, a copolymer of a C2 to Cs
ber. Instances of mechanical damage to the piston and unsaturated hydrocarbon, or a hydrogenated polymer or
piston rings caused by these deposits have been observed. copolymer of a C2 to Cs unsaturated hydrocarbon having
Considerable work has been conducted to determine 40 a molecular weight in the range from about 500 to 3500
the nature and cause of the intake valve deposits. The is employed at a concentration from about 0.01 to 0.20
deposits themselves are composed essentially of the by volume percent in the motor fuel composition for a spark
products of fuel combustion and lubricating oil deteriora ignited internal combustion engine. This motor fuel com
tion. Analysis of the deposits indicates that the viscosity position is effective for preventing or inhibiting the for
index improvers contained in the lubricating oil act as 45
mation of deposits on the intake valves and parts of the
binders for the deposits. Polymethacrylate viscosity in engine.
dex improvers, as an example, are one class of materials SUMMARY OF THE INVENTION
Which appear to contribute materially to the deposits
build-up. The fuel composition of the inventor comprises a mix
An understanding of engine operation will show how ture of hydrocarbons in the gasoline boiling range con
lubricating oil deterioration can contribute to deposits in 50 taining minor amounts of an additive combination of an
the fuel intake System. A spark-ignited internal combus N-polyamine-substituted alkenyl succinamic acid or cor
tion engine contains a reservoir of lubricating oil in the responding Succinimide with a polymer, copolymer or
crankcase. When the engine is in operation, the primary hydrogenated polymer or copolymer of a C to C un
lubrication is effected by the crankcase oil being splashed 55
Saturated hydrocarbon. More specifically, the fuel com
up on the operating parts of the engine and on the cylinder position of the invention contains from about 0.0004 to
Walls. A portion of this oil, however, is pumped under 0.1 weight percent based on said composition of an N
preSSure to the upper parts of the engine to lubricate the polyamine-substituted alkenyl succinamic acid or suc
Working parts therein. In an overhead valve engine, a small cinimide, prepared by reacting an alkenyl succinic acid
stream of the oil pumped to the upper section of the engine 60
or anhydride having the structural unit:
is constantly run down the intake and exhaust valve stems R-CHCO
to insure that they are constantly lubricated in their guides ÖH,Co
during operation. The oil trickling down the intake valve
stem, the valve head and around the intake port is ap in which R is a hydrocarbon radical having a molecular
parently pyrolyzed under the temperatures prevailing, 65 Weight from about 400 to about 3000 with from one-half
thereby contributing to the formation and build-up of the to two equivalent amounts of a polyamine having the
above-noted deposits. formula:
This deposit problem is not encountered to any material
extent in or around the exhaust ports or valves. This is
believed to be due to the high temperatures existing at the 70
exhaust valve during the exhaust cycle and to the action in which x is an integer and R is hydrogen or a low
of the expelled exhaust gases which do not permit a lay molecular weight alkyl radical, and from about 0.003 to
3,676,089
3 4
0.20 volume percent of (1) a homopolymer of a C to diethylene triamine. The mixture is heated and the water
Cs unsaturated hydrocarbon, (2) a copolymer of C2 to toluene azeotrope is collected in a Dean-Stark trap. The
Cs unsaturated hydrocarbons, or (3) the corresponding reaction is complete when no more water is collected. The
hydrogenated polymer or copolymer, said polymer, co mixture is then heated to 100° C. at reduced pressure to
polymer or hydrogenated derivative having a molecular remove the toluene and yield a product consisting mainly
weight in the range from about 500 to 3500. The method of the bispolyisobutenyl succinimide.
of the invention comprises supplying to and burning in a In another typical preparation, a polyisobutylene suc
spark-ignited internal combustion gasoline engine the cinic anhydride is prepared by the reaction of one mole
above-described motor fuel composition. of a polybutene having a molecular weight of about 1300
The discovery of an improved motor fuel composition O with 196 grams (2 moles) of maleic anhydride at 200
from the combination of an N-polyamine-substituted C. for 24 hours. The reaction mixture is cooled and 750
alkenyl succinamic acid or corresponding succinimide with ml. of hexane added. The solution is filtered to remove
a polymer, copolymer or hydrogenated polymer or co Solid contaminants and then stripped under full vacuum
polymer of a Ca to Cs unsaturated hydrocarbon having a to remove the hexane. The temperature is then raised to
molecular weight from about 500 to 3500 was most sur 5 about 200 C. to remove all traces of the excess maleic
prising and unexpected. The reason for this is that an N anhydride.
polyamine-substituted alkenyl succinamic acid or corre To a mixture of 1 mole of the polyisobutenyl succinic
sponding succinimide in a motor fuel composition has anhydride and 400 ml. of toluene there is added slowly
essentially no effect on the prevention of intake valve and at room temperature 0.5 mole of ethylene diamine. The
port deposits in a spark-ignited internal combustion engine. 20 mixture is heated and the water-toluene azeotrope is col
As more fully described in the above-noted patents, the lected in a Dean-Stark trap. The reaction is complete
N-polyamine-substituted alkenyl succinamic acid or suc when no more water is collected. The mixture is then
cinimide is prepared by reacting an alkenyl succinic acid heated to about 100° C. at reduced pressure to remove
or anhydride having the structural unit: the toluene and yield a product consisting mainly of the
25 bis-polyisobutenyl succinimide.
R-HC O The polymer which is employed in the motor fuel of
CBCO the invention is a polymer prepared from an unsaturated
in which R is a hydrocarbon radical having a molecular hydrocarbon, i.e. a monoolefin, diolefin or copolymer of
weight from about 400 to about 3000 with from one-half either having an average molecular weight in the range
to two equivalent amounts of a polyamine having the 30 of about 500 to 3500. Mixtures of olefin polymers with
formula: an average molecular weight falling within the foregoing
range are also effective. Olefins which can be employed
RN(CHHNR)
R
R. to prepare the polyolefin polymers include ethylene,
propylene, 1-butene, 2-butene, isobutylene, amylene,
in which x is an integer and R is hydrogen or a low molec heXylene, butadiene and isoprene. In general, the olefin
ular weight alkyl radical, R radical in the above formula monomers from which the polyolefins are prepared are
is a hydrocarbon radical preferably derived from an olefin unsaturated hydrocarbons having from two to six car
containing from 2 to 5 carbon atoms. Suitable olefins bon atoms. The polyolefin polymers from C and C.
from which R is derived are ethylene, propylene, 1-butene, olefins, such as propylene and isobutylene, are partic
2-butene, isobutylene and the amylenes. The R radical 40 ularly preferred for the practice of this invention. Other
generally has a molecular weight ranging from about 400 polyolefins which can be employed are those prepared
to 3000, corresponding to approximately 30 to 200 carbon by Cracking polyolefin polymers or copolymers of high
atoms, with the preferred molecular weight being from molecular weight to a polymer in the above-noted molec
800 to 1200. ular weight range. Derivatives of the noted polymers ob
The R radical in the polyamine is hydrogen or a low 45 tained by Saturating the polymers by hydrogenation are
molecular weight alkyl radical having from 1 to 3 carbon also effective and are a part of this invention. The word
atoms. X is an integer from 1 to about 6 and preferably "polymers' is intended to include the polyolefin homo
from 2 to 4. Suitable polyamines and polyalkylene poly polymers and copolymers and their corresponding hydro
amines represented by the formula are ethylene diamine, genated derivatives.
propylene diamine, butylene diamine, diethylene triamine, 50 The molecular weight of the polymer or polymer de
triethylene tetramine, tetraethylene pentamine, penta rivative component is important in preparing an effec
ethylene hexamine, dipropylene triamine, tripropylene tive fuel composition according to this invention. Effec
tetramine, Dialkylaminoalkylene amines, such as dimethyl tive fuel compositions of the invention require a poly
aminomethylamine, dimethylaminopropylamine, diethyl ner or hydrogenated polymer derivative having an aver
aminopropylamine and the like are also included. 55 age molecular weight in the range of 500 to 3500 as deter
From one-half to two equivalents of the polyamine are mined by an osmometer method. Highly effective fuel
reacted with the alkenyl succinic acid or anhydride to compositions are obtained with polymers and derivatives
form the reaction product. Equivalency of the polyamine having molecular weights in the preferred range from
reactant is based on the nitrogen content, ethylene diamine 650 to 2600. Very effective fuel compositions can be
having two equivalents per mole. It is preferred to react 60 prepared from relatively low molecular weight poly
approximately one equivalent of the alkenyl succinic acid mers, that is using polymers having molecular weights
or anhydride with about one equivalent of the polyamine. in the range of 650 to 995.
In a typical preparation, a polyisobutylene succinican The method of preparation of the olefin homopolymer,
hydride is prepared by the reaction of one mole of a poly copolymers, hydrogenated polymers or copolymers is
butene having a molecular weight of about 1000 with 196 65 Well known in the art and is not part of the present in
grams (2 moles) of maleic anhydride at 200° C. for 24 vention. The average molecular weight determination
hours. The reaction mixture is cooled and 750 m. hexane for the polymers is determined by the ASTM Osmometer
added. The solution is filtered to remove solid con Method identified as ASTM D-2503-67. Examples of
taminants and then stripped under full vacuum to re polymers which are effective in the fuel composition of
move the hexane. The temperature is raised to about 70 the invention together with their average molecular
200 C. to remove all traces of the excess maleic an weight are as follows: polypropylene 800, polypropylene
hydride. 975, polypropylene 1120, polypropylene 1150, polypropyl
To a mixture of 1100 grams (1 mole) of the polyiso ene 1370, polypropylene 2560, polybutene-1 800, poly
butenyl Succinic anhydride and 400 ml. toluene there is butene-1 1200, polyisobutylene 850, polyisobutylene
added slowly at room temperature 52 grams (0.5 mole) 75 1000, polyisobutylene 1200, polyisobutylene 1575, hy.
3,676,089
5
drogenated polybutene 1100, ethylene-butylene copoly Road heavy
mer 810. idle load load
The method for preparing the N-polyamine-substituted Operation Stage stage Stage
alkenyl succinamic acid or succinimide is also well known Speed, r.p.m.------------------------ 1,000-i6 2,250-15 2,250-15
as shown by the patents noted above. It is to be noted Load, b.h.p.----
Air-fuel ratio---------------- -
0
1.5-0.5
301, 5
12.20.4
75:1. 5
a 2.2
that suitable N-polyamine-substituted alkenyl succinamic Spark Advanceb, BTDC- 30 40 34
acid or succinimides or mixtures containing same are Exhaust back press, in. Hg
Intake air temp., F.
a 0.2
1402
100,1
1402
a 3.5
40-2
commercially available. It is convenient to employ the Jacket-out temp., 2002 2002 2002
reaction product dissolved in a mineral oil carrier. Clkc's oil temp., F--- a 200 234-2 2342
The base fuel of the invention comprises a mixture of 10 a Typical values, not controlled
hydrocarbons boiling in the gasoline boiling range. This b Approximate values-spark advance set 6 BTDC at 600 r.p.m.
base fuel may consist of straight chain or branched chain Upon completion of a run, the cylinder heads and
paraffins, cycloparaffins, olefins and aromatic hydrocar valves are removed and the valves visually rated for the
bons or any mixture of these. This fuel can be derived extent of deposit build-up on the valve tulip surface. The
from straight run naphtha, polymer gasoline, natural 5 intake valve deposits are rated according to a merit rating
gasoline or from catalytically cracked or thermally scale running from 10 to 1. A rating of 10 indicates a per
cracked hydrocarbons and catalytically reformed stocks fectly clean valve while the rating of 1 is applied to an
and boils in the range from about 90° to 450 F. The extremely heavily coated valve. Deposits around the port
composition of the base fuel is not critical nor does the opening are rated T-trace, L-light, M-medium and
octane level of the base fuel have any material effect 20 H-heavy.
on the invention. Any conventional motor fuel base The following examples illustrate the practice of this
may be employed in the practice of this invention. invention.
The fuel composition of the invention may contain The base fuel employed was a typical premium grade
any of the additives normally employed in a motor fuel. gasoline containing about 3 cc. of tetraethyllead per gal
For example, the base fuel may be blended with an anti 25 lon. This base fuel consisted of 25 percent aromatic, 20
knock compound, such as a tetraalkyl lead compound, percent olefinic and 55 percent aliphatic hydrocarbons as
including tetraethyl lead, tetramethyl lead, tetrabutyl lead determined by FIA analysis. This gasoline had an ASTM
and mixtures thereof generally in a concentration from distillation IBP of 87 F., an E.P. of 376 F. and a Re
about 0.5 to 40 cc. per gallon of gasoline. The tetra search Octane Number of about 100. Except where other
ethyl lead mixture commercially available for automo 30 wise noted, this base fuel contained 0.5 volume percent
tive use contains an ethylene chloride-ethylene bromide of a commercial corrosion inhibitor-mineral oil additive
mixture as a scavenger for removing lead from the com mixture. This additive mixture has no significant effect on
bustion chamber in the form of a volatile lead halide. the test for intake valve and port deposits.
The motor fuel composition may also contain any of A typical commercial gasoline without the additive of
the conventional anti-icing additives, corosion inhibitors, 35 the invention gives an intake valve rating of about 6.0 and
dyes, and the like as illustrated by U.S. 2,632,695; a port rating of heavy. An improvement in the valve
2,844,449; 3,325,260; 3,232,724; 2,622,018 and 2,922,708. rating of 0.5 unit above the base fuel and an acceptable
The novel fuel composition of this invention is prepared port rating, Trace or Light, is a significant improvement.
by mixing suitable amounts of the prescribed N-poly An improvement of 1.0 unit or more generally to 7.0 or
amine-substituted alkenyl succinamic acid or succinimide 40
above and a passing port rating is a very substantial im
and polymer additives to the base gasoline. The additives provement in engine cleanliness,
are readily soluble and may be added in any manner or
order. ABLE I-BUCKINOUCTION SYSTEM DEPOSIS, TEST
The test employed for testing the fuel compositions was Fuel composition and additive Hours Wave Port
the Buick Induction System Deposits Test conducted using 45 Concentration on test rating rating
a 1964 Buick 425 CID V-8 engine. The fuels employed (l) Base fuel------------------------------ 96 5.8 M to H.
in the tests were evaluated basis deposit ratings of the (2) Base fuel plus 0.013 wt. percent of the 96 5.
Succinimide reaction product of mole
intake valves and ports of the engine as more fully de polyisobutenyl-1000-stuccinic anhydride
and 0.5 mole diethylene triamine.1
scribed below. (3) Base fuel plus 0.1 vol. percent poly- 96 8.0 T to L.
The test is conducted using the noted engine equipped 50 propene 800.
(4) Base fuel plus 0.05 vol. percent poly- 96 6.2 M.
with a PCV (Positive Crankcase Ventilation) valve and propene 800.
installed on a dynamometer test stand with supporting (5) Base fuel plus 0.075 vol. percent poly-
propene 800.
384 7.5 L.
equipment to control speed, load and engine temperatures. (6) Base fuel plus 0.075 vol. percent poly- 384 8.4 L.
This test requires approximately 350 gallons of fuel and propene 800 plus 0.013 wt. percent of the
Succinimide reaction product of i mole
4 gallons of lubricant per run. 55 polyisobutenyl-1000-succinic anhydride
Prior to each run, the cylinder heads are completely and 0.5 mole diethylene triamine.
(7) Base fuel plus 0.043 vol. percent poly- 92 7.5 I.
reconditioned and new intake valves installed. Special propene 800 plus 0.013 wt. percent of the
care must be taken to insure that the inlet valve-to-valve succinimide reaction product of i mole
polyisobutenyl-1000-succinic anhydride
guide clearance be maintained between 0.0035 and 0.0045 and 0.5 mole diethylene triamine.
inch. In addition, the valve seat widths are maintained 60 1 Contained no corrosion inhibited-mineral oil additive.
between 364 and %4 inch. The engine block is completely
overhauled in accordance with the procedures stated in Examples 6 and 7 are representative of the present
the 1964 Buick Service Manual when blow-by or oil invention and show surprisingly enhanced cleanliness of
consumption become excessive. the engine intake valves and ports brought about by the
The engine is charged with four quarts of oil and flushed 65 unexpected cooperation of the additive combination of
for 15 minutes at 1500 r.p.m. Following an oil drain, four the invention in this test.
quarts of new oil are added and the fuel tests begun. The Obviously, many modifications and variations of the
engine is operated on a four-stage six-hour cycle for a invention, as hereinbefore set forth, may be made without
total of 16 cycles or 96 hours as follows: departing from the spirit and scope thereof, and therefore
Cycle 70 only such limitations should be imposed as are indicated
time Hours Operation in the appended claims.
0- Idle.
We claim:
1-4 3 Road load. 1. A motor fuel composition comprising (A) a hydro
4-5
5-6
1 Heavy load.
1 Rest. carbon base fuel consisting of a mixture of hydrocarbons
75 in the gasoline boiling range, (B) from 0.0004 to 0.1
3,676,089
7 8
weight percent based on said composition of an N-poly -Hc O
amine-substituted succinimide prepared by reacting an CHCO
alkenyl succinic acid or anhydride having the structural
unit: in which R is a hydrocarbon radical having a molecular
R-CHCO Weight from about 800 to 1200 with from one-half to
CaCO
two equivalent amounts of diethylene triamine and (C)
from 0.003 to 0.20 volume percent of (1) a homopolymer
of a Cato Ce unsaturated hydrocarbon, (2) a copolymer
in which R is a hydrocarbon radical having a molecular of C2 to Cs unsaturated hydrocarbons or (3) the corre
weight from about 800 to 1200 with from one-half to two sponding hydrogenated polymer or copolymer, said poly
equivalent amounts of diethylene triamine and (C) from O mer, copolymer or hydrogenated derivative having a mo
0.003 to 0.20 volume percent of (1) a homopolymer of lecular weight in the range from about 500 to 3500.
a C2 to C6 unsaturated hydrocarbon, (2) a copolymer of 5. A method according to claim 4 in which said N-poly
C2 to Cs unsaturated hydrocarbons or (3) the correspond amine-substituted alkenyl succinimide is the reaction prod
ing hydrogenated polymer or copolymer, said polymer, uct of polybutene-1000-succinimide and diethylene tri
copolymer or hydrogenated derivative having a molecular 5 amine.
weight in the range from about 500 to 3500. 6. A method according to claim 4 in which said polymer
2. A motor fuel composition according to claim 1 in of an unsaturated hydrocarbon is polypropene 800.
which said N-polyamine-substituted alkenyl succinimide
is the reaction product of polybutene-1000-succinic anhy 20 References Cited
dride and diethylene triamine. UNITED STATES PATENTS
3. A motor fuel composition according to claim 1 in
which said polymer of an unsaturated hydrocarbon is 3,223,495 12/1965 Calvino et al. ---------- 44-71
polypropene 800. 3,280,033 10/1966 Drummond ------ 44-71 X
4. A method for preventing the build-up of intake valve 3,307,928 3/1967 Chaikivsky et al. ------- 44-63
and port deposits in a spark-ignited, internal combustion 25 3,401,118 9/1968 Benoit ------------ 44-63 X
gasoline engine which comprises supplying to and burning. 3,443,918 5/1969 Kautsky et al. ---------- 44-63
in said engine a motor fuel composition comprising (A) 3,502,451 3/1970 Moore et al. ---------- 44-58
a hydrocarbon base fuel consisting of a mixture of hydro
carbons in the gasoline boiling range, (B) from 0.0004 30 DANIEL E. WYMAN, Primary Examiner
to 0.1 weight percent based on said composition of an W. J. SHINE, Assistant Examiner
N-polyannine-substituted succinimide prepared by reacting
an alkenyl succinic acid or anhydride having the structural U.S. C. X.R.
unit: 44-63
Potrebbero piacerti anche
- Egeberg 2010 - Hydrotreating in The Production ofDocumento13 pagineEgeberg 2010 - Hydrotreating in The Production ofNadia RizanedewiNessuna valutazione finora
- Hydrocracking TechnologyDocumento11 pagineHydrocracking TechnologyAsad SaeedNessuna valutazione finora
- Matter Sphere For Mage The AscensionDocumento2 pagineMatter Sphere For Mage The AscensionBeth100% (3)
- Thomas I.J. Dugmore, Moray S. Stark: SciencedirectDocumento6 pagineThomas I.J. Dugmore, Moray S. Stark: SciencedirectSurahmanNessuna valutazione finora
- Isomerization Process in A Petroleum Refinery: Submitted To-Prof. T Panda Name-Yadav Ritik Ranjan ROLL-CH14B090Documento22 pagineIsomerization Process in A Petroleum Refinery: Submitted To-Prof. T Panda Name-Yadav Ritik Ranjan ROLL-CH14B090Ritikranjan YadavNessuna valutazione finora
- United States Patent Office: 2 Cating Oil. From A Practical Point of View, 0.25% To 10%Documento9 pagineUnited States Patent Office: 2 Cating Oil. From A Practical Point of View, 0.25% To 10%Tristan Tabago ConsolacionNessuna valutazione finora
- Crude Oil. The Process of Refining Involves The Following StepsDocumento4 pagineCrude Oil. The Process of Refining Involves The Following StepsSai Ram MotupalliNessuna valutazione finora
- US2960514Documento4 pagineUS2960514PRASSAN SHAHNessuna valutazione finora
- "Heptaldehyde From Castor Oil": A Report OnDocumento12 pagine"Heptaldehyde From Castor Oil": A Report OnVandana AgrawalNessuna valutazione finora
- Lecture 6Documento47 pagineLecture 6Musah HarunaNessuna valutazione finora
- Patent Application Publication (10) Pub. No.: US 2008/00663.74 A1Documento8 paginePatent Application Publication (10) Pub. No.: US 2008/00663.74 A1AdhityaEkoBagusNessuna valutazione finora
- Glossary of Petroleum Refinery Terms: AbsorberDocumento17 pagineGlossary of Petroleum Refinery Terms: AbsorberAbdul MoeedNessuna valutazione finora
- DeC-6 2007Documento10 pagineDeC-6 2007Kun ConNessuna valutazione finora
- Power Plant Simulator Training Institute: Bakreswar Thermal Power Project: WBPDCLDocumento13 paginePower Plant Simulator Training Institute: Bakreswar Thermal Power Project: WBPDCLRaj KumarNessuna valutazione finora
- Mechanisms of HC Formation in SI Engines.... Contd. The Lecture ContainsDocumento7 pagineMechanisms of HC Formation in SI Engines.... Contd. The Lecture ContainsAnkush AgarwalNessuna valutazione finora
- C CC CC: CCCCCCCCCCCCCCCCDocumento27 pagineC CC CC: CCCCCCCCCCCCCCCCMuhammadTanzeeLUsmanNessuna valutazione finora
- 017 Catalytic CrackingDocumento10 pagine017 Catalytic CrackingJess TobiasNessuna valutazione finora
- Processing Biomass in Conventional Oil RefineriesDocumento10 pagineProcessing Biomass in Conventional Oil RefineriesSpafiu Paula RalucaNessuna valutazione finora
- Hydrogen From Used OilDocumento7 pagineHydrogen From Used Oilrvsingh100% (1)
- The Oil: CrackingDocumento2 pagineThe Oil: CrackingFinka Pertama PutriNessuna valutazione finora
- Down-Hole Heavy Crude Oil Upgrading by CAPRI Effect of HydrogenDocumento10 pagineDown-Hole Heavy Crude Oil Upgrading by CAPRI Effect of HydrogenAli ShahNessuna valutazione finora
- Less Emissions Through Waste Heat RecoveryDocumento10 pagineLess Emissions Through Waste Heat Recoveryhpss77Nessuna valutazione finora
- Distillation Characteristics of Petroleum DieselDocumento30 pagineDistillation Characteristics of Petroleum DieselT Richie100% (2)
- Measurement of Lubricant Flow in A Gasoline Engine: Key WordsDocumento16 pagineMeasurement of Lubricant Flow in A Gasoline Engine: Key WordsYamill Najar LermaNessuna valutazione finora
- Petroleum Refining Process Control and RT OptimizationDocumento11 paginePetroleum Refining Process Control and RT Optimizationdemos2011Nessuna valutazione finora
- Oliver Van Rheinberg, Klaus Lucka, Heinrich Köhne, Thomas Schade, Jan T. AnderssonDocumento9 pagineOliver Van Rheinberg, Klaus Lucka, Heinrich Köhne, Thomas Schade, Jan T. AnderssonbassamjavedNessuna valutazione finora
- Crude OilDocumento21 pagineCrude OilAlex LochaiNessuna valutazione finora
- Liquid FuelsDocumento21 pagineLiquid FuelsvaibhavNessuna valutazione finora
- Dewaxing of Distilate Oil Fraction (400 - 500 ºC) Using UreaDocumento15 pagineDewaxing of Distilate Oil Fraction (400 - 500 ºC) Using UreaNima FakherNessuna valutazione finora
- Emulsion Fuel Technology in Combustion FurnacesDocumento3 pagineEmulsion Fuel Technology in Combustion Furnacesvuongcoi102Nessuna valutazione finora
- Patente de Resina Metacrilica UsosDocumento3 paginePatente de Resina Metacrilica UsosGhiu BrittgoNessuna valutazione finora
- Purifier Maintenance 1Documento54 paginePurifier Maintenance 1Noel Nico FernandoNessuna valutazione finora
- Comaprison of Tray Position in Distillation ProcessDocumento11 pagineComaprison of Tray Position in Distillation ProcessShieeplNessuna valutazione finora
- Production of Diesel Fuel From Used Engine OilDocumento6 pagineProduction of Diesel Fuel From Used Engine OilNorBertoChavezNessuna valutazione finora
- Petroleum Refining Process Control and Real-Time OptimizationDocumento11 paginePetroleum Refining Process Control and Real-Time OptimizationLuís Roberto Cavalcanti da SilvaNessuna valutazione finora
- Chemcad Cc5 ExampleDocumento37 pagineChemcad Cc5 ExampleBabulu BalarkanNessuna valutazione finora
- Performance Characteristics of A Low Heat Rejection Diesel Engine Operating With BiodieselDocumento7 paginePerformance Characteristics of A Low Heat Rejection Diesel Engine Operating With BiodieselbalakaleesNessuna valutazione finora
- Alkylation Is The Transfer of An Alkyl Group From One Molecule To AnotherDocumento2 pagineAlkylation Is The Transfer of An Alkyl Group From One Molecule To AnotherAnthony BasantaNessuna valutazione finora
- Combating Green Oil PDFDocumento5 pagineCombating Green Oil PDFkulaspiroNessuna valutazione finora
- Evaporation of Biomass Fast Pyrolysis Oil: Evaluation of Char FormationDocumento8 pagineEvaporation of Biomass Fast Pyrolysis Oil: Evaluation of Char FormationyemresimsekNessuna valutazione finora
- Process Description - ARU Rev BDocumento8 pagineProcess Description - ARU Rev BPhani Raj MNessuna valutazione finora
- AT6504 AFL Notes PDFDocumento52 pagineAT6504 AFL Notes PDFmeetbalakumarNessuna valutazione finora
- Production of Fatty Alcohols From Fatty Acids: ManufacturingDocumento3 pagineProduction of Fatty Alcohols From Fatty Acids: ManufacturingPutri Defriska SiagianNessuna valutazione finora
- 2001 Elemental Sulfur As An Effective Promoter For The Catalytic Hydrocracking of Arabian Vacuum ResidueDocumento5 pagine2001 Elemental Sulfur As An Effective Promoter For The Catalytic Hydrocracking of Arabian Vacuum Residueيا بقية الله ادركناNessuna valutazione finora
- Lec 13Documento5 pagineLec 13Ghazy alshyalNessuna valutazione finora
- Crude Oil Distillation ProcessesDocumento109 pagineCrude Oil Distillation ProcessesFlowealthNessuna valutazione finora
- AADocumento30 pagineAAAhmed MajidNessuna valutazione finora
- ProductionofunleadedGasolineinRiyadhOilRefinery Al-MutazDocumento9 pagineProductionofunleadedGasolineinRiyadhOilRefinery Al-MutazQwERTyNessuna valutazione finora
- 4-Commercial-Scale Demonstration of Pollutant Emission Reduction and Energy Saving For Industrial Boilers by Employing Water - Oil Emulsified FuelDocumento6 pagine4-Commercial-Scale Demonstration of Pollutant Emission Reduction and Energy Saving For Industrial Boilers by Employing Water - Oil Emulsified FuelBerkefedeNessuna valutazione finora
- Us 2845438Documento5 pagineUs 2845438Pat22 22patNessuna valutazione finora
- Career Development: The Chemical Engineer - Issue 839 - May 2011Documento4 pagineCareer Development: The Chemical Engineer - Issue 839 - May 2011Thupten Gedun Kelvin OngNessuna valutazione finora
- Fuels For Ic EnginesDocumento43 pagineFuels For Ic EnginesNipun AroraNessuna valutazione finora
- Subject Name: Petroleum Refining and Petrochemicals Semester - Viii (Chem) Chapter Name: Treatment TechniquesDocumento9 pagineSubject Name: Petroleum Refining and Petrochemicals Semester - Viii (Chem) Chapter Name: Treatment TechniquesMohit SutharNessuna valutazione finora
- Fuel Injection System Solution DG Question Bank by Saidul Islam (Jahed) 35th Batch of BMFADocumento22 pagineFuel Injection System Solution DG Question Bank by Saidul Islam (Jahed) 35th Batch of BMFAkh al aminNessuna valutazione finora
- Patent US2748063Documento6 paginePatent US2748063Gökhan Kürşat demirNessuna valutazione finora
- Comparison of Diesel and Petrol EnginesDa EverandComparison of Diesel and Petrol EnginesValutazione: 2.5 su 5 stelle2.5/5 (3)
- The Production of Olefine-Containing and Fuel GasesDa EverandThe Production of Olefine-Containing and Fuel GasesNessuna valutazione finora
- 12) United States Patent: Tripathi Et ADocumento10 pagine12) United States Patent: Tripathi Et AAlex KattamisNessuna valutazione finora
- United States Patent (10) Patent No.: US 6,577,512 B2: Tripathi Et Al. (45) Date of Patent: Jun. 10, 2003Documento6 pagineUnited States Patent (10) Patent No.: US 6,577,512 B2: Tripathi Et Al. (45) Date of Patent: Jun. 10, 2003Alex KattamisNessuna valutazione finora
- Jose, CA (US) 95.125 Joseph Zappel, E. "E. E. 35,: (12) United States Patent (10) Patent No.: US 6,332,229 B1Documento41 pagineJose, CA (US) 95.125 Joseph Zappel, E. "E. E. 35,: (12) United States Patent (10) Patent No.: US 6,332,229 B1Alex KattamisNessuna valutazione finora
- SW4 T T T: (12) United States Patent (10) Patent No.: US 7,995,047 B2Documento18 pagineSW4 T T T: (12) United States Patent (10) Patent No.: US 7,995,047 B2Alex KattamisNessuna valutazione finora
- United States Patent: Shteynberg Et Al. Patent No.: Oct. 2, 2007 Date of PatentDocumento29 pagineUnited States Patent: Shteynberg Et Al. Patent No.: Oct. 2, 2007 Date of PatentAlex KattamisNessuna valutazione finora
- GRS Is: (12) United States Patent (10) Patent No.: US 6,285,139 B1Documento9 pagineGRS Is: (12) United States Patent (10) Patent No.: US 6,285,139 B1Alex KattamisNessuna valutazione finora
- United States Patent: (10) Patent No.: (45) Date of PatentDocumento19 pagineUnited States Patent: (10) Patent No.: (45) Date of PatentAlex KattamisNessuna valutazione finora
- United States Patent (10) Patent No.: US 8,590,068 B2Documento10 pagineUnited States Patent (10) Patent No.: US 8,590,068 B2Alex KattamisNessuna valutazione finora
- United States Patent (10) Patent No.: US 6,202,227 B1: Gurowitz (45) Date of Patent: Mar. 20, 2001Documento9 pagineUnited States Patent (10) Patent No.: US 6,202,227 B1: Gurowitz (45) Date of Patent: Mar. 20, 2001Alex KattamisNessuna valutazione finora
- United States Patent: (75) Inventors: Michael Belscher, Cary, NC (US)Documento11 pagineUnited States Patent: (75) Inventors: Michael Belscher, Cary, NC (US)Alex KattamisNessuna valutazione finora
- United States Patent (10) Patent No.: US 6,715,162 B2: Bordentown, NJ (US) Robert Michael 2. A 3. RDocumento26 pagineUnited States Patent (10) Patent No.: US 6,715,162 B2: Bordentown, NJ (US) Robert Michael 2. A 3. RAlex KattamisNessuna valutazione finora
- US1372148Documento3 pagineUS1372148Alex KattamisNessuna valutazione finora
- United States Patent (19) : 11 Patent Number: (45) Date of PatentDocumento6 pagineUnited States Patent (19) : 11 Patent Number: (45) Date of PatentAlex KattamisNessuna valutazione finora
- United States Patent Office: Patented Feb. 9, 1971Documento7 pagineUnited States Patent Office: Patented Feb. 9, 1971Alex KattamisNessuna valutazione finora
- Us 4927814Documento11 pagineUs 4927814Alex KattamisNessuna valutazione finora
- United States Patent: Rwick Et AlDocumento10 pagineUnited States Patent: Rwick Et AlAlex KattamisNessuna valutazione finora
- Us 7068503Documento51 pagineUs 7068503Alex KattamisNessuna valutazione finora
- United States Patent: Plastic Pad Secured To The Bottom of The Cushion and Having ADocumento5 pagineUnited States Patent: Plastic Pad Secured To The Bottom of The Cushion and Having AAlex KattamisNessuna valutazione finora
- United States Patent (19) 11 Patent Number: 5,626,614: Hart 45 Date of Patent: May 6, 1997Documento10 pagineUnited States Patent (19) 11 Patent Number: 5,626,614: Hart 45 Date of Patent: May 6, 1997Alex KattamisNessuna valutazione finora
- Jan. 12, 1971 M. R. Fechillas 3,554,788: Filed Oct. 9, 1968Documento4 pagineJan. 12, 1971 M. R. Fechillas 3,554,788: Filed Oct. 9, 1968Alex KattamisNessuna valutazione finora
- United States Patent: UehlingDocumento5 pagineUnited States Patent: UehlingAlex KattamisNessuna valutazione finora
- Seeeeeeee F: 950,347. Patented Feb. 22, 1910Documento3 pagineSeeeeeeee F: 950,347. Patented Feb. 22, 1910Alex KattamisNessuna valutazione finora
- United States Patent: Rwick Et AlDocumento10 pagineUnited States Patent: Rwick Et AlAlex KattamisNessuna valutazione finora
- United States Patent (10) Patent No.: US 7,116,517 B1: He Et Al. (45) Date of Patent: Oct. 3, 2006Documento11 pagineUnited States Patent (10) Patent No.: US 7,116,517 B1: He Et Al. (45) Date of Patent: Oct. 3, 2006Alex KattamisNessuna valutazione finora
- March 23, 1971 D. Rosenberg 3,572,375: Filed March 5, 1968 2. Sheets-Sheet LDocumento6 pagineMarch 23, 1971 D. Rosenberg 3,572,375: Filed March 5, 1968 2. Sheets-Sheet LAlex KattamisNessuna valutazione finora
- March 23, 1971 D. Rosenberg 3,572,375: Filed March 5, 1968 2. Sheets-Sheet LDocumento6 pagineMarch 23, 1971 D. Rosenberg 3,572,375: Filed March 5, 1968 2. Sheets-Sheet LAlex KattamisNessuna valutazione finora
- Us 3572375Documento9 pagineUs 3572375Alex KattamisNessuna valutazione finora
- United States Patent 19Documento14 pagineUnited States Patent 19Alex KattamisNessuna valutazione finora
- Us 7856985Documento15 pagineUs 7856985Alex KattamisNessuna valutazione finora
- As 4873.1-2005 Recommended Practice For Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) Principles andDocumento8 pagineAs 4873.1-2005 Recommended Practice For Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) Principles andSAI Global - APACNessuna valutazione finora
- Bongsuwan D 2008Documento7 pagineBongsuwan D 2008Liona MargaritaNessuna valutazione finora
- Zinc Chloride Sol Msds PDFDocumento6 pagineZinc Chloride Sol Msds PDFJunia Alfa NessaNessuna valutazione finora
- Chemicals Zetag MSDS Inverse Emulsion Zetag 8849 FS - 0710Documento6 pagineChemicals Zetag MSDS Inverse Emulsion Zetag 8849 FS - 0710PromagEnviro.comNessuna valutazione finora
- 2561 Rubber Based Adhesives For Automobile IndustryDocumento14 pagine2561 Rubber Based Adhesives For Automobile IndustryKaushik SenguptaNessuna valutazione finora
- Schultz 1987Documento18 pagineSchultz 1987Ruiz ManuelNessuna valutazione finora
- S K HazraDocumento6 pagineS K HazraRicky MenonNessuna valutazione finora
- I - 6 Batch 2022 Project ReportDocumento72 pagineI - 6 Batch 2022 Project Reportvilla srisuryaNessuna valutazione finora
- Chapter 5: Analytic Techniques: by Julia C. Drees, Matthew S. Petrie, Alan H.B. WuDocumento15 pagineChapter 5: Analytic Techniques: by Julia C. Drees, Matthew S. Petrie, Alan H.B. WuTanveerNessuna valutazione finora
- 01-0105 Pyrocatechol Developer FormulaDocumento2 pagine01-0105 Pyrocatechol Developer FormulaEugene KulikovNessuna valutazione finora
- Helicopter StructureDocumento10 pagineHelicopter StructuredivyaNessuna valutazione finora
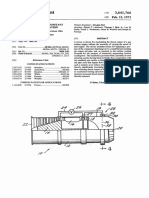
- Piercing Extrusion of Long Hollow Component With Subsidiary TensionDocumento4 paginePiercing Extrusion of Long Hollow Component With Subsidiary TensionDhan CNessuna valutazione finora
- Studi Bioekivalensi Amoksisilin Generik Dan Dagang Menggunakan Matriks UrinDocumento7 pagineStudi Bioekivalensi Amoksisilin Generik Dan Dagang Menggunakan Matriks UrinEA12345aeNessuna valutazione finora
- Advances and Challenges in Alkaline Anion Exchange Membrane Fuel CellsDocumento35 pagineAdvances and Challenges in Alkaline Anion Exchange Membrane Fuel CellsJosePPMolinaNessuna valutazione finora
- Failure Rate Data Analysis For High Technology ComponentsDocumento9 pagineFailure Rate Data Analysis For High Technology ComponentsganeshdhageNessuna valutazione finora
- Synergism in Solvent ExtractionDocumento26 pagineSynergism in Solvent ExtractionabrahanNessuna valutazione finora
- Causes of KickDocumento8 pagineCauses of KickAyman64Nessuna valutazione finora
- D2671-13 Standard Test Methods For Heat-Shrinkable Tubing For Electrical UseDocumento19 pagineD2671-13 Standard Test Methods For Heat-Shrinkable Tubing For Electrical UseOmar Alejandro SalazarNessuna valutazione finora
- Introductory Experiment: Calibration of Volumetric GlasswareDocumento4 pagineIntroductory Experiment: Calibration of Volumetric GlasswareOcampo AmyNessuna valutazione finora
- Temperature Dependence and ZTC Bias Point Evaluation of Sub 20nm Bulk Multigate DevicesDocumento16 pagineTemperature Dependence and ZTC Bias Point Evaluation of Sub 20nm Bulk Multigate DevicesYgor AguiarNessuna valutazione finora
- Problemas de Espectroscopia Organica IDocumento5 pagineProblemas de Espectroscopia Organica IGabriel Alejandro Socias EsquivelNessuna valutazione finora
- Group Two'S Seminar Work: Topic: Enzyme Regulation Allosteric Regulation and Models OutlineDocumento13 pagineGroup Two'S Seminar Work: Topic: Enzyme Regulation Allosteric Regulation and Models OutlineOluwasegun ModupeNessuna valutazione finora
- Continuum Electromechanics Cem - 811Documento637 pagineContinuum Electromechanics Cem - 811kgrhoads100% (1)
- Heat-Stable Salts and Amine Unit Performance: Ralph WeilandDocumento4 pagineHeat-Stable Salts and Amine Unit Performance: Ralph WeilandAzimzadeh FamNessuna valutazione finora
- Interrelationship Phases of The Hydrologic CycleDocumento37 pagineInterrelationship Phases of The Hydrologic CycleHeart DaclitanNessuna valutazione finora
- BPhO Round 1 2013 PRT 1Documento6 pagineBPhO Round 1 2013 PRT 1Karn KumarNessuna valutazione finora
- Bpo C Chapter 18Documento74 pagineBpo C Chapter 18Cristiano Hamdiansyah SempadianNessuna valutazione finora
- Recommended Practices ON Static Electricity: OISD-110 OISD - 110 (Rev.1)Documento35 pagineRecommended Practices ON Static Electricity: OISD-110 OISD - 110 (Rev.1)manuppm100% (1)
- Hosting Offer For Marie Sklodowska-Curie Postdoctoral Fellowships (PF) 2022 at University of Antwerp/Research Group A-Sense Lab/Centre of Excellence NanolabDocumento3 pagineHosting Offer For Marie Sklodowska-Curie Postdoctoral Fellowships (PF) 2022 at University of Antwerp/Research Group A-Sense Lab/Centre of Excellence Nanolabznadeempk_423992578Nessuna valutazione finora