Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Thermo
Caricato da
AcfMacCopyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Thermo
Caricato da
AcfMacCopyright:
Formati disponibili
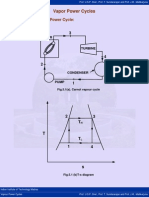
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M .
Mallikarjuna
Vapor Power Cycles
5.1 Carnot Vapor Power Cycle:
3
BOILER
TURBINE
CONDENSER
PUMP 1
Fig.5.1(a). Carnot vapour cycle
2 3
TH
T
TL
1 4
s
Fig.5.1 (b)T-s diagram
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Process 1-2: Reversible adiabatic compression process from P1 to P2.
Process 2-3: Reversible isothermal heat addition process at constant
temperature TH.
Process 3-4: Reversible adiabatic expansion process from P3 to P4.
Process 4-1: Reversible isothermal heat rejection process at constant
temperature TL.
Saturated vapor leaves the boiler at state 3, enters the turbine and expands to state 4.
The fluid then enters the condenser, where it is cooled to state 1 and then it is
compressed to state 2 in the pump. The efficiency of the cycle is as follows:
TH - TL ⎡ T ⎤
ηcarnot = = ⎢1 - L ⎥
TH ⎣ TH ⎦
Practically, it is very difficult to add or reject heat to or from the working fluid at constant
temperature. But, it is comparatively easy to add or reject heat to or from the working
fluid at constant pressure. Therefore, Carnot cycle is not used as an idealized cycle for
steam power plants. However, ideal cycle for steam power plant is Rankine cycle in
which heat addition and rejection takes place at constant pressure process.
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Refrigeration Cycles
6.0 Introduction:
The term “refrigeration” is used to denote maintenance of a system or body at a
temperature lower than that of its surroundings. The system maintained at the lower
temperature is known as refrigerated system, while the equipment used to maintain this
lower temperature is known as the refrigerating system or refrigerator.
Applications of Refrigeration:
1) Industrial Applications:
a) Processing of food products.
b) Processing of farm crops.
c) Processing of textiles, printing work, photographic materials, etc.
d) Cooling of concrete for dams.
e) Treatment of air for blast furnace.
f) Processing of tobacco, petroleum and other chemical products.
2) Preservation of Perishable Goods:
a) Manufacturing of ice.
b) Freezing or chilling, storage and transportation of food stuffs including
beverages, meat, poultry products, dairy products, fish, fruits, vegetables, fruit
juices, etc.
c) Preservation of photographic films, archeological documents etc.
3) Providing comfortable environment:
a) Industrial air-conditioning.
b) Comfort air-conditioning of hospitals, residences, hotels, restaurants,
theatres, offices, etc.
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
4.1 Carnot Cycle :
A Carnot gas cycle operating in a given temperature range is shown in the T-s diagram
in Fig. 4.1(a). One way to carry out the processes of this cycle is through the use of
steady-state, steady-flow devices as shown in Fig. 4.1(b). The isentropic expansion
process 2-3 and the isentropic compression process 4-1 can be simulated quite well by
a well-designed turbine and compressor respectively, but the isothermal expansion
process 1-2 and the isothermal compression process 3-4 are most difficult to achieve.
Because of these difficulties, a steady-flow Carnot gas cycle is not practical.
The Carnot gas cycle could also be achieved in a cylinder-piston apparatus (a
reciprocating engine) as shown in Fig. 4.2(b). The Carnot cycle on the p-v diagram is as
shown in Fig. 4.2(a), in which processes 1-2 and 3-4 are isothermal while processes 2-3
and 4-1 are isentropic. We know that the Carnot cycle efficiency is given by the
expression.
TL T T
ηth = 1 - =1- 4 =1- 3
TH T1 T2
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
T
1 2
TH
TL
4 3
(a)
heat in Isentropic
isothermal Turbine
Turbine Work
Work out
out
1 2
3 4
Work Work
in in
Isothermal heat out Isentropic
Compressor compressor
Fig.4.1. Steady flow Carnot engine
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Process 1 2: isothermal
p 1 Process 2 3: isentropic
Process 3 4: isothermal
Process 4 1: isentropic
2
4
3
(a)
Piston
displacement
(b)
Fig.4.2. Reciprocating Carnot engine
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Volume
3 4
T3=T4
T2=T1
2 1
Entropy
Fig.4.3. Carnot cycle on p-v and T-s diagrams
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
High temperature Perfectly insulated walls
Source
T3 K Piston
T1 K
Low temperature Perfect insulator cum
Sink Perfet conductor
Fig.4.4. Working of Carnot engine
Since the working fluid is an ideal gas with constant specific heats, we have, for the
isentropic process,
γ−1 γ−1
T1 ⎛ V4 ⎞ T ⎛V ⎞
=⎜ ⎟ ; 2 = ⎜ 3⎟
T4 ⎝ V1 ⎠ T3 ⎝ V3 ⎠
Now, T1 = T2 and T4 = T3, therefore
v4 v
= 3 = r = compression or expansion ratio
v1 v2
Carnot cycle efficiency may be written as,
1
ηth = 1 -
rγ - 1
From the above equation, it can be observed that the Carnot cycle efficiency increases
as ‘r’ increases. This implies that the high thermal efficiency of a Carnot cycle is
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
obtained at the expense of large piston displacement. Also, for isentropic processes we
have,
γ−1 γ−1
T1 ⎛p ⎞ γ T ⎛p ⎞ γ
=⎜ 1⎟ and 2 = ⎜ 2 ⎟
T4 ⎝ p4 ⎠ T3 ⎝ p3 ⎠
Since, T1 = T2 and T4 = T3, we have
p1 p
= 2 = rp = pressure ratio
p4 p3
Therefore, Carnot cycle efficiency may be written as,
1
ηth = 1 - γ −1
γ
rp
From the above equation, it can be observed that, the Carnot cycle efficiency can be
increased by increasing the pressure ratio. This means that Carnot cycle should be
operated at high peak pressure to obtain large efficiency.
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
5.2 Rankine Cycle:
Rankine cycle is the idealized cycle for steam power plants. This cycle is shown on p-v,
T-v, h-s, diagram in the above figures. It consists of following processes:
3
BOILER
TURBINE
CONDENSER
PUMP 1
Fig.5.2(a). Rankine vapour power cycle
3''
2' 3' 3 5
2
1 1' 4' 4 4''
a b s
Fig.5.2(b). T-s diagram Rankine power cycle
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
2 2' 3' 3 3''
1 4' 4 4''
Fig.5.2(c). p-v diagram Rankine power cycle
3''
3
3'
h
2 2'
4''
4' 4
1
s
Fig.5.2(d). h-s diagram Rankine power cycle
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Process 1-2: Water from the condenser at low pressure is pumped into the boiler at
high pressure. This process is reversible adiabatic.
Process 2-3: Water is converted into steam at constant pressure by the addition of heat
in the boiler.
Process 3-4: Reversible adiabatic expansion of steam in the steam turbine.
Process 4-1: Constant pressure heat rejection in the condenser to convert condensate
into water.
The steam leaving the boiler may be dry and saturated, wet or superheated. The
corresponding T-s diagrams are 1-2-3-4-1; 1-2-3’-4’-1 or 1-2-3”-4”-1.
Thermal Efficiency of Rankine Cycle:
Consider one kg of working fluid, and applying first law to flow system to various
processes with the assumption of neglecting changes in potential and kinetic energy,
we can write,
δq - δw = dh
For process 2-3, δw = 0 (heat addition process), we can write,
( δq )boiler = ( dh )boiler = ( h3 - h2 )
For process 3-4; δq = 0 (adiabatic process)
( δw )turbine = - ( dh )turbine = ( h 3 - h4 )
Similarly,
( δq )cond = ( h1 - h 4 )
( δw )pump = ( h1 - h 2 )
( δw )net = ( δw )turbine + ( δw )pump = (h 3 - h 4 ) + (h1 - h 2 ) = (h 3 - h 4 ) - (h 2 - h1 )
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Net work ( δw )net
Now, Thermal efficiency = ηth = =
heat sup plied ( δq )boiler
( h3 - h 4 ) - (h 2 - h1 ) area 122 '341
ηrankine = ηth = =
(h 3 - h 2 ) area a22 '3ba
The pump work ( δw )pump is negligible, because specific volume of water is very small.
Therefore,
h3 - h 4 area 12 '341
ηrankine = = (Neglecting pump work)
h3 - h 2 area a12 '3ba
Note that the rankine cycle has a lower efficiency compared to corresponding Carnot
cycle 2’-3-4-1’ with the same maximum and minimum temperatures. The reason is that
the average temperature at which heat is added in the rankine cycle lies between T2
and T12 and is thus less than the constant temperature T12 at which heat is added to the
Carnot cycle.
Reasons for Considering Rankine Cycle as an Ideal Cycle For Steam
Power Plants:
1) It is very difficult to build a pump that will handle a mixture of liquid and vapor
at state 1’ (refer T-s diagram) and deliver saturated liquid at state 2’. It is
much easier to completely condense the vapor and handle only liquid in the
pump.
2) In the rankine cycle, the vapor may be superheated at constant pressure from
3 to 3” without difficulty. In a Carnot cycle using superheated steam, the
superheating will have to be done at constant temperature along path 3-5.
During this process, the pressure has to be dropped. This means that heat is
transferred to the vapor as it undergoes expansion doing work. This is difficult
to achieve in practice.
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
6.1 Reversed Carnot Cycle:
Reversed Carnot cycle is shown in Fig.6.1. It consists of the following processes.
Process a-b: Absorption of heat by the working fluid from refrigerator at constant low
temperature T2 during isothermal expansion.
Process b-c: Isentropic compression of the working fluid with the aid of external work.
The temperature of the fluid rises from T2 to T1.
Process c-d: Isothermal compression of the working fluid during which heat is rejected
at constant high temperature T1.
Process d-a: Isentropic expansion of the working fluid. The temperature of the working
fluid falls from T1 to T2.
q1
d c
d T1
c
T
P
a q2
T2 a b
b
V S
Fig.6.1. Reversed Carnot cycle
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
COP of Refrigerator:
Heat absorbed Heat absorbed
COP = =
Work supplied Heat rejected - Heat absorbed
T2 (s b - sa ) T2
= =
T1 (s b - s a ) - T2 (s b - sa ) (T1 - T2 )
Practically, the reversed Carnot cycle cannot be used for refrigeration purpose as the
isentropic process requires very high speed operation, whereas the isothermal process
requires very low speed operation.
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
5.3 Mean Temperature of Heat Addition:
Tmax
5 Tmean
T 6
2
Tmin
1 4
Fig.5.3. Mean temperature of heat addition
If Tm is the mean temperature of heat addition as shown in the above figure, so that the
area under curve 2-3 is equal to area under curve 5-6, then heat added.
Q1 = H.A = (h 3 - h 2 ) = Tm (s3 - s 2 )
(h 3 - h 2 )
Tm =
(s3 - s 2 )
If Q2 = heat rejected = ( h 4 - h1 ) = Tmin ( s 4 - s1 ) = Tmin ( s3 - s 2 )
Q2 T (s - s 2 )
ηrankine = 1 - = 1 - min 3
Q1 Tm (s3 - s 2 )
Tmin
ηrankine = 1 -
Tm
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
6.2 Methods of Refrigeration:
a) Natural Method:
The natural method includes the utilization of ice or snow obtained naturally in cold
climate. Ice melts at 00C. So when it is placed in space or system warmer than 00C,
heat is absorbed by the ice and the space is cooled. The ice then melts into water by
absorbing its latent heat at the rate of 324 kJ/kg. But, now-a-days, refrigeration
requirements have become so high that the natural methods are inadequate and
therefore obsolete.
b) Mechanical or Artificial Refrigeration:
Atmosphere
(T1)
δQ1
Refrigerating System (R)
T2 δW
δQ2
Refrigerated
System (T3)
Fig.6.2. Reversed Carnot engine
A mechanical refrigeration system works on the principle of reversed Carnot cycle as
shown in Fig.6.2. Work δw is delivered to the refrigerating system, causing it to remove
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
heat δQ2 from the body or system (at lower temperature T3) and to deliver it along with
work, δw, to another body at higher temperature, T1, so that,
δQ1 = δw + δQ 2
There can be two methods by which the temperature T2 < T3 may be attained within the
refrigerating system.
i) By lowering the temperature of the working substance in the refrigerating
system to the level of T2. In this case, the heat will be absorbed due to
temperature difference and T3 will decrease as heat δQ2 flows out.
ii) By evaporating some fluid at an appropriate pressure. In this case, a constant
temperature T2 will be maintained and latent heat of fluid will be absorbed as
δQ2.
Depending upon the above method used, there are two types of mechanical
refrigerating systems :
i) Air systems: Uses air as a working fluid. Air does not undergo any change of
phase, but absorbs heat due to temperature difference.
ii) Chemical Agent Systems: The working fluid changing its phase while boiling
from liquid to vapor state, thereby it absorbs the latent heat.
Unit of Refrigeration:
Capacity of refrigeration unit is generally defined in ton of refrigeration. A ton of
refrigeration is defined as the quantity of heat to be removed in order to form one ton
(1000 kg) of ice at 00C in 24 hrs, from liquid water at 00C. This is equivalent to 3.5 kJ/s
(3.5 kW) or 210 kJ/min.
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
4.2 Stirling Cycle (Regenerative Cycle) :
The Carnot cycle has a low mean effective pressure because of its very low work
output. Hence, one of the modified forms of the cycle to produce higher mean effective
pressure whilst theoretically achieving full Carnot cycle efficiency is the Stirling cycle. It
consists of two isothermal and two constant volume processes. The heat rejection and
addition take place at constant temperature. The p-v and T-s diagrams for the Stirling
cycle are shown in Fig.4.2.
Volume
Temperature
3 4
T3=T4
T2=T1
2 1
Entropy
Fig.4.2. Stirling cycle processes on p-v and T-s diagrams
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Stirling Cycle Processes:
(a) The air is compressed isothermally from state 1 to 2 (TL to TH).
(b) The air at state-2 is passed into the regenerator from the top at a temperature
T1. The air passing through the regenerator matrix gets heated from TL to TH.
(c) The air at state-3 expands isothermally in the cylinder until it reaches state-4.
(d) The air coming out of the engine at temperature TH (condition 4) enters into
regenerator from the bottom and gets cooled while passing through the
regenerator matrix at constant volume and it comes out at a temperature TL,
at condition 1 and the cycle is repeated.
(e) It can be shown that the heat absorbed by the air from the regenerator matrix
during the process 2-3 is equal to the heat given by the air to the regenerator
matrix during the process 4-1, then the exchange of heat with external source
will be only during the isothermal processes.
Now we can write, Net work done = W = Qs - QR
Heat supplied = QS = heat supplied during the isothermal process 3-4.
⎛V ⎞ v
= P3 V3 ln ⎜ 4 ⎟ ; r = 4 = CR
⎝ V3 ⎠ v3
= mRTH ln ( r )
Heat rejected = QR = Heat rejected during the isothermal compression process, 1-2.
⎛v ⎞
= P1V1 ln ⎜ 1 ⎟
⎝ v2 ⎠
= mR TL ln ( r )
Wnet = m R ln ( r ) [ TH - TL ]
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Now,
Wnet m R ln ( r )( TH - TL ) TH - TL
ηth = =
Qs m R ln ( r ) TH TH
and
TL
ηth = 1 -
TH
Thus the efficiency of Stirling cycle is equal to that of Carnot cycle efficiency when both
are working with the same temperature limits. It is not possible to obtain 100% efficient
regenerator and hence there will be always 10 to 20 % loss of heat in the regenerator,
which decreases the cycle efficiency. Considering regenerator efficiency, the efficiency
of the cycle can be written as,
R ln ( r )( TH - TL )
ηth =
R TH ln ( r ) + (1 - ηR ) CV ( TH - TL )
Where, ηR is the regenerator efficiency.
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
5.4 Capacity of Steam Power Plant:
Steam rate: It is defined as the rate of steam flow (kg/hr) required for producing unit
shaft output (1 kW), therefore,
3600
Steam rate = ( kg/kWh )
w net
Heat rate: It is rate of heat input (Q1) required for producing unit work output (1 kW).
3600
Heat rate = . Q1 ( kJ/kWh )
w net
where, Q1 is heat added per kg of steam
Effect of Varying the Operating Conditions on the Efficiency of the
Simple Rankine Cycle:
(a) Effect of Superheat:
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
3'
2' 3
2
1
4 4'
A B C s
Fig.5.4(a). Effect of superheating
In the above figure two Rankine cycles are compared, cycle 1-2-3-4-1 using dry
saturated steam at the exit of the boiler and cycle 1-2-3’-4’-1 using superheated steam
at the exit of the boiler. The superheat steam cycle delivers more work and this excess
work is represented by area 3-3’-4’-4, and also it takes in more amount of heat and this
excess is represented by area 3-3’-C-B. The net effect is to increase the thermal
efficiency of the cycle. This increase could have been anticipated from second law,
because superheating increases the average temperature of heat addition to the cycle.
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
(b) Effect of Maximum Pressure:
3'
3
p'
p
T
2'
2
1 4' 4
A B C s
Fig.5.4(b). Effect of maximum pressure on Rankine cycle
The two cycles are shown above 1-2-3-4-1 and 1-2’-3’-4’-1 have the same minimum
pressure but different maximum pressures. As the result of increasing the maximum
pressure from p to p’, the net work output has increased by the area shown by
horizontal hatching and decreased by the area shown by vertical hatching. Since, these
two areas are nearly equal, the network is nearly the same, but the net heat rejected
decreases by the area 4’-4-C-B. Hence, the thermal efficiency increases.
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
(c) Effect of Condenser Pressure:
T 2
P4
2'
1 P4' 4
1'
4'
A B C S
Fig.5.4(c). Effect of condenser pressure on Rankine cycle
If the condenser pressure is reduced from p4 to p14, the net work is increased by area 1-
4-4’-1’-2’-2-1. And the heat supplied to steam increases by the area A-2’-2-B. These
two areas are nearly equal; however, the net effect is to increase the thermal efficiency.
This could be expected because the average temperature of heat rejection of the cycle
decreases with decrease in condenser pressure.
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
4.3 Ericsson Cycle:
The Ericsson cycle consists of two isothermal and two constant pressure processes.
The processes are:
Process 1-2: Reversible isothermal compression.
Process 2-3: Constant pressure heat addition.
Process 3-4: Reversible isothermal expansion.
Process 4-1: Constant pressure heat rejection.
The heat addition and rejection take place at constant pressure as well as isothermal
processes. Since the process 2-3 and 3-4 are parallel to each other on the T-s diagram,
the net effect is that the heat need to be added only at constant temperature T3=T4 and
rejected at the constant temperature T1=T2. The cycle is shown on p-v and T-s
diagrams in Fig.4.3. The advantage of the Ericsson cycle over the Carnot and Stirling
cycles is its smaller pressure ratio for a given ratio of maximum to minimum specific
volume with higher mean effective pressure.
2 3
1 4
Volume
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Temperature
3 4
T3=T4
T2=T1
2 1
Entropy
Fig.4.3. Ericsson cycle on p-v and T-s diagrams
The thermal efficiency of Ericsson cycle is given by, (derivation is same as that of
Stirling cycle),
TH - TL ⎡ T ⎤
ηth = = ⎢1 - L ⎥
TH ⎣ TH ⎦
The Ericsson cycle does not find practical application in piston engines but is
approached by a gas turbine employing a large number of stages with heat exchangers,
insulators and reheaters.
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
6.3 Air Refrigeration System And Bell-Coleman Cycle Or
Reversed Brayton Cycle:
qc
5 3
H.E
Compressor
Expander
6 Evaporator 2
qe
Motor
6.3 (a) Air refrigeration system
5 p2 3
4
1
6 p1 2
V
6.3 (b) Air refrigeration system
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
p2
3
p1
T 5
2
6.3 (c) Air refrigeration system
The components of the air refrigeration system are shown in Fig.6.3(a). In this system,
air is taken into the compressor from atmosphere and compressed. The hot
compressed air is cooled in heat exchanger upto the atmospheric temperature (in ideal
conditions). The cooled air is then expanded in an expander. The temperature of the air
coming out from the expander is below the atmospheric temperature due to isentropic
expansion. The low temperature air coming out from the expander enters into the
evaporator and absorbs the heat. The cycle is repeated again. The working of air-
refrigeration cycle is represented on p-v and T-s diagrams in Fig.6.3(b) and (c).
Process 1-2 represents the suction of air into the compressor. Process 2-3 represents
the isentropic compression of air by the compressor. Process 3-5 represents the
discharge of high pressure air from the compressor into the heat exchanger. The
reduction in volume of air from v3 to v5 is due to the cooling of air in the heat exchanger.
Process 5-6 represents the isentropic expansion of air in the expander. Process 6-2
represents the absorption of heat from the evaporator at constant pressure.
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
6.3.1. Analysis of Bell-Coleman Cycle:
The air refrigeration system works on Bell-Coleman cycle.
Assumptions:
1) The compression and expansion processes are reversible adiabatic
processes.
2) There is a perfect inter-cooling in the heat exchanger.
3) There are no pressure losses in the system.
Net refrigeration effect
COP =
Net work sup plied
Work done per kg of air for the isentropic compression process 2-3 is given by,
WC = C p (T3 - T2 )
Work developed per kg of air for the isentropic expansion process 5-6 is given by,
WE = Cp (T5 - T6 )
Net work required = Wnet = (WC - WE ) = Cp (T3 - T2 ) - Cp (T5 - T6 )
Net refrigerating effect per kg of air is given by,
R net = Cp (T2 - T6 )
R net Cp (T2 - T6 )
COP = =
Wnet Cp {(T3 - T2 ) - (T5 - T6 )}
For perfect inter-cooling, the required condition is T5 = T2
(T2 - T6 )
COP =
(T3 - T2 ) - (T2 - T6 )
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
1
= (for isentropic process)
( T3 - T2 )
- 1
( T2 - T6 )
1
=
T3 (1 - T2 / T3 )
- 1
T2 (1 - T6 / T2 )
For isentropic compression process 2-3 and for expansion process 5-6, we have,
γ -1 γ -1
T3 ⎛P ⎞ γ T ⎛P ⎞ γ
= ⎜ 1⎟ and 5 = ⎜ 2 ⎟
T2 ⎝ P2 ⎠ T6 ⎝ P1 ⎠
T3 T T T
Therefore, = 5 or 6 = 2 Q (T5 = T2 )
T2 T6 T5 T3
T2
COP =
T3 - T2
Advantages:
a) Air is a cheaper refrigerant and available easily compared to other
refrigerants.
b) There is no danger of fire or toxic effects due to leakage.
c) The total weight of the system per ton of refrigerating capacity is less.
Disadvantages:
(a) The quantity of air required per ton refrigerating capacity is far greater than
other systems.
(b) The COP is low and hence maintenance cost is high.
(c) The danger of frosting at the expander valves is more as the air taken into the
system always contains moisture.
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Work done during Compression and Expansion Processes (for
polytropic processes) (Refer P-V-diagram) (For problem solving)
P3 V3 - P2 V2
WC = P3 V3 + - P2 V2
n -1
( P3 V3 - P2 V2 )
= ( P3 V3 - P2 V2 ) +
n - 1
n
= ( P3 V3 - P2 V2 )
n -1
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
4.4 Air Standard Otto Cycle:
The air-standard-Otto cycle is the idealized cycle for the spark-ignition internal
combustion engines. This cycle is shown above on p-v and T-s diagrams. The Otto
cycle 1-2-3-4 consists of following four process:
Process 1-2: Reversible adiabatic compression of air.
Process 2-3: Heat addition at constant volume.
Process 3-4: Reversible adiabatic expansion of air.
Process 4-1: Heat rejection at constant volume.
2
4
Volume
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
2
4
1
Entropy
Fig.4.4. Otto cycle on p-v and T-s diagrams
Air Standard Efficiency:
Net workdone
ηth =
Net heat added
Since processes 1-2 and 3-4 are adiabatic processes, the heat transfer during the cycle
takes place only during processes 2-3 and 4-1 respectively. Therefore, thermal
efficiency can be written as,
Heat added - Heat rejected
ηth =
Heat added
Consider ‘m’ kg of working fluid,
Heat added = mCV ( T3 - T2 )
Heat Rejected = mCV ( T4 - T1 )
mCV ( T3 - T2 ) - mCV ( T4 - T1 ) T4 - T1
ηth = =1-
mCV ( T3 - T2 ) T3 - T2
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
For the reversible adiabatic processes 3-4 and 1-2, we can write,
γ -1 γ -1
T4 ⎛v ⎞ T ⎛V ⎞
=⎜ 3⎟ and 1 = ⎜ 2 ⎟
T3 ⎝ v4 ⎠ T2 ⎝ V1 ⎠
v 2 = v3 and v 4 = v1
γ−1
T4 T T -T ⎛V ⎞
= 1 = 4 1 =⎜ 2⎟
T3 T2 T3 - T2 ⎝ V1 ⎠
γ -1
T1 ⎛V ⎞
η th = 1 - = 1- ⎜ 2 ⎟
T2 ⎝ V1 ⎠
V1
The ratio is called as compression ratio, r.
V2
γ -1
⎛1⎞
ηth =1- ⎜ ⎟
⎝r⎠
From the above equation, it can be observed that the efficiency of the Otto cycle is
mainly the function of compression ratio for the given ratio of Cp and Cv. If we plot the
variations of the thermal efficiency with increase in compression ratio for different
gases, the curves are obtained as shown in Fig.4.4.1. Beyond certain values of
compression ratios, the increase in the thermal efficiency is very small, because the
curve tends to be asymptotic. However, practically the compression ratio of petrol
engines is restricted to maximum of 9 or 10 due to the phenomenon of knocking at high
compression ratios.
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
γ=1.67
γ=1.40
γ=1.30
Compression ratio,r
Effect of CR and γ on efficiency for Otto cycle.
Fig.4.4.1. Variation of thermal efficiency with compression ratio
Mean Effective Pressure:
Generally, it is defined as the ratio of the net workdone to the displacement volume of
the piston.
Let us consider ‘m’ kg of working substance.
Net work done = m Cv {( T3 - T2 ) - ( T4 - T1 )}
Displacement Volume = ( V1 - V2 )
⎛ 1⎞ m R T1 ⎛ r - 1 ⎞
= V1 ⎜1 - ⎟ = ⎜ ⎟
⎝ r ⎠ P1 ⎝ r ⎠
m C v ( γ- 1) T1 ⎧ r - 1 ⎫
= ⎨ ⎬
P1 ⎩ r ⎭
since, R = Cv ( γ - 1)
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
m C v ⎡⎣( T3 - T2 ) - ( T4 - T1 ) ⎤⎦
mep =
m C v ( γ -1) T1 ⎧⎛ r - 1 ⎞ ⎫
⎨⎜ ⎟⎬
P1 ⎩⎝ r ⎠ ⎭
⎛ 1 ⎞ ⎛ p1 ⎞ ⎛ r ⎞
= ⎜ ⎟⎜ ⎟⎜ ⎟ {( T3 - T2 ) - ( T4 - T1 )}
⎝ γ - 1 ⎠ ⎝ T1 ⎠ ⎝ r - 1 ⎠
γ -1
Now, T2 = T1 ( r )
P3 T3
Let, rp = = = Pressure ratio
P2 T2
P3
T3 = T2 = rp T2 = rp r γ -1 T1 (for V = C)
P2
γ -1 γ -1
⎛1⎞ γ -1 ⎛1⎞
So, T4 = T3 ⎜ ⎟ = rp r T1 ⎜ ⎟ = rp T1
⎝r⎠ ⎝r⎠
mep =
P1 r
( r - 1) ( γ - 1) {( rp r γ -1 - r γ -1 ) - ( rp - 1)}
⎪
= P1 r ⎨⎜
p (
⎧⎛ r γ -1 r - 1 - r - 1 ⎞ ⎫
p
⎟ ⎪⎬
) ( )
⎪⎩⎜⎝ ( γ - 1) ( r - 1) ⎟⎪
⎠⎭
⎪
mep = P1 r ⎨
p(
⎧ r γ -1 - 1 r - 1 ⎫
⎪ )( )
⎬
⎪⎩ ( r - 1) ( γ - 1) ⎭⎪
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
6.4 Chemical Agent Refrigeration System:
These machines are based on the fact that a liquid can be vaporized at any desired
temperature by changing its pressure. Further, heat is required to be added to the liquid
during vaporization, when the liquid phase changes to the gaseous phase. Therefore, a
vaporizing liquid can be used to produce refrigeration at any temperature. For instance,
at a pressure of about 1 atm, ammonia boils at –330C, and at a pressure of about 5 atm,
Freon-22 boils at 00C and Freon-12 at 1 atm, boils at –300C.
Evaporating chamber
B(1 atm)
A
Fr-12 Liquid T2 = -300C
T3 = 00C
Refrigerated system
Fig.6.4. Refrigeration by using a chemical agent (Fr-12)
Hence, in an arrangement in which a system containing some refrigerant in liquid form
at a certain pressure (corresponding to a temperature T2) is exposed to another system
at temperature T3, the latter will be refrigerated, if T2 < T3. A simple scheme of such an
arrangement is shown in Fig.6.4.
Liquid Fr-12 is supplied to the evaporating chamber through a valve at a pressure of
about 1 atm. Since, the boiling temperature of Fr-12 at this pressure is –300C, heat
flows from the sarrounding space at 00C and makes Fr-12 to boil. Thus, the space will
be cooled as long as there is a supply of liquid Fr-12 to the evaporating chamber.
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
But, the above arrangement, however suffers from two important draw backs.
1. The cost of replacing Fr-12 will be more as the evaporated vapor leaks out to
the atmosphere.
2. If the system was to use some refrigerant like ammonia, it may become
hazardous to life due to its discharge into atmoshphere, since it is a highly
toxic and irritating fluid.
To eliminate these drawbacks, it will be necessary to make the refrigerant work in a
closed loop and be used again and again. Therefore, the vapor at B, the exit of the
evaporating chamber, should be collected and be converted into liquid state again, so
that it could be supplied to the chamber for re-evaporation. Hence, in reality such a
mechanical refrigerating system will use refrigerant alternatively between vapor and
liquid phases. To condense the vapor at state B, its condensing temperature (thereby its
pressure) should be brought to the level higher than that of some freely available natural
cooling medium like air or water. This arrangement is shown in Fig.6.4.1.
Vapor
A -300C,1bar
T2=-300C Compressor
B
∆Ο2 ∆w
T3=00C
C
FR-12 SYSTEM
D
Condenser
Liquid
350C, 8.46 bar Heat rejected
∆Q1
Fig.6.4.1. Closed cycle chemical agent refrigeration cycle
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Depending upon the equipment employed in the system, the chemical agent
refrigeration systems are classified into:
1. Vapor compression refrigeration systems.
2. Vapor absorption refrigeration systems.
3. Steam jet refrigeration systems.
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
5.5 Modified Rankine Cycle:
1 2
4 3
6
Fig.5.5(a). p-v diagram of modified Rankine cycle
2
T
1 5
4 6 3
s
Fig.5.5(b). T-s diagram of modified Rankine cycle
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Process 1-2 represents the admission of high pressure steam into the engine cylinder,
process 2-3 is the reversible adiabatic expansion of steam in the cylinder and process
3-4 is the exhaust of steam into condenser. Net work done is represented by the area 1-
2-3-4-1.
Observe that the area 3-6-5 is very small and in order to obtain this small work, the
cylinder volume must be increased from v6 to v3.This makes cylinder very bulky. For this
reason, the expansion process is terminated at point 5. So that indicator diagram
becomes 1-2-5-6-4. The work lost is small but there is large saving in cylinder volume.
Process 5-6 represents the release of steam into the condenser, thus causing the
cylinder pressure to drop from P5 to P6. Process 6-4 is the exhaust of steam at constant
pressure. Cycle 1-2-5-6-4 is called as the “modified Rankine cycle”.
Thermal Efficiency:
Considering the unit mass of working fluid,
Heat supplied = h 2 - h1
Net workdone = {w 2-5 + w 5-6 + w 4-1 }
6
= ( h 2 - h 5 ) - ∫ vdp + ( h 4 - h1 )
5
= ( h 2 - h 5 ) + v5 ( p5 - p6 ) + ( h 4 - h1 )
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
v5 = specific volume of steam at state 5.
Net workdone (h 2 - h 5 ) + v5 (p5 - p6 ) + (h 4 - h1 )
ηth = =
Heat sup plied (h 2 - h1 )
If pump work is neglected, then h 4 ≈ h1
(h 2 - h 5 ) + v5 (p5 - p6 )
ηth =
(h 2 - h 4 )
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
4.5 Air Standard Diesel Cycle:
Air standard diesel cycle is a idealized cycle for diesel engines. It is as shown on P-v
and T-s diagrams. The processes in the cycle are as follows:
2 3
Volume
2
4
Entropy
Fig.4.5. Air standard diesel cycle on p-v and T-s diagrams.
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Process 1-2: Reversible adiabatic Compression.
Process 2-3: Constant pressure heat addition.
Process 3-5: Reversible adiabatic Compression.
Process 4-1: Constant volume heat rejection.
Consider ‘m’ kg of working fluid. Since the compression and expansion processes are
reversible adiabatic processes, we can write,
Heat sup plied = m Cp ( T3 - T2 ) = ( h3 - h2 )
Heat rejected = m Cv ( T4 - T1 ) = ( u4 - u1 )
Workdone = m Cp ( T3 - T2 ) - m C v ( T4 - T1 )
Now, we can write, thermal efficiency as,
m Cp ( T3 - T2 ) - m C v ( T4 - T1 )
ηth =
m Cp ( T3 - T2 )
1 ⎛ T4 - T1 ⎞
= 1 - ⎜ ⎟
γ ⎝ T3 - T2 ⎠
v1 v
T2 = T1 r γ -1 ; r = = 4
v2 v2
T3 v
= 3 = rc = cutoff ratio
T2 v2
T3 = rc T2 = rc T1 r γ -1
γ -1 γ -1
⎛v ⎞ ⎛v ⎞
T4 = T3 ⎜ 3 ⎟ = T3 ⎜ 4 ⎟
⎝ v4 ⎠ ⎝ v3 ⎠
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
1-γ 1- γ
⎛v v ⎞ ⎛r ⎞
= T3 ⎜ 4 . 2 ⎟ = T3 ⎜ ⎟
⎝ v 2 v3 ⎠ ⎝ rc ⎠
1- γ
γ -1 ⎛ r ⎞
= rc T1 r ⎜ ⎟ ; T4 = rcγ T1
⎝ rc ⎠
1 ⎧⎪ rcγ T1 - T1 ⎫⎪
Hence, ηth = 1 - ⎨ ⎬
γ ⎩⎪ rc r γ -1 T1 - r γ -1 T1 ⎭⎪
⎧⎪ r γ -1 ⎫⎪
= 1 - r1-γ ⎨ c ⎬
⎪⎩ γ ( rc -1) ⎭⎪
From the above equation, it is observed that, the thermal efficiency of the diesel engine
can be increased by increasing the compression ratio, r, by decreasing the cut-off ratio,
α2, or by using a gas with large value of γ. Since the quantity (rγ-1)/γ(rp-1) in above
equation is always greater than unity, the efficiency of a Diesel cycle is always lower
than that of an Otto cycle having the same compression ratio. However, practical Diesel
engines uses higher compression ratios compared to petrol engines.
Mean effective Pressure:
Net workdone
mep =
Displacement volume
m Cp ( T3 - T2 ) - m C v ( T4 - T1 )
=
v1 - v 2
⎛ v ⎞ ⎛ 1⎞
v1 - v 2 = v1 ⎜ 1 - 2 ⎟ = v1 ⎜1 - ⎟
⎝ v1 ⎠ ⎝ r⎠
⎛ r - 1⎞
= m R T1 ⎜ ⎟
⎝ r ⎠
m C v ( γ -1) T1 ⎛ r - 1 ⎞
= ⎜ ⎟
P1 ⎝ r ⎠
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
m Cp ( T3 - T2 ) - m C v ( T4 - T1 )
mep =
⎛ γ - 1⎞⎛ r - 1⎞
m C v T1 ⎜ ⎟⎜ ⎟
⎝ P1 ⎠ ⎝ r ⎠
⎛ P r ⎞ ⎛ 1 ⎞ ⎪⎧ ⎛ T3 - T2 ⎞ ⎛ T4 - T1 ⎞ ⎪⎫
= ⎜ 1 ⎟⎜ ⎟ ⎨γ ⎜ ⎟-⎜ ⎟⎬
⎝ r - 1 ⎠ ⎝ γ - 1 ⎠ ⎪⎩ ⎝ T1 ⎠ ⎝ T1 ⎠ ⎭⎪
= P1 r ⎨
c (
⎧ γ r γ -1 ( r - 1) - r γ - 1 ⎫
⎪ c )
⎪
⎬
⎪⎩ ( r - 1)( γ - 1)
⎭⎪
Difference between Actual Diesel and the Otto Engines:
Otto Engine Diesel Engine
1. Homogenous mixture of fuel and air 1. No carburetor is used. Air alone is
formed in the carburetor is supplied supplied to the engine cylinder. Fuel is
to engine cylinder. injected directly into the engine
cylinder at the end of compression
stroke by means of a fuel injector.
Fuel-air mixture is heterogeneous.
2. Ignition is initiated by means of an 2. No spark plug is used. Compression
electric spark plug. ratio is high and the high temperature
of air ignites fuel.
3. Power output is controlled by varying 3. No throttle value is used. Power output
the mass of fuel-air mixture by is controlled only by means of the
means of a throttle valve in the mass of fuel injected by the fuel
carburetor. injector.
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
5.6 Deviation of Actual Cycle from Ideal Cycle:
The actual cycle deviates from the ideal cycle for the following reasons.
3
T 2'
2
1 4 4'
s
Fig.5.6. T-s diagram of actual and ideal cycle
1) Turbine Losses:
During the expansion of steam in the turbine there will be heat transfer to the
surroundings and the expansion instead of being isentropic will be polytropic as shown
in the figure.
3 − 4 → Isentropic expansion
3 − 4 ' → Acutal expansion
h 3 - h ′4
Turbine efficiency = η t =
h3 - h 4
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
2) Pump Losses:
There are losses in the pump due to irreversibility and the process of compression is
polytropic instead of isentropic as shown above.
h 2 - h1
Pump efficiency = ηp =
h ′2 - h1
3) Condenser Losses:
Due to pressure loss in the condenser, fluid cools below the saturation temperature,
which requires additional heat energy to bring the liquid to the saturation temperature.
Methods of Increasing the Efficiency of Simple Rankine Cycle:
1) Rankine Cycle With Reheat:
3
BOILER
TURBINE
I II
4
5 6
2
PUMP 1
CONDENSER
(a)
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
5
T 4
2
1
6
s
(b)
Fig.5.6.1(a & b). Rankine cycle with reheat
In reheat Rankine cycle, the expansion of steam is carried out in several stages and the
steam is reheated by addition of heat between the stages of turbine. Thus excessive
moisture in the low-pressure stages of the turbine is avoided.
Above figure shows schematic and corresponding T-s, p-v diagrams of a reheat
Rankine cycle with two turbine stages. Steam is expanded from the boiler pressure P3
to some intermediate pressure P4 in the first stage of the turbine. It is then reheated in
the boiler from state 4 to state 5 and finally expanded from P4 = P5 to the exhaust
pressure P1 = P6, in the second stage of the turbine. Note that we can employ any
number of turbine stages.
Reheating does not result in any appreciable gain in thermal efficiency, because the
average temperature of heat addition is not changed. The main advantage is that the
moisture content of steam is reduced to a safe value.
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Thermal efficiency of Reheat cycle:
Net workdone
ηreheat =
Heat sup plied
( h3 - h4 ) + ( h5 - h6 ) - (h2 - h1 )
=
( h3 - h 2 ) + ( h5 - h4 )
Neglecting pump work,
(h 3 - h 4 ) + (h 5 - h 6 )
ηreheat =
(h 3 - h1 ) + (h 5 - h 4 )
Optimum Intermediate Pressure and Temperature for Reheat Cycle:
The reheat Rankine cycle will perform efficiently when intermediate pressure for
reheating is optimized. First, the intermediate temperature is determined as follows:
h3 - h 2
T4 =
s3 - s 2
And then, the intermediate pressure will be equal to saturation pressure corresponding
to the above temperature.
2) Regenerative Feed Heating Cycles:
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
3
2'
x
T
2
1 4
a b s
Fig.5.6.1(c). T-s diagram
The object of regenerative feed heating cycle is to supply the working fluid to the boiler
at some state between 2 and 2’, thereby increasing the average temperature of heat
addition to the cycle.
(a) Single stage regenerative cycle
(i) Open feed water heater
(ii) Closed feed water heater
(b) Multiple stage regenerative cycle.
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
6.5 Simple Vapor Compression Refrigeration System:
A simple vapor compression refrigeration system consists of the following equipments:
i) Compressor ii) Condenser iii) Expansion valve iv) Evaporator.
B COMPRESOR
C
∆Q1
200C
FLUID TO
BE COOLED EVAPORATOR
w CONDENSER
COOLING
WATER
(-300C) (350C)
00C
∆Q2 EXPANSION VALVE
A D
Fig.6.5. Simple vapour compression system
The schematic diagram of the arrangement is as shown in Fig.6.5. The low
temperature, low pressure vapor at state B is compressed by a compressor to high
temperature and pressure vapor at state C. This vapor is condensed into high pressure
vapor at state D in the condenser and then passes through the expansion valve. Here,
the vapor is throttled down to a low pressure liquid and passed on to an evaporator,
where it absorbs heat from the surroundings from the circulating fluid (being
refrigerated) and vaporizes into low pressure vapor at state B. The cycle then repeats.
The exchange of energy is as follows:
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
a) Compressor requires work, δw. The work is supplied to the system from the
surroundings.
b) During condensation, heat δQ1 the equivalent of latent heat of condensation
etc, is lost from the refrigerator.
c) During evaporation, heat δQ2 equivalent to latent heat of vaporization is
absorbed by the refrigerant.
d) There is no exchange of heat during throttling process through the expansion
valve as this process occurs at constant enthalpy.
6.5.1 Simple Vapor Compression Cycle:
C"
T C
350c D C'
T1
-300C B"
T2
T A B' B
P Q R' R S
Fig.6.5.1. T-s diagram of refrigeration cycle
Figure 6.5.1 shows a simple vapor compression refrigeration cycle on T-s diagram for
different compression processes. The cycle works between temperatures T1 and T2
representing the condenser and evaporator temperatures respectively. The various
process of the cycle A-B-C-D (A-B’-C’-D and A-B”-C”-D) are as given below:
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
i) Process B-C (B’-C’ or B”-C”): Isentropic compression of the vapor from state
B to C. If vapor state is saturated (B), or superheated (B”), the compression is
called dry compression. If initial state is wet (B’), the compression is called
wet compression as represented by B’-C’.
ii) Process C-D (C’-D or C”-D): Heat rejection in condenser at constant
pressure.
iii) Process D-A: An irreversible adiabatic expansion of vapor through the
expansion value. The pressure and temperature of the liquid are reduced.
The process is accompanied by partial evaporation of some liquid. The
process is shown by dotted line.
iv) Process A-B (A-B’ or A-B”) : Heat absorption in evaporator at constant
pressure. The final state depends on the quantity of heat absorbed and same
may be wet (B’) dry (B) or superheated (B”).
6.5.2 COP of Vapor Compression Cycle:
Heat extracted at low tempreature
COP =
Work sup plied
Heat extracted at low temperature = Heat transfer during the process A-B =
refrigerating effect.
q 2 = (h B - h A )
Work of compression = w = (hc-hB) (adiabatic compression).
⎧ h - hA ⎫
So, COP = ⎨ B ⎬
⎩ hc - hB ⎭
Now, heat rejected to the condenser, = q1 = w + q 2
= (h C - h B ) + (h B - h A )
= (h C - h A ) = (h C - h D )
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
6.5.3 Comparison of Simple Vapor Compression Cycle with Carnot
Cycle:
350c D C'
T1 S
-300C
T2
E T A B' B
O L P Q R' R
S
Fig.6.5.3. Comparison of simple vapor compression cycle with Carnot cycle
a. In vapor compression cycle, de-superheating between C and C’ is at constant
pressure rather than constant temperature. Therefore, more work has to be
supplied to the compressor. There is an equivalent amount of increase in the
magnitude of heat rejected.
b. In vapor compression cycle, no work is done by the system during the throttling
process. Hence, the network supplied to the cycle increases further by area EDT
as compared to the reversed Carnot cycle. Because,
{(Area RSDO – Area RBEO) – Area EDT } = Area BSDT
c. In vapor compression cycle, there is a loss of refrigeration effect equivalent to
area PQAT due to increase in entropy during the irreversible throttling expansion.
d. The effect of all these deviations is to increase the compression work required or
to decrease the refrigeration effect and therefore the COP of the vapor
compression cycle will be less than that of reversed Carnot cycle.
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
6.5.4 Factors Affecting the Performance of Vapor Compression
Refrigeration System:
(a) Sub-cooling of Liquids:
In the Fig.6.5.4(a) of simple vapor compression cycle, condensation process CD
resulted in the liquid at saturated state D. If it was possible to further cool down the
liquid to some lower value say upto D’, then the net refrigeration effect will be increased
as (hB – h’A) > (hB - hA). Hence, the sub cooling of the liquid increases the refrigerating
effect without increasing the work requirement. Thus COP is improved. The sub cooling
may be achieved by any of the following methods:
8.46 D' D 350C C' C C''
P
(bar)
1.00 -300C
A' A B''
B
59.05 175.81 192.60 212.70 235.60
h(kj/kg)
Fig.6.5.4(a). Subcooling and superheating of refrigerant
(i) By passing the liquid refrigerant from condenser through a heat exchanger
through which the cold vapor at suction from the evaporator is allowed to flow
in the reversed direction. This process subcools the liquid but superheats the
vapor. Thus, COP is not improved though refrigeration effect is increased.
(ii) By making use of enough quantity of cooling water so that the liquid
refrigerant is further cooled below the temperature of saturation. In some
cases, a separate subcooler is also made use of for this purpose. In this case,
COP is improved.
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
(b) Superheating of Vapor:
If the vapor at the compressor entry is in the superheated state B”, which is produced
due to higher heat absorption in the evaporator, then the refrigerating effect is increased
as (h”B - hA) > (hB – hA). However, COP may increase, decrease or remain unchanged
depending upon the range of pressure of the cycle.
(c) Change in suction pressure (PS):
p'd D' 500C C"
12.10
pd D 350C L C C'
8.46
P
(bar)
M
ps A A" -50C B
2.60
p's A' -300c B'
1.00
69.63 85.37 187.91 209.25 219.59
h(kj/kg)
Fig.6.5.4(c). Effect of change in evaporator and condenser pressure
Let the suction pressure or the evaporating pressure in a simple refrigeration cycle be
reduced from PS to P’S. It will be clear from the figure that:
The refrigerating effect is reduced to: (h ′B - h ′A ) < (h B - h A )
The work of compression is increased to: (h C′′ - h ′′B ) > (h C - h B )
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Hence, the decrease in suction pressure decreases the refrigeration effect and at the
same time increases the work of compression. But, both the effects tend to decrease
the COP.
(d) Change in discharge pressure (Pd):
In Fig.6.5.4(c), let us assume that the pressure at the discharge or the condensing
pressure is increased from Pd to P’d. It will have effects as follows:
′′ - h B ) > (h C - h B )
The compressor work requirement is increased to: (h C
The refrigerating effect is reduced to: (h B - h ′′A ) < (h B - h A )
Therefore, the increase in discharge pressure results in lower COP. Hence, the
discharge pressure should be kept as low as possible depending upon the temperature
of the cooling medium available.
(e) Effect of Volumetric Efficiency of Compressor:
C' DISCHARGE C
pd
SUCTION
PS B
B" B'
VC V2 V V1
Fig.6.5.4(e). Effect of volumetric efficiency
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
The factors like clearance volume, pressure drop through discharge and suction values,
leakage of vapor along the piston and superheating of cold vapor due to contact with
hot cylinder walls, affects the volume of the vapor actually pumped by the compressor.
The volumetric efficiency of a compressor is defined as;
Actual mass of vapor drawn at suction conditions
ηvol =
Theoritical mass that can be filled in the displacement volume
Figure 6.5.4(e) represents the p-v diagram of a compressor. Now, during suction stroke
B”–B, the vapor filled in clearance space at pressure Pd expands along C’-B’ and the
suction valve opens only when the pressure has dropped down to pS. Therefore, the
actual amount of vapor sucked during the suction stroke is (v1 - v2) while the stroke
volume is (v1 - vc). Volumetric efficiency decreases the refrigeration effect.
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
6.6 Analysis of Simple Vapor Compression
Refrigeration Cycle:
(a) Mass of Refrigerant in Circulation:
Refrigeration effect = (hB – hA) kJ/kg of refrigerant
3.5 * 60
Or, mass of refrigerant in circulation, m r = kg / min- ton
(h B - h A )
(b) Piston Displacement:
Let the specific volume of the vapor at B i.e at suction of the compressor be, vB and let
the volumetric efficiency of the compressor be ηvol , then piston displacement required
per min.
vB mr
Piston displacement = (m3 / min- ton)
ηvol
(c) Power Required by Compressor:
(i) If the compression is isentropic, then,
Work of compression = (hC – hB) kJ/kg
m r (h C - h B )
Hence, Power required = (kw / ton)
60
(ii) If the compression is polytropic (Pvn = C).
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
n
Work of compression = (pC vC - p B v B ) (N - m / kg)
n -1
n (pC vC - p B v B )
Or Power required = m r * * (kW/ton)
n-1 60 * 1000
(d) Heat Rejected to Cylinder Jacket:
⎧ n
Q jacket = m r ⎨ ( pC vC - pB vB ) - ( h C - h B )⎫⎬ (kJ / min- ton)
⎩ n -1 ⎭
(e) Heat Rejected in Condenser:
Heat rejected in condenser = (h C - h D ) (kJ / kg)
Total heat rejected = m r (h C - h D ) (kJ / min- ton)
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
4.6 Limited Pressure Cycle (or Dual Cycle):
This cycle is also called as the dual cycle, which is shown in Fig.4.6. Here the heat
addition occurs partly at constant volume and partly at constant pressure. This cycle is a
closer approximation to the behavior of the actual Otto and Diesel engines because in
the actual engines, the combustion process does not occur exactly at constant volume
or at constant pressure but rather as in the dual cycle.
Process 1-2: Reversible adiabatic compression.
Process 2-3: Constant volume heat addition.
Process 3-4: Constant pressure heat addition.
Process 4-5: Reversible adiabatic expansion.
Process 5-1: Constant volume heat rejection.
3 4
Volume
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Constant Volume
Constant Pressure
4
2
5
Entropy
Fig.4.6. Dual cycle on p-v and T-s diagrams
Air Standard Efficiency:
Heat sup plied = m C v ( T3 - T2 ) + m Cp ( T4 - T3 )
Heat rejected = m C v ( T5 - T1 )
Net work done = m C v ( T3 - T2 ) + m Cp ( T4 - T3 ) - m CV ( T5 - T1 )
m C v ( T3 - T2 ) + m Cp ( T4 - T3 ) - m C v ( T5 - T1 )
ηth =
m C v ( T3 - T2 ) + m Cp ( T4 - T3 )
T5 - T1
ηth = 1 -
( T3 - T2 ) + γ ( T4 - T3 )
P3 v v
Let, = rp ; 4 = rc ; 1 = r
P2 v3 v2
T2 = T1 r γ - 1
T3 = T2 rp = T1 r γ - 1 rp
T4 = T3 rc = T1 r γ - 1 rp rc
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
γ -1 γ -1 γ -1
T5 ⎛v ⎞ ⎛v v ⎞ ⎛r ⎞
=⎜ 4⎟ = ⎜ 4. 2⎟ =⎜ c⎟
T4 ⎝ v5 ⎠ ⎝ v 2 v5 ⎠ ⎝r⎠
γ -1
⎛r ⎞
T5 = T4 ⎜ c ⎟ = T1 rp rcγ
⎝r⎠
T1 rp rcγ - T1
ηth = 1 -
{( T r
1
γ -1
) (
rp - T1 r γ - 1 + γ T1 r γ - 1 rp rc - T1 r γ - 1 rp )}
= 1-
( rp rcγ - 1)
{( rp r γ - 1 - r γ - 1 ) + γ ( rp rc r γ - 1 - rp r γ - 1 )}
1 ⎧⎪ rp rcγ - 1 ⎫⎪
ηth 1 - γ -1 ⎨ ⎬
r ( )
⎪⎩ rp - 1 + γrp ( rc - 1) ⎭⎪
From the above equation, it is observed that, a value of rp > 1 results in an increased
efficiency for a given value of rc and γ. Thus the efficiency of the dual cycle lies between
that of the Otto cycle and the Diesel cycle having the same compression ratio.
Mean Effective Pressure:
Workdone
mep =
Displacement volume
m C v ( T3 - T2 ) + m Cp ( T4 - T3 ) - m C v ( T5 - T1 )
=
v1 - v 2
m C v ( γ - 1) T1 ⎛ r - 1 ⎞
v1 - v 2 = ⎜ ⎟
p1 ⎝ r ⎠
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
p1 r ⎧⎪ T3 - T2 γ ( T4 - T3 ) T5 - T1 ⎫⎪
mep = ⎨ + - ⎬
( r -1)( γ - 1) ⎪⎩ T1 T1 T1 ⎭⎪
=
p1 r
( )( )
r - 1 γ - 1
{ ( ) (
r γ - 1 rp - 1 + γ r γ - 1 rp ( rc - 1) - rp rcγ - 1 )}
=
p1 r
( )( )
r - 1 γ - 1
{ {( ) } (
r γ - 1 rp - 1 + γ rp ( rc - 1) - rp rcγ - 1 )}
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
5.7 Open Feed Water Heater:
5
BOILER
1 kg
TURBINE
(1-m1) kg
6
7
4 m1 kg
CONDENSER
HEATER
3
2
PUMP (1-m1) kg
PUMP
1
(a)
1 kg
5
4 m1 kg
T
3 6
2 (1-m1) kg
1 7
s
(b)
Fig.5.7. Open feed water heater cycle
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
A regenerative cycle having a single stage of feed water heating is shown above. Steam
enters the turbine at state 5. After expansion to state 6, part of this steam is extracted
and supplied to the feed water heater while the remainder continues to expand to state
7. Other processes are as shown above. The above T-s diagram is not the exact one,
(because the mass flow rate is changing at all the state points) but, it simply shows
various states of the working fluid.
Let m1 = mass of steam extracted at state 6 then, heat balance for heater gives,
m1h 6 + (1 - m1 ) h 2 = h 3
m1h 6 + h 2 - m1h 2 = h 3
m1 (h 6 - h 2 ) = (h 3 - h 2 )
(h 3 - h 2 )
m1 =
(h 6 - h 2 )
if, h 2 ≈ h1
(h 3 - h1 )
m1 =
(h 6 - h1 )
The amount is so adjusted that the liquid leaving the feed water heater at state 3 is
saturated.
Thermal Efficiency:
Turbine work = (h 5 - h 6 ) + (1 - m1 )(h 6 - h 7 )
Heat supplied = (h 5 - h 4 ) ≈ (h 5 - h 3 )
(h 5 - h 6 ) + (1 - m1 )(h 6 - h 7 )
Therefore, ηth =
(h 5 - h 3 )
(h 5 - h 7 ) - m1 (h 6 - h 7 )
=
(h 5 - h 3 )
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
5.8 Closed Feed Water Heater:
TURBINE
5 (1-m1)
I II
1 kg
6 m1 kg 7
BOILER PUMP
3
CONDE
4
NSER
x 2
m1
3 1
DRAINCOOLER 2
1 kg
PUMP
(a)
1 kg 5
4 m1 kg
3
T 6
2 (1-m1) kg
1 7
s
(b)
Fig.5.8. Closed feed water heater
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Regeneration here is single stage, while turbine is of two stages. The extracted steam
of mass m1 kg is completely condensed in the heater and this liquid is first passed
through a drain cooler and then enters the condenser where it mixes with the main
condensate of mass (1-m1) kg. This liquid from the condenser is first heated from state
2 to state x in the drain cooler and then from state x to state 3 in heater. If we assume
perfect heat exchange in water heater, then the feed water as well as the condensate of
the extracted steam will leave the feed water heater at state 3. Similarly in the drain
cooler, the liquid coming from heater will get cooled to the temperature t2 of the
condensate from the pump.
Let, m1 = mass of extracted steam per kg steam supplied to the turbine.
Heat balance for drain cooler gives,
m1 (h 3 - h 2 ) = 1(h x - h 2 )
h x = h 2 + m1 (h 3 - h 2 )
Heat balance for feed heater gives,
m1 (h 6 - h 3 ) = (h 3 - h x ) = h 3 - h 2 - m1 (h 3 - h 2 )
m1 {(h 6 - h 3 ) + (h 3 - h 2 )} = (h 3 - h 2 )
h3 - h 2
m1 =
h6 - h2
Since, h 2 ≈ h1
h 3 - h1
m1 =
h 6 - h1
(h 5 - h 7 ) - m1 (h 6 - h 7 )
ηth = (neglecting pump work)
(h 5 - h 3 )
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
4.7 Comparison of Otto, Diesel and Dual Cycles:
The important variable factors which are used as the basis for comparison of the cycles
are compression ratio, peak pressure, heat addition, heat rejection and the net work. In
order to compare the performance of the Otto, Diesel and Dual combustion cycles,
some of the variable factors must be fixed. In this section, a comparison of these three
cycles is made for the same compression ratio, same heat addition, constant maximum
pressure and temperature, same heat rejection and net work output. This analysis will
show which cycle is more efficient for a given set of operating conditions.
Case 1: Same Compression Ratio and Heat Addition:
The Otto cycle 1-2-3-4-1, the Diesel cycle 1-2-3'-4'-1 and the Dual cycle 1-2-2”-3”-4”-1
are shown in p-V and T-θ diagram in Fig.4.7.1 (a) and (b) respectively for the same
compression ratio and heat input.
2' 3"
2 3'
4'
4"
4
1
Isentropic Process
Volume
(a)
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Constant Pressure 3
2' 3"
2 3'
4'
4 4"
1
constant Volume
5 Entropy 6 6"6'
(b)
Fig.4.7.1. Same compression ratio and heat addition
From the T-s diagram, it can be seen that Area 5-2-3-6 = Area 5-2-3'-6’ = Area 5-2-2"-
3"-6" as this area represents the heat input which is the same for all cycles. All the
cycles start from the same initial state point 1 and the air is compressed from state 1 to
2 as the compression ratio is same. It is seen from the T-s diagram for the same heat
input, the heat rejection in Otto cycle (area 5-1-4-6) is minimum and heat rejection in
Diesel cycle (5-1-4'-6') is maximum.. Consequently, Otto cycle has the highest work
output and efficiency. Diesel cycle has the least efficiency and Dual cycle having the
efficiency between the two.
One more observation can be made i.e., Otto cycle allows the working medium to
expand more whereas Diesel cycle is least in this respect. The reason is heat is added
before expansion in the case of Otto cycle and the last portion of heat supplied to the
fluid has a relatively short expansion in case of the Diesel cycle.
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Case 2: Same Compression Ratio and Heat Rejection:
2 3'
Isentropic Process 1
Volume
(a)
3'
2
4
1
Entropy
(b)
Fig.4.7.2. Same compression ratio and heat rejection
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Efficiency of Otto cycle is given by [Figs.4.7.2 (a) and (b)],
QR
ηotto = 1 -
QS
Where, Qs is the heat supplied in the Otto cycle and is equal to the area under the curve
2-3 on the T-s diagram [Fig.4.7.2 (b)]. The efficiency of the Diesel cycle is given by,
QR
ηDiesel = 1 -
Qs′
Where Q’s is heat supplied in the Diesel cycle and is equal to the area under the curve
2-3' on the T-s diagram [Fig.4.7.2. (b)]. From the T-s diagram in Fig.4.7.2, it is clear that
Qs > Q’s i.e., heat supplied in the Otto cycle is more than that of the Diesel cycle. Hence,
it is evident that, the efficiency of the Otto cycle is greater than the efficiency of the
Diesel cycle for a given compression ratio and heat rejection.
Case 3: Same Peak Pressure, Peak Temperature and Heat Rejection:
Figures 4.7.3 (a) and (b) show the Otto cycle 1-2-3-4 and Diesel cycle 1-2'-3-4 on p-V
and T-s coordinates, where the peak pressure and temperature and the amount of heat
rejected are the same.
The efficiency of the Otto cycle,
QR
ηotto = 1 -
QS
Where, Qs in the area under the curve 2-3 in Fig.4.7.3 (b). The efficiency of the Diesel cycle, 1-
2-3'-3-4 is,
QR
ηDiesel = 1 -
Qs′
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
2' 3
2
4
1
Volume
(a)
2'
2 4
5 Entropy 6
(b)
Fig.4.7.3. Same peak pressure and temperature
It is evident from Fig.4.7.3 that Qs > Q’s. Therefore, the Diesel cycle efficiency is greater
than the Otto cycle efficiency when both engines are built to withstand the same thermal
and mechanical stresses.
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Case 4: Same Maximum Pressure and Heat Input:
2' 3' 3
2 4
4'
1
Volume
(a)
3
3'
2'
2 4
4'
1
5 Entropy 6' 6
(b)
Fig.4.7.4. Same maximum pressure and heat input.
For same maximum pressure and heat input, the Otto cycle (1-2-3-4-1) and Diesel cycle
(1-2'-3'-4'-1) are shown on p-V and T-s diagrams in Fig.4.7.4 (a) and (b) respectively. It
is evident from the figure that the heat rejection for Otto cycle (area 1-5-6-4 on T-s
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
diagram) is more than the heat rejected in Diesel cycle (1-5-6'-4'). Hence Diesel cycle is
more efficient than Otto cycle for the condition of same maximum pressure and heat
input. One can make a note that with these conditions, the Diesel cycle has higher
compression ratio than that of Otto cycle. One should also note that the cycle which is
having higher efficiency allows maximum expansion. The Dual cycle efficiency will be
between these two.
Case 5: Same Maximum Pressure and Work Output:
The efficiency, η can be written as
Work done Work done
η = =
Heat sup plied Work done + Heat rejected
Refer to T-s diagram in Fig.4.7.4 (b). For same work output the area 1-2-3-4 (work
output of Otto cycle) and area 1-2'-3'-4' (work output of Diesel cycle) are same. To
achieve this, the entropy at 3 should be greater than entropy at 3' .It is clear that the
heat rejection for Otto cycle is more than that of diesel cycle. Hence, for these
conditions, the Diesel cycle is more efficient than the Otto cycle. The efficiency of Dual
cycle lies between the two cycles.
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
6.7 Vapor Absorption Refrigeration System:
Weak
solution
NH3
Strong
solution Absorber
10
NH3
generator qa(Ta=T )
qn(Th)
5
9 6 4
7 8
Pump
T-valve Heat exchager q0(T0)
qP Evaporator
Rectifier
qc(Tc=T )
1 2 3
Condenser
T-valve
Fig.6.7. Vapor absorption refrigeration system
Some liquids like water have great affinity for absorbing large quantities of certain
vapors (NH3) and reduce the total volume greatly. The absorption refrigeration system
differs fundamentally from vapor compression system only in the method of
compressing the refrigerant. An absorber, generator and pump in the absorption
refrigerating system replace the compressor of a vapor compression system.
Figure 6.7 shows the schematic diagram of a vapor absorption system. Ammonia vapor
is produced in the generator at high pressure from the strong solution of NH3 by an
external heating source. The water vapor carried with ammonia is removed in the
rectifier and only the dehydrated ammonia gas enters into the condenser. High pressure
NH3 vapor is condensed in the condenser. The cooled NH3 solution is passed through a
throttle valve and the pressure and temperature of the refrigerant are reduced below the
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
temperature to be maintained in the evaporator. The low temperature refrigerant enters
the evaporator and absorbs the required heat from the evaporator and leaves the
evaporator as saturated vapor. Slightly superheated, low pressure NH3 vapor is
absorbed by the weak solution of NH3 which is sprayed in the absorber as shown in
Fig.6.7.
Weak NH3 solution (aqua–ammonia) entering the absorber becomes strong solution
after absorbing NH3 vapor and then it is pumped to the generator through the heat
exchanger. The pump increases the pressure of the strong solution to generator
pressure. The strong NH3 solution coming from the absorber absorbs heat form high
temperature weak NH3 solution in the heat exchanger. The solution in the generator
becomes weak as NH3 vapor comes out of it. The weak high temperature ammonia
solution from the generator is passed to the heat exchanger through the throttle valve.
The pressure of the liquid is reduced to the absorber pressure by the throttle valve.
Comparison between Vapor Compression and Absorption system:
Absorption system Compression System
a) Uses low grade energy like heat. a) Using high-grade energy like
Therefore, may be worked on mechanical work.
exhaust systems from I.C engines,
etc.
b) Moving parts are only in the pump, b) Moving parts are in the compressor.
which is a small element of the Therefore, more wear, tear and noise.
system. Hence operation is smooth.
c) The system can work on lower c) The COP decreases considerably with
evaporator pressures also without decrease in evaporator pressure.
affecting the COP.
d) No effect of reducing the load on d) Performance is adversely affected at
performance. partial loads.
e) Liquid traces of refrigerant present in e) Liquid traces in suction line may
piping at the exit of evaporator damage the compressor.
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
constitute no danger.
f) Automatic operation for controlling f) It is difficult.
the capacity is easy.
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
4.8. Atkinson Cycle:
Atkinson cycle is an ideal cycle for Otto engine exhausting to a gas turbine. In this cycle
the isentropic expansion (3-4) of an Otto cycle (1-2-3-4) is further allowed to proceed to
the lowest cycle pressure so as to increase the work output. With this modification the
cycle is known as Atkinson cycle. The cycle is shown on p-v and T-s diagrams in
Fig.4.8. Processes involved are:
Process 1-2: Reversible adiabatic compression (v1 to v2).
Process 2-3: Constant volume heat addition.
Process 3-4: Reversible adiabatic expansion (v3 to v4).
Process 4-1: Constant pressure heat rejection.
2
4'
1 4
Volume
(a)
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
4'
2
4
Entropy
(b)
Fig.4.8. Atkinson cycle on p-v and T-s diagrams
Thermal Efficiency:
Heat supplied = C v ( T3 - T2 )
Heat rejected = Cp ( T4 - T1 )
Net workdone = C v ( T3 - T2 ) - Cp ( T4 - T1 )
C v ( T3 - T2 ) - Cp ( T4 - T1 )
ηth =
C v ( T3 - T2 )
γ ( T4 - T1 )
= 1-
( T3 - T2 )
v1
Let, r = = CR
v2
T2 = T1 r γ - 1
T3 P
= 3 = rp = Pressure ratio
T2 P2
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
T3 = T2 rp = T1 r γ - 1 rp
γ -1 γ -1 γ -1 γ -1 γ -1
T3 ⎛P ⎞ γ ⎛P ⎞ γ ⎛P P ⎞ γ ⎛ P ⎞ γ
=⎜ 3⎟ = ⎜ 3⎟ = ⎜ 3. 2⎟ = ⎜ rp . 2 ⎟ = rp γ . r γ−1
T4 ⎝ P4 ⎠ ⎝ P1 ⎠ ⎝ P2 P1 ⎠ ⎝ P1 ⎠
since,
γ
P2 ⎛v ⎞
= ⎜ 1 ⎟ = rγ
P1 ⎝ v2 ⎠
and
T1 rp r γ - 1
1
T3
T4 = γ -1
= γ -1
= T1 rpγ
rp γ rγ - 1 rp γ rγ - 1
⎡ 1 ⎤
⎢ rγ - 1 ⎥
=1-γ ⎢ ⎥
p
ηth
(
⎢ rp - 1 r γ - 1 ⎥
⎢ ⎥
)
⎣ ⎦
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
5.9 Multi–Stage Regenerative Cycles:
TURBINE
10
I II III IV
(1-m1-m2-m3)
BOILER 11 m1 12 m2 13 m3 14
PUMP 7 5 3
8 6 4
9
7 5
(m1+m2) x 2 1
m1
(m1+m2+m3) 3
PUMP
2
Fig.5.9(a). Three stage regenerative cycle
1 kg
9 10
8 m1
(1-m1)
7 11
T 6 m1+m2
(1-m1-m2)
5 12
4 (m1+m2+m3) (1-m1-m2-m3)
3 13
2
1 (1-m1-m2-m3)
kg 1 14
Fig.5.9(b). T-s diagram
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Above figure shows an arrangement in which there are 3 stages of feed water heating
employing closed heaters. Steam to the 1st, 2nd and 3rd heaters is supplied at states 11,
12 and 13 respectively. The feed water leaving each heater is at the saturation
temperature corresponding to the pressure of bled steam supplied to that heater. The
corresponding T-s diagram for the cycle is shown above.
Considering one kg of steam leaving the boiler and entering the turbine at state 10.
Let, m1 = mass of steam supplied to 1st heater.
m2 = mass of steam supplied to 2nd heater.
m3 = mass of steam supplied to 3rd heater.
Heat balance for 1st heater gives,
m1 (h11 - h 7 ) = (h 7 - h 6 ) ≈ (h 7 - h 5 )
(h 7 - h 5 )
m1 =
(h11 - h 7 )
Heat balance for 2nd heater gives,
h 4 + m 2 h12 + m1h 7 = h 5 + h 5 (m1 + m 2 )
m 2 (h12 - h 5 ) = (h 5 - h 4 ) + m1 (h 5 - h 7 )
(h 5 - h 3 ) - m1 (h 7 - h 5 )
m2 = ; Q (h 4 ≈ h 3 )
(h12 - h 5 )
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Also, heat balance for 3rd heater + drain cooler, gives,
m3 h13 + (m1 + m 2 )h 5 + h 2 = (m1 + m 2 + m3 ) h 2 + h 3
m3 h13 + (m1 + m 2 )h 5 + (1 - m1 - m 2 - m3 )h 2 = h 3
m3 (h13 - h 2 ) = (h 3 - h 2 ) - (m1 + m 2 )(h 5 - h 2 )
m3 (h13 - h1 ) ≈ (h 3 - h1 ) - (m1 + m 2 )(h 5 - h1 )
(h 3 - h1 ) - (m1 + m 2 )(h 5 - h1 )
m3 =
(h13 - h1 )
⎧⎪( h10 - h11 ) + (1 - m1 ) (h11 - h12 ) + (1 - m1 - m 2 )(h12 - h13 ) ⎫⎪
Turbine work = ⎨ ⎬
⎩⎪+ (1 - m1 - m 2 - m3 )(h13 - h14 ) ⎭⎪
= (h10 - h14 ) - m1 (h11 - h14 ) - m 2 (h12 - h14 ) - m3 (h13 - h14 )
Heat sup plied = (h10 - h 7 )
work done
η =
Heat sup plied
⎪⎧( h10 - h11 ) + (1 - m1 ) (h11 - h12 ) + (1 - m1 - m 2 )(h12 - h13 ) ⎪⎫
⎨ ⎬
⎪+ (1 - m1 - m 2 - m3 )(h13 - h14 )
⎩ ⎭⎪
η =
(h10 - h 7 )
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
6.8 Steam Jet Refrigeration System:
Control valve
Steam nozzle
Thermocompresser
Steam
ejector
boiler
Water returned
A.C-plant
Vapor
Spray Condenser
Flash chamber
Pump
Cold water to
A.C-plant
Pump Make-up-water
Fig.6.8. Steam jet refrigeration system
This system uses the principle of boiling the water below 1000C. If the pressure on the
surface of the water is reduced below atmospheric pressure, water can be made boil at
low temperatures. Water boils at 60C, when the pressure on the surface is 5 cm of Hg
and at 100C, when the pressure is 6.5 cms of Hg. The very low pressure or high
vacuum on the surface of the water can be maintained by throttling the steam through
jets or nozzles. The general arrangement of the system is shown in the Fig.6.8.
Consider a flash chamber contains 100 kg of water. If suddenly 1 kg of water is
removed by boiling, as pressure is reduced due to throttling of steam through nozzles.
Approximately 2385 kJ of heat will be removed from the water, which is equivalent to
heat of evaporation of water. The fall in temperature of the remaining water will be,
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Q = m Cp dT
2385
dT = = 5.70 C
99 * 4.187
Evaporating one more kg of water reduces the remaining water temperature by 5.70C
further. Thus by continuing this process, the remaining water can be made to freeze.
Water is the refrigerant used in the steam jet refrigeration system. As water freezes at
00C, then either refrigeration has to be stopped or some device is required to pump the
ice.
Operation:
High pressure steam is supplied to the nozzle from the boiler and it is expanded. Here,
the water vapor originated from the flash chamber is entrained with the high velocity
steam jet and it is further compressed in the thermo compressor. The kinetic energy of
the mixture is converted into static pressure and mass is discharged to the condenser.
The condensate is usually returned to the boiler. Generally, 1% evaporation of water in
the flash chamber is sufficient to decrease the temperature of chilled water to 60C. The
chilled water in the flash chamber is circulated by a pump to the point of application.
The warm water from the load is returned to the flash chamber. The water is sprayed
through the nozzles to provide maximum surface area for cooling. The water, which is
splashed in the chamber and any loss of cold water at the application, must be replaced
by makeup water added to the cold water circulating system.
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Advantages:
a) It is flexible in operation; cooling capacity can be easily and quickly changed.
b) It has no moving parts as such it is vibration free.
c) It can be installed out of doors.
d) The weight of the system per ton of refrigerating capacity is less.
e) The system is very reliable and maintenance cost is less.
f) The system is particularly adapted to the processing of cold water used in
rubber mills,, distilleries, paper mills, food processing plants, etc.
g) This system is particularly used in air-conditioning installations, because of
the complete safety of water as refrigerant and ability to adjust quickly to load
variations and no hazard from the leakage of the refrigerant.
Disadvantages:
a) The use of direct evaporation to produce chilled water is usually limited as
tremendous volume of vapor is to be handled.
b) About twice as much heat must be removed in the condenser of steam jet per
ton of refrigeration compared with the vapor compression system.
c) The system is useful for comfort air-conditioning, but it is not practically
feasible for water temperature below 40C.
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
5.10 Binary Vapor Power Cycle:
Thermal efficiency of Rankine cycle can be increased by:
1) Increasing the average temperature of heat addition.
2) Decreasing the average temperature of heat rejection.
Maximum temperature of the cycle is limited by practical considerations. For steam as a
working fluid, the following difficulties arise at maximum temperature.
1) Critical temperature of steam is equal to 3740C and critical pressure is 221.2
bar. It is not possible to work at this pressure.
2) Latent heat of vaporization decreases as the pressure increases.
3) If high pressure steam is expanded, high degree of moisture content will be
present at the end of process.
The minimum temperature of the cycle is usually limited to natural water temperature of
250C. At this temperature, the saturation pressure of water will be 0.0318 bar. It means
that the condenser has to work at vacuum. This is very difficult. So, ideal working fluid
for Rankine cycle should fulfill the following requirements.
1) Reasonable saturation pressure at maximum temperature.
2) Steep saturated vapor line to minimize moisture problem.
3) Saturation pressure higher than atmospheric at minimum temperature.
4) Low liquid specific heat so that most of the heat is added at maximum
temperature.
5) Non-toxic and non-corrosive.
All the above requirements are not met by any single working fluid. In binary cycle two
working fluids are used in order to obtain good results. Mercury and steam are most
commonly used working fluids. Saturation pressure and saturation temperature of
mercury is 20.6 bar and 5400C at critical point.
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
4.9 Lenoir Cycle:
1 3
Volume
(a)
Entropy
(b)
Fig.4.9. Lenoir cycle on p-v and T-s diagrams
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
The Lenoir cycle consists of the following processes:
• Process 1-2: Constant volume heat addition
• Process 2-3: Reversible adiabatic expansion
• Process 3-4: Constant pressure heat rejection.
• No compression process.
The thermal efficiency can be derived as follows:
Heat added - Heat rejected C v ( T2 - T1 ) - Cp ( T3 - T1 )
ηth = =
Heat added C v ( T2 - T1 )
⎛T -T ⎞
ηth = 1 - γ ⎜ 3 1 ⎟
⎝ T2 - T1 ⎠
P2
Let, = rp = Pressure ratio
P1
T2 = rp T1
γ -1 γ -1 γ -1
1
⎛P ⎞ ⎛P ⎞ ⎛ 1 ⎞
( rp )
T3 γ γ γ -1
=⎜ 3⎟ =⎜ 1⎟ =⎜ ⎟ = γ
T2 ⎝ P2 ⎠ ⎝ P2 ⎠ ⎝ α1 ⎠
1 1 1
( ) ( ) ( )
-1 -1
T3 = T2 rp γ = T1 rp rp γ = T1 rp γ
⎛ 1 ⎞
γ rp - 1⎟
⎜ γ
⎜ ⎟
ηth =1- ⎝ ⎠
(
rp - 1 )
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
6.9 Vortex Tube (Non-Conventional):
Compressed air
Cold Hot tube
Air Hot
air
Chamber
Diaphragm Valve
Nozzle
Fig.6.9. Vortex tube
It is one of the non-conventional type refrigerating systems for the production of
refrigeration. The schematic diagram of vortex tube is shown in the Fig.6.9. It consists of
nozzle, diaphragm, valve, hot-air side, cold-air side. The nozzles are of converging or
diverging or converging-diverging type as per the design. An efficient nozzle is designed
to have higher velocity, greater mass flow and minimum inlet losses. Chamber is a
portion of nozzle and facilities the tangential entry of high velocity air-stream into hot
side. Generally the chambers are not of circular form, but they are gradually converted
into spiral form. Hot side is cylindrical in cross section and is of different lengths as per
design. Valve obstructs the flow of air through hot side and it also controls the quantity
of hot air through vortex tube. Diaphragm is a cylindrical piece of small thickness and
having a small hole of specific diameter at the center. Air stream traveling through the
core of the hot side is emitted through the diaphragm hole. Cold side is a cylindrical
portion through which cold air is passed.
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Working:
Compressed air is passed through the nozzle as shown in Fig.6.9. Here, air expands
and acquires high velocity due to particular shape of the nozzle. A vortex flow is created
in the chamber and air travels in spiral like motion along the periphery of the hot side.
This flow is restricted by the valve. When the pressure of the air near valve is made
more than outside by partly closing the valve, a reversed axial flow through the core of
the hot side starts from high-pressure region to low-pressure region. During this
process, heat transfer takes place between reversed stream and forward stream.
Therefore, air stream through the core gets cooled below the inlet temperature of the air
in the vortex tube, while air stream in forward direction gets heated up. The cold stream
is escaped through the diaphragm hole into the cold side, while hot stream is passed
through the opening of the valve. By controlling the opening of the valve, the quantity of
the cold air and its temperature can be varied.
Advantages:
1) It uses air as refrigerant, so there is no leakage problem.
2) Vortex tube is simple in design and it avoids control systems.
3) There are no moving parts in vortex tube.
4) It is light in weight and requires less space.
5) Initial cost is low and its working expenses are also less, where compressed
air is readily available.
6) Maintenance is simple and no skilled labours are required.
Disadvantages:
Its low COP, limited capacity and only small portion of the compressed air appearing as
the cold air limits its wide use in practice.
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Applications:
1) Vortex tubes are extremely small and as it produce hot as well as cold air. It
may be of use in industries where both are simultaneously required.
2) Temperature as low as –500C can be obtained without any difficulty, so it is
very much useful in industries for spot cooling of electronic components.
3) It is commonly used for body cooling of the workers in mines.
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
4.10 Brayton Cycle (Simple Gas Turbine Cycle):
2 3
4'
1 4
Volume
(a)
4'
2 4
Entropy
(b)
Fig.4.10. Brayton cycle on p-v and T-s diagram
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
The Brayton cycle is a theoretical cycle for simple gas turbine. This cycle consists of two
isentropic and two constant pressure processes. Figure.4.10 shows the Brayton cycle
on p-v and T-s coordinates. The cycle is similar to the Diesel cycle in compression and
heat addition. The isentropic expansion of the Diesel cycle is further extended followed
by constant pressure heat rejection. The thermal efficiency is given by,
Heat added - Heat rejected
ηth =
Heat added
mCp ( T3 - T2 ) - mCp ( T4 - T1 ) T4 - T1
ηth = =1-
mCp ( T3 - T2 ) T3 - T2
For isentropic processes, we have,
γ -1 γ -1
T2 ⎛p ⎞ γ T3 ⎛p ⎞ γ
= ⎜ 2⎟ and = ⎜ 3⎟
T1 ⎝ p1 ⎠ T4 ⎝ p4 ⎠
But, p2 = p3 and p1 = p4, thus,
T2 T
= 3
T1 T4
and we can write,
T4 T
ηth = 1 - =1- 1
T3 T2
T4 T v 1
= 1 = 2 = γ -1
T3 T2 v1 r
1 ⎫γ - 1
γ -1⎧ γ -1
⎛v ⎞ ⎪⎛ p 2 ⎞ γ ⎪
1
rγ - 1
=⎜ 2⎟ ⎨⎜ ⎟ ⎬ ( )
= rp γ
⎝ v1 ⎠ ⎪⎝ p1 ⎠ ⎪
⎩ ⎭
1
ηth = 1 - γ -1
rp γ
Indian Institute of Technology Madras
Gas Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Deviation of Actual Cycles from Air Standard Cycles:
The actual Otto and Diesel engines show marked deviations form the air-standard
cycles described above. Above fig shows p-v-diagram for a high-speed diesel engine
would be very similar in appearance. The main differences between the actual and
theoretical cycles are as follows.
(a) Compression and expansion are not friction less adiabatic processes. A Certain amount
of friction is always present and there is considerable heat transfer between the gases
and cylinder wall.
(b) Combustion does not occur either at constant volume or at constant pressure.
(c) The thermodynamics properties of the gases after combustion are different than those of
the fuel-air mixture before combustion..
(d) The combustion may be incomplete.
(e) The specific heats of the working fluid are not constant but increases with temperature.
(f) The cylinder pressure during exhaust process is higher than the atmosphere. As a
result, more work has to be done by the piston on the gases to expel them out of the
cylinder, than work done by the gases on the piston during the intake stroke. This
difference in work, called pumping work, is represented by the pumping loop shown by
hatched area. Note that this work is negative and represents loss of work called pumping
loss.
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
5.11 Mercury-Steam Binary Vapor Cycle:
STEAM SUPER HEATER
AND MERCURY BOILER 4
3 STEAM
TURBINE
C MERCURY 5
TURBINE
D CONDE
NSER
A 1
B 2
PUMP PUMP
(a)
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
T
4
B
A D
3
2
1 5
s
(b)
Fig.5.11. Binary vapour power cycle
Above figure shows the schematic diagram of a mercury-steam binary cycle. The
corresponding T-s diagram is also shown. There are two distinct circuits, one for
mercury and the other for steam. Saturated mercury vapor from the mercury boiler at
state C enters the mercury turbine, expands to state D, and is condensed at state A.
The condensate is pumped back to the boiler by the mercury pump.
The heat rejected in the mercury condenser is used to vaporize water into steam at
state 3. Thus, the mercury condenser also acts as the steam boiler. Note that there is a
considerable temperature differential between condensing mercury and boiling water.
Saturated steam is then superheated to state 4 as shown, expanded in the steam
turbine to state 5 and then condensed. The mercury cycle is represented by A-B-C-D-A
and the steam cycle by 1-2-3-4-5-1 on the T-s diagram.
Indian Institute of Technology Madras
Vapour Power Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
Let x = mass of mercury per kg of steam.
Then,
x ( h D - h A ) = 1(h 3 - h 2 ) ≈ (h 3 - h1 )
(h 3 - h1 )
x =
(h D - h A )
⎧Hg turbine work + steam turbine work ⎫
Net work done = ⎨ ⎬
⎩ + Hg pump work + steam pump work ⎭
w net = x(h C - h D ) + x(h A - h B ) + (h 4 - h 5 ) + (h1 - h 2 )
Neglecting pump work:
w net = x(h C - h D ) + (h 4 - h 5 )
Heat supplied per kg of steam = x(h C - h B ) + (h 4 - h 3 ) ≈ x(h C - h A ) + (h 4 - h 3 )
⎧ x(h C - h D ) + (h 4 - h 5 ) ⎫
ηth = ⎨ ⎬
⎩ x(h C - h 4 ) + (h 4 - h 3 ) ⎭
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
6.10 Multi-Stage Refrigeration System:
When number of applications at same temperatures is to be taken up by the
refrigerating plant, multi-stage refrigeration systems are generally used.
Assuming T1, T2, and T3 are the refrigerating loads on evaporator E1, E2 and E3 as
shown in Fig.6.10, then the refrigerant flowing through E1, E2 and E3 are given by,
3.5 * T1 * 60
M1 = (kg/min)
H 2 - H1
3.5 * T2 * 60
M2 = (kg/min)
H 2 - H1
3.5 * 60 * T3
M3 = (kg/min)
H 2 - H1
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
2 2 2
CONDENSER Compressor
E3 T1 E2 T2 E1 T3
1 1 1
T1,T2 & T3 are refrigerating
loads in tons of refrigeration
4 3
P
1 2
Fig.6.10. Multi-stage refrigeration system
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
As the temperatures in all evaporators are same, the same thermodynamic cycle will be
used for all. The power required for the compressor is given by,
(M1 + M 2 + M 3 )(H 2 - H1 )
Power = (kW)
60
and
H 2 - H1
COP =
H3 - H 2
Indian Institute of Technology Madras
Refrigeration Cycles Prof. U.S.P. Shet , Prof. T. Sundararajan and Prof. J.M . Mallikarjuna
6.11 Required Properties of Ideal Refrigerant:
1) The refrigerant should have low boiling point and low freezing point.
2) It must have low specific heat and high latent heat. Because high specific
heat decreases the refrigerating effect per kg of refrigerant and high latent
heat at low temperature increases the refrigerating effect per kg of
refrigerant.
3) The pressures required to be maintained in the evaporator and condenser
should be low enough to reduce the material cost and must be positive to
avoid leakage of air into the system.
4) It must have high critical pressure and temperature to avoid large power
requirements.
5) It should have low specific volume to reduce the size of the compressor.
6) It must have high thermal conductivity to reduce the area of heat transfer in
evaporator and condenser.
7) It should be non-flammable, non-explosive, non-toxic and non-corrosive.
8) It should not have any bad effects on the stored material or food, when any
leak develops in the system.
9) It must have high miscibility with lubricating oil and it should not have
reacting properly with lubricating oil in the temperature range of the system.
10) It should give high COP in the working temperature range. This is
necessary to reduce the running cost of the system.
11) It must be readily available and it must be cheap also.
Important Refrigerants:
Properties at -150C
(1) Ammonia (NH3)(R-717)
Latent heat = 1312.75 kJ/Kg
Specific volume = 0.509 m3/kg
(2) Dichloro–Difluoro methane (Freon–12) (R-12) [C Cl2 F2]
Latent heat = 162 kJ/Kg
Specific volume = 0.093 m3/kg
(3) Difluoro monochloro methane – or Freon-22 (R-22) [CH Cl F2]
Latent heat = 131 kJ/Kg
Specific Volume = 0.15 m3/kg.
Indian Institute of Technology Madras
Potrebbero piacerti anche
- Burnham Boiler v8h - ManualDocumento100 pagineBurnham Boiler v8h - ManualEdwin GeeqNessuna valutazione finora
- Boiler Massachusetts Explosionkaza Raporu 10082013Documento105 pagineBoiler Massachusetts Explosionkaza Raporu 10082013Osman SökeNessuna valutazione finora
- Boilers - ClassificationDocumento77 pagineBoilers - Classificationkabbilaash kumarNessuna valutazione finora
- Borderer Boiler Complete ManualDocumento228 pagineBorderer Boiler Complete ManualNitrogeno MPDNessuna valutazione finora
- Vdocuments - MX Boiler Book Cleaver BrooksDocumento1.391 pagineVdocuments - MX Boiler Book Cleaver BrooksTecnico A Lazaro CardenasNessuna valutazione finora
- Watertube Boilers HaveDocumento175 pagineWatertube Boilers Havenathaniel villanueva100% (1)
- 02 Feedstocks & Products PDFDocumento124 pagine02 Feedstocks & Products PDFNavneet KumarNessuna valutazione finora
- Jinnan Boilers ManualDocumento282 pagineJinnan Boilers ManualMuhummad Tanzeel RanaNessuna valutazione finora
- Petroleum Refining Technology: CHEG 437 4 Credit CourseDocumento61 paginePetroleum Refining Technology: CHEG 437 4 Credit Courseprathamesh singhNessuna valutazione finora
- 11 BPFuelOilSystems PDFDocumento120 pagine11 BPFuelOilSystems PDFBaher Saleh100% (1)
- Experimental Investigation of Tube Rupture Under Boiler Dynamic Operating Conditions (Mossaedyousid Alawwad Id 199656100) Dec 2013Documento152 pagineExperimental Investigation of Tube Rupture Under Boiler Dynamic Operating Conditions (Mossaedyousid Alawwad Id 199656100) Dec 2013rajanarsu100% (1)
- Flow Diagram SymbolsDocumento5 pagineFlow Diagram SymbolshussainNessuna valutazione finora
- BoilerDocumento65 pagineBoilermangal SinghNessuna valutazione finora
- Group 15. Mechanics of Fluids: Vocabulary of Mechanics in Five Languages: English/German/French/Polish/Russian, Vol. 2Da EverandGroup 15. Mechanics of Fluids: Vocabulary of Mechanics in Five Languages: English/German/French/Polish/Russian, Vol. 2Valutazione: 1.5 su 5 stelle1.5/5 (3)
- Boiler TrainingDocumento117 pagineBoiler TrainingAlvinNessuna valutazione finora
- Boiler Afbc PDFDocumento101 pagineBoiler Afbc PDFdika wahyu0% (1)
- Steam System Q&ADocumento115 pagineSteam System Q&Atajshah283Nessuna valutazione finora
- Madukismo Water TreatmentDocumento119 pagineMadukismo Water TreatmentFadli Ryan ArikundoNessuna valutazione finora
- ArticleDocumento14 pagineArticlejaime100% (1)
- مشروع عبدالشافي النهايةDocumento121 pagineمشروع عبدالشافي النهايةMostafa MahmodNessuna valutazione finora
- BoilersDocumento162 pagineBoilersAli SarfrazNessuna valutazione finora
- Boiler EfesiensiDocumento161 pagineBoiler Efesiensirapuy90Nessuna valutazione finora
- Chapter 4 - Steam Generators (Boilers)Documento55 pagineChapter 4 - Steam Generators (Boilers)rrhoshackNessuna valutazione finora
- Steam BoilerDocumento7 pagineSteam BoilerVishnu VardhanNessuna valutazione finora
- Ch5.2 Boiler ControlDocumento131 pagineCh5.2 Boiler ControlSanthosh GskNessuna valutazione finora
- Catalytic Cracking: Fluid Catalytic Cracking and HydrocrackingDocumento16 pagineCatalytic Cracking: Fluid Catalytic Cracking and HydrocrackingdGNessuna valutazione finora
- AALBORG Boiler ControlDocumento185 pagineAALBORG Boiler ControlBatuhan ZerenerNessuna valutazione finora
- Nano-Emulsion: Advantages and Disadvantages Preparation Stability Sign ApplicationDocumento10 pagineNano-Emulsion: Advantages and Disadvantages Preparation Stability Sign ApplicationsyarifaNessuna valutazione finora
- Power Plant Instrumentation - TsDocumento205 paginePower Plant Instrumentation - TsGrishma WarkeNessuna valutazione finora
- Boiler Integrity & EfiiciencyDocumento45 pagineBoiler Integrity & EfiiciencySumaira KhanNessuna valutazione finora
- FALLSEM2020-21 CHE1014 TH VL2020210101682 Reference Material I 19-Aug-2020 Catalytic Cracking Different Types PDFDocumento77 pagineFALLSEM2020-21 CHE1014 TH VL2020210101682 Reference Material I 19-Aug-2020 Catalytic Cracking Different Types PDFJateni GedaNessuna valutazione finora
- Syllabus - CBB - CP ExamDocumento7 pagineSyllabus - CBB - CP ExamRAJKUMARNessuna valutazione finora
- Chapter 6 Heat Transfer EquipmentDocumento76 pagineChapter 6 Heat Transfer EquipmentHải MâyNessuna valutazione finora
- PRE 2 MidT SepProc 10 Jun 2014 PArt 1Documento34 paginePRE 2 MidT SepProc 10 Jun 2014 PArt 1Shabbir AliNessuna valutazione finora
- Thermal Fluid Techniques in Power PlantsDocumento136 pagineThermal Fluid Techniques in Power PlantsDiego Martínez FernándezNessuna valutazione finora
- Distillation Column-3Documento36 pagineDistillation Column-3Sana BashirNessuna valutazione finora
- Ponchon Savarit MethodDocumento17 paginePonchon Savarit MethodRose Dane Escobedo DiestaNessuna valutazione finora
- 413 Topic IV-3 (Fossil Fuels and Boiler Efficiency)Documento60 pagine413 Topic IV-3 (Fossil Fuels and Boiler Efficiency)Sabina Suljic100% (1)
- Sipat - 660 MW Super Critical Boiler PresentationDocumento55 pagineSipat - 660 MW Super Critical Boiler PresentationParveen NakwalNessuna valutazione finora
- Boiler Mechanical Engineering Question BookDocumento103 pagineBoiler Mechanical Engineering Question BookMayank GuptaNessuna valutazione finora
- The Chemistry of Petroleum and Its Products-2nd 2022Documento168 pagineThe Chemistry of Petroleum and Its Products-2nd 2022محمود صابرNessuna valutazione finora
- M.F. Menoufy, H.S. Ahmed, M.A. Betiha, M.A. Sayed: Highlights GraphicalDocumento5 pagineM.F. Menoufy, H.S. Ahmed, M.A. Betiha, M.A. Sayed: Highlights GraphicalTya ArisandiNessuna valutazione finora
- Technical Waterchem PDFDocumento153 pagineTechnical Waterchem PDFsbktceNessuna valutazione finora
- Auxiliary Boiler Construction Details: BY Mohammed Arif M Mechanical Engineer 41256Documento81 pagineAuxiliary Boiler Construction Details: BY Mohammed Arif M Mechanical Engineer 41256Arif MechanicalNessuna valutazione finora
- Khor2016Khor, C. S., & Varvarezos, D. (2016) - Petroleum Refinery Optimization. Optimization and EngineeringDocumento47 pagineKhor2016Khor, C. S., & Varvarezos, D. (2016) - Petroleum Refinery Optimization. Optimization and EngineeringKhaled BeheryNessuna valutazione finora
- Visbreaking ModelDocumento13 pagineVisbreaking ModelMaileen Julissa Hoyos CastellanosNessuna valutazione finora
- Seawater Flue Gas DesulphurisationDocumento2 pagineSeawater Flue Gas DesulphurisationNestramiNessuna valutazione finora
- Petroleum Refinery Engg. - SyllabusDocumento7 paginePetroleum Refinery Engg. - SyllabusPradeep MunnaNessuna valutazione finora
- Basic of BoilerDocumento107 pagineBasic of Boilerjohn_kadier651Nessuna valutazione finora
- Dec. 15, 1959 L. W. Pollock 2,917,564: Hydrocarbon Cracking Furnace and Its Operation Filed Jan. 5, 1959Documento6 pagineDec. 15, 1959 L. W. Pollock 2,917,564: Hydrocarbon Cracking Furnace and Its Operation Filed Jan. 5, 1959regina pramuditaNessuna valutazione finora
- Fischer-Tropsch ProcessDocumento5 pagineFischer-Tropsch ProcessBilal Arif100% (1)
- S Announcement 20872 PDFDocumento90 pagineS Announcement 20872 PDFHey YoNessuna valutazione finora
- Ppe Exp.3 ROLLNO 1Documento9 paginePpe Exp.3 ROLLNO 1Aniket SinghNessuna valutazione finora
- 34 Boiler AccessoriesDocumento21 pagine34 Boiler AccessoriesSwaraj TodankarNessuna valutazione finora
- Organic Rankine Cycles: IIT BombayDocumento19 pagineOrganic Rankine Cycles: IIT BombayShubham PunjabiNessuna valutazione finora
- CW Treatment (Corrosion and Scale) : BY P.Srivastava Sr. Manager (Chem.)Documento58 pagineCW Treatment (Corrosion and Scale) : BY P.Srivastava Sr. Manager (Chem.)Nitin SinghNessuna valutazione finora
- Design of Gasifier System For Diesel Engine PPT FinaleDocumento28 pagineDesign of Gasifier System For Diesel Engine PPT FinaleNitu ShresthaNessuna valutazione finora
- Cheema Boiler Limited: Presented To: MR - Deepak Bhandari (Documento32 pagineCheema Boiler Limited: Presented To: MR - Deepak Bhandari (angenious100% (1)
- CVPCDocumento2 pagineCVPCapi-3830954Nessuna valutazione finora
- 2 Carnot CycleDocumento6 pagine2 Carnot CyclecaptainhassNessuna valutazione finora
- Col Line HDocumento1 paginaCol Line HAcfMacNessuna valutazione finora
- 800 Col Line: Bill of Material Sketch ListDocumento1 pagina800 Col Line: Bill of Material Sketch ListAcfMacNessuna valutazione finora
- Course On: Wastewater Pumping Stations DesignDocumento26 pagineCourse On: Wastewater Pumping Stations DesignAcfMacNessuna valutazione finora
- 1 - Lab - ICE - Engine Energy BalanceDocumento7 pagine1 - Lab - ICE - Engine Energy BalanceAcfMacNessuna valutazione finora
- Lecture (7) : Introduction To G U IDocumento15 pagineLecture (7) : Introduction To G U IAcfMacNessuna valutazione finora
- Graphics PDFDocumento5 pagineGraphics PDFAcfMacNessuna valutazione finora
- Design of Doubly-Reinforced Beams: Lecture 2.c.2 by Engr. Jerry B. Maratas and Engr. Ricardo FornisDocumento12 pagineDesign of Doubly-Reinforced Beams: Lecture 2.c.2 by Engr. Jerry B. Maratas and Engr. Ricardo FornisGodfrey RuizNessuna valutazione finora
- The Gravity Tunnel in A Non-Uniform EarthDocumento8 pagineThe Gravity Tunnel in A Non-Uniform EarthAlain RiverosNessuna valutazione finora
- Moment Redistribution in BeamsDocumento12 pagineMoment Redistribution in BeamsChukwuka WayemeruNessuna valutazione finora
- Tomas Liko and Louis H Kauffman - Knot Theory and A Physical State of Quantum GravityDocumento29 pagineTomas Liko and Louis H Kauffman - Knot Theory and A Physical State of Quantum GravityLopmazNessuna valutazione finora
- Automatic Control, Basic Course FRTF05: ReglerteknikDocumento6 pagineAutomatic Control, Basic Course FRTF05: ReglerteknikAl-ShukaNessuna valutazione finora
- Determine-The-Optimal-Span-Between-Pipe Supports-For-Thin-Walled-Piping-Systems PDFDocumento10 pagineDetermine-The-Optimal-Span-Between-Pipe Supports-For-Thin-Walled-Piping-Systems PDFLuis Pereira PeñaNessuna valutazione finora
- Tensile Splice ACI 350-06Documento4 pagineTensile Splice ACI 350-06YioYinNessuna valutazione finora
- Flexibility Method - Temp. and Pre-StrainDocumento32 pagineFlexibility Method - Temp. and Pre-StrainSarah SullivanNessuna valutazione finora
- Pass Schedule of Wire Drawing Process To Prevent Delamination For High Strength Steel Cord WireDocumento9 paginePass Schedule of Wire Drawing Process To Prevent Delamination For High Strength Steel Cord WireSmruti Ranjan PattanayakNessuna valutazione finora
- Module 2 Physical and Mechanical Properties of Rocks 2Documento64 pagineModule 2 Physical and Mechanical Properties of Rocks 2Flora FigueredoNessuna valutazione finora
- RADIOSS For Impact Analysis v12 Rev20130214 PDFDocumento328 pagineRADIOSS For Impact Analysis v12 Rev20130214 PDFKevin ChackochanNessuna valutazione finora
- Metamaterials Presentation 4Documento31 pagineMetamaterials Presentation 4Iim MafahirNessuna valutazione finora
- NotedDocumento4 pagineNotedJason OrquiaNessuna valutazione finora
- 1 Lab Manual-Final-Control-System-1Documento35 pagine1 Lab Manual-Final-Control-System-1Shimalis RetaNessuna valutazione finora
- Tutorial 2 - Failure Theories ReliabilityDocumento10 pagineTutorial 2 - Failure Theories ReliabilitysharleenNessuna valutazione finora
- L-9 Pure Substance Ideal Gases-IDocumento25 pagineL-9 Pure Substance Ideal Gases-IShailin SequeiraNessuna valutazione finora
- Experiment # 3: Objective:: Fig: 3.1 (Pelton Wheel Turbine)Documento3 pagineExperiment # 3: Objective:: Fig: 3.1 (Pelton Wheel Turbine)waseemjuttNessuna valutazione finora
- GodaDocumento22 pagineGodaLazaros NtoanidisNessuna valutazione finora
- Chapter Two Review of Related Literature and Studies 2.1 EarthquakesDocumento8 pagineChapter Two Review of Related Literature and Studies 2.1 EarthquakesJholo BuctonNessuna valutazione finora
- 6 Waves SDocumento52 pagine6 Waves SRuddyMartiniNessuna valutazione finora
- Energy Loss in PipesDocumento8 pagineEnergy Loss in PipesRo Win83% (6)
- VIII Friction 1Documento6 pagineVIII Friction 1smi_santhoshNessuna valutazione finora
- Class TestDocumento15 pagineClass TestMitul KaziNessuna valutazione finora
- Module Thermodynamics 1Documento37 pagineModule Thermodynamics 1Ryan Philip CatapangNessuna valutazione finora
- Goyal Brothers Prakashan Physics Solutions Class 10 Chapter 1 ForceDocumento46 pagineGoyal Brothers Prakashan Physics Solutions Class 10 Chapter 1 ForceCherryNessuna valutazione finora
- Design of Flat Slab by DDMDocumento16 pagineDesign of Flat Slab by DDMNafees Imitaz100% (1)
- Mass Spring Damper SystemDocumento2 pagineMass Spring Damper SystemDivya Bharathi SNessuna valutazione finora
- Diffraction: Lesson 17: Interference and DiffractionDocumento4 pagineDiffraction: Lesson 17: Interference and DiffractionJohn John SierraNessuna valutazione finora
- NSCP 2015 Seismic Analysis (Static Force Procedure)Documento2 pagineNSCP 2015 Seismic Analysis (Static Force Procedure)Al Patrick Dela CalzadaNessuna valutazione finora
- Chapter - 5 The Step Potential (Continued From Chapter 4)Documento5 pagineChapter - 5 The Step Potential (Continued From Chapter 4)solomon mwatiNessuna valutazione finora