Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
The of Supported Platinum Catalysts: Structure
Caricato da
joseramonfachadoDescrizione originale:
Titolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
The of Supported Platinum Catalysts: Structure
Caricato da
joseramonfachadoCopyright:
Formati disponibili
The Structure of Supported
Platinum Catalysts
EXAMINATION BY ELECTRON MICROSCOPY
By R. L. MOSS,M.Sc., Ph.D.
Ministry of Technology, Warren Spring Laboratory
metal catalysts (diffusion limitations apart)
Modern techniques are steadily increas- may be related not only to the metal area but
ing our knowledge of the structure and to the actual size of the metal crystallites
properties of the supported platinum responsible for that area. The latest standard
metal catalysts so widely used in electron microscopes can resolve the smallest
chemical processing. The main func- aggregates of platinum atoms which may be
tion of the support, such as charcoal, described as crystallites and hence provide
alumina or silica, is to increase the valuable information on the sizes and numbers
surface area of the platinum metal and of crystallites present. Further, the distribu-
so to enhance catalyst performance, and tion of platinum throughout the support and
methods for studying the dispersion of characteristics of the support itself may be
the platinum are therefore of consider- examined.
able importance. This article describes
the application of electron microscopy Appearance under the Electron
to the problem and compares the results Microscope
given by this and other methods. Suitable specimens for electron microscopy
can be prepared by cutting extremely thin
sections (300 to 500 A) with an ultra-micro-
The study of the state of dispersion of the tome from catalyst particles embedded in,
metal in supported platinum catalysts is based for example, “Araldite”. An alternative
on indirect methods such as gas chemi- method is ultrasonic dispersion of the
sorption, and on direct methods such as catalyst in butyl alcohol. Remembering the
X-ray diffraction and electron microscopy. very small area under examination, a number
The chemisorption method depends on of specimens must be prepared and surveyed
finding conditions of temperature and pres- in order to obtain representative electron
sure at which a gas-hydrogen or carbon micrographs.
monoxide-will chemisorb to monolayer
coverage on the platinum but not on the Platinum/Silica
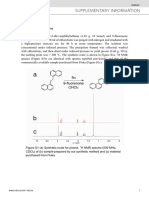
support. The volume of gas taken up shows Fig. I shows an electron micrograph of a
the extent to which the platinum has been 3 per cent platinum/silica catalyst made by
dispersed. For example, it was shown (I) impregnating silica gel with chloroplatinic
that a freshly prepared reforming catalyst acid solution, drying at 120°C and reducing
(0.6 wt. per cent platinum on ?-alumina) had in hydrogen. At a magnification of IOO,OOO X ,
most of the platinum atoms exposed, probably the platinum shows up as dark spots evenly
as islands or as very small crystallites less than distributed as minute crystallites in the pore
10 a in size. There is, however, a growing system of the silica gel. Electron diffraction
awareness that the performance of supported patterns from selected areas with a high
Platinum Metals Rev., 1967, 11, (4), 141-145 141
Fig. 1 3per centplatinunt/
silica catalyst, magnifica-
tion 100,000 X , showing
platinum crystallites as
dark spots on grey silica
background
concentration of dark spots confirm the are predominantly in sizes below about 50 A;
presence of platinum. in the 10 A size crystallites most of the
About 1000 platinum crystallites in this atoms (datomic=z.77 A) are “surface” atoms.
electron micrograph were sized in terms of Although the 10 to 50 A crystallites account
their diameters, since they appear approxi- for only one-quarter of the total weight of
mately spherical, in increments of 10A, and platinum present on the support, nevertheless
Fig. z shows the number of crystalljtes they provide almost half of the available
observed in each size range. The crystallites platinum area. Hence the platinum pri-
marily responsible for the performance of this
supported catalyst can be “seen” by electron
microscopy.
Electron micrographs of the silica support
itself showed spherical particles of, very
a
roughly, IOO diameter. Assuming no loss
of area when the particles contact, the cal-
culated surface area is -250 m2/g. This
rapid estimate agrees reasonably with the
BET gas adsorption value.
Platinum/Charcoal or Alumina
Fig. 3 shows an electron micrograph of a
platinum on charcoal catalyst at a magnifica-
tion of IOO,OOO A . Chemisorption methods
showed the very high platinum area of a
Johnson Matthey 5 per cent platinum/
charcoal catalyst which formed the basis of the
sample examined. The catalyst was then
subjected to a vigorous sintering treatment
(firing at 300°C in air) to encourage crystallite
growth. Nevertheless, as the electron micro-
graph shows, the platinum crystallites were
Platinum Metals Rev., 1967, 11, (4) 142
Fig.3 Sper centplatinuml
charcoal catalyst, heated i n
air at 300”C, magni5cation
100,000 X , showing very
small platinum crystallites
still very numerous and extremely small in the relation existing between the number of
gas molecules adsorbed at this point and
size, yielding a large catalytically-active the number of surface metal atoms.
platinum surface.
The equipment required (2), however, is
Fig. 4 shows an electron micrograph
relatively simple, for example, a conventional
(35,000 X ) of a catalyst with 2.5 per cent
volumetric apparatus such as might be used
platinum supported on a low-area alumina.
for BET surface area determinations, a
Measurement of the platinum area by carbon
vacuum micro-balance or a flow system linked
monoxide chemisorption showed that it was
to a gas chromatograph. If the observed
closely similar to the area of the platinum/
metal area is S, the mean crystallite size, d,,
silica catalyst discussed above (Fig. I), but
is calculated from dS=6/Sp where p is the
the electron micrographs are in marked
density of the metal; it is assumed that the
contrast. Whereas the platinum crystallites
crystallites are spheres or any regular poly-
in the silica-supported catalyst are widely
hedron except the tetrahedron. This dia-
distributed, this alumina-supported catalyst
meter, d,, is the surface-average diameter
shows dark patches of platinum. At still
defined by Cnidt/Cnid,2, where there are ni
higher magnifications (IOO,OOO x ), these
crystallites of diameter, di. From the crystal-
patches were clearly resolved as groups of
lite size distribution (Fig. 2) obtained from
small platinum crystallites (Fig. 5) and the
electron micrographs the diameter, d,, is
platinum area is obviously higher than it
readily calculated for comparison with the
might at first seem.
mean size obtained by chemisorption.
The determination of crystallite size by
Comparison with Chemisorption X-ray diffraction depends on the fact that
and X-ray Diffraction below about 1000 A size, X-ray reflections
The chemisorption method for measuring are broadened beyond the normal ‘‘instru-
the metal area of a supported catalyst has mental” breadth. Thus the method involves
already been briefly discussed. Its main measuring the breadth of one or more X-ray
problems are : reflections, preferably using an X-ray counter-
difiactometer which provides a chart-record-
chemisorbing gas on the metal but not on the
support; ing of the position, profile and intensity of
choice of a criterion for monolayer coverage; each reflection. The Scherrer equation relates
Platinum Metals Rev., 1967, 11, (4) 143
Fig. 4 A 2.5 per cent platinumlalumina Fig. 5 Same catalyst, magni$cution 100,000 X ,
catalyst, magnification 35,000 >( ,showing plat- resolving individual platinum crystallites
inum as dark patches
the excess breadth to the mean crystallite done for the 3 per cent platinum/silica
size, 6, which is a volume-weighted average catalyst (Figs. I and z), taking into account
diameter, Cnidt/Cnidt. Again, this type of the different types of average involved, with
average diameter can be calculated from the results shown in the table. Some satis-
electron microscope observations for com- factory conclusions can be drawn from these
parison with X-ray results. T h e main prob- results :
lems with the X-ray diffraction method are: the assumptions involved in the chemisorption
method (carbon monoxide at 25OC, COiPt
the smaller platinum crystallites, perhaps ratio=^, no adsorption on silica) seem
those less than 50 A, when measured with reasonably justified;
standard equipment, are not detected yet
provide much of the available platinum the electron microscope resolved most of the
area of the catalyst. The proportion of platinum crystallites contributing to the
platinum remaining undetected can, how- platinum area;
ever, be estimated (3); the ‘particles’ viewed in the electron rnicro-
crystallite size is measured whereas the plati- scope were also the crystallites detected by
num can be present as particles, that js, X-ray diffraction.
agglomerates of crystallites, with interior
surfaces inaccessible to gas molecules, Relation to Catalyst Performance
With the problems involved in measuring There are two important consequences,
crystallite size (and platinum area) by at least, for catalyst performance arising
chemisorption or X-ray diffraction, it is there- from an increase in crystallite size, perhaps
fore of some interest to compare such results as a result of sintering during use.
with the crystallite size distribution obtained T h e more obvious is the rapid loss in metal
from electron micrographs. This has been area accompanying crystallite growth. The
Average Crystallite Size in 3 per cent Platinum-Silica Catalyst
Mean diameter, d,, of all crystallites:
by chemisorption 45 A
by electron microscopy 55 A
Mean diameter, d,, of crystallites 50 A size and above:
by X-ray diffraction 60 A
by electron microscopy 65 A
Platinum Metals Rev., 1967, 11, (4) 144
Fig. 6 Models of small
platinum crystallites:
left-hand represents
approximately 10 A
diameter with mainly
( 1 1 1 ) crystal planes ex-
posed; right-hand re-
presents approximately
I 2 A with predomi-
nantly (100) planes ex-
posed
-
left-hand model in Fig. 6 represents a very
small crystallite, 10A diameter, containing
38 atoms ofwhich 31 are exposed (82 per cent),
activity per unit metal area. Now a
further step forward would be to record
the structure of the catalyst, at least in
excluding those which only contact the sup- part, by determining the crystallite
port. The slightly larger right-hand model, size distribution from electron micro-
-12 A diameter, represents 62 atoms but graphs.
now only 44 (71 per cent) are present at the (ii) Apart from duofunctional reforming
surface. One important factor in choosing the catalysts where both platinum and
support material is the stability which it can alumina provide catalytically active
impart to the crystallites against sintering sites, it is believed that the specific
together. activity may sometimes be altered by
Further reference to the models (Fig. 6) the nature of the support. This means
shows a less obvious consequence of crystallite that, in addition to its traditional roles
growth. Whereas the smaller crystallite which include extending the metal
displays mainly ( I I I) crystal planes, addition area, the support co-operates somehow
of a single layer of atoms to.form the slightly in the catalytic process. However,
larger crystallite yields a surface with pre- changing the support can alter the
dominantly (100)planes exposed. As these crystallite size distribution and again
small crystallites grow, other crystal planes electron micrographs may help to
and atomic arrangements rapidly form and disentangle the effects of crystallite size
change, each with its characteristic catalytic and support on catalyst performance.
properties. For example, when n-heptane
was reformed over a series of platinum/ Acknowledgement
alumina catalysts (4, dehydrocyclisation The author wishes to acknowledge the con-
tribution of Miss J. Holroyd who prepared the
activity was decreased and isomerisation in- electron micrographs used to illustrate this article.
creased as the mean crystallite size varied
References
from 10 to 450 A.
Some applications of the electron-micro- I L. Spenadel and M. Boudart,J. Phys. Chem.,
1960164, 204
scope to supported catalyst research are 2 R. L. Moss, The Chemical Engineer, 1966,
therefore apparent. (1991,(June), CE 114
3 T. A. Dorling and R. L. Moss, J . Catalysis,
(i) When the performance of a supported 1966,5,111
catalyst is being assessed, often the 4 H.J. Maat and L. Moscou, in Proceedings of
metal area is measured in order to the Third International Congress on Cataly-
sis, Amsterdam, 1964,p. 1277 (Amsterdam:
report the specific activity, that is, North-Holland Publ. Co, 1965)
Platinum Metals Rev., 1967, 11, (4) 145
Potrebbero piacerti anche
- Seventeen, Jan 2011Documento4 pagineSeventeen, Jan 2011emediageNessuna valutazione finora
- Gas Sensing Characteristics of Ultrathin Tio2-X Films Investigated With and in Situ XPS, TPD Resistance MeasurementsDocumento5 pagineGas Sensing Characteristics of Ultrathin Tio2-X Films Investigated With and in Situ XPS, TPD Resistance MeasurementsRizky SåbillyNessuna valutazione finora
- Effect of Donor, Acceptor, and Donor-Acceptor Codoping On BSTDocumento3 pagineEffect of Donor, Acceptor, and Donor-Acceptor Codoping On BSThichiku4uNessuna valutazione finora
- Monolayer-Protected Metal Nanoparticle: Toluene) Auc L Toluene) N (C H Auc L N (C HDocumento27 pagineMonolayer-Protected Metal Nanoparticle: Toluene) Auc L Toluene) N (C H Auc L N (C HmichsantosNessuna valutazione finora
- Magnetic Properties of Some Ferrite Micropowders: Additional Information On J. Appl. PhysDocumento3 pagineMagnetic Properties of Some Ferrite Micropowders: Additional Information On J. Appl. Physdita wulansariNessuna valutazione finora
- ABanuJOAMv12n5 p1189Documento5 pagineABanuJOAMv12n5 p1189Alexandra BanuNessuna valutazione finora
- 2007 Eletrodeposição ZN GomesDocumento10 pagine2007 Eletrodeposição ZN GomesJuliermes CarvalhoNessuna valutazione finora
- (Sici) 1521-4095 (199810) 10 14 1100 Aid-Adma1100 3.0.co 2-2Documento5 pagine(Sici) 1521-4095 (199810) 10 14 1100 Aid-Adma1100 3.0.co 2-2Walid EbaiedNessuna valutazione finora
- Kadlecikova Optik 2018Documento7 pagineKadlecikova Optik 2018Sissi DadiNessuna valutazione finora
- Lead Dioxide 4Documento4 pagineLead Dioxide 4Khobaib HayatNessuna valutazione finora
- Electrochromism of Sputtered Fluorinated Titanium Oxide Thin FilmsDocumento4 pagineElectrochromism of Sputtered Fluorinated Titanium Oxide Thin FilmsTanveer ZiaNessuna valutazione finora
- Crystallites - Structure and DefectsDocumento8 pagineCrystallites - Structure and Defectschiuchan888Nessuna valutazione finora
- Silicon Solar Cell Enhancement by Using Au NanoparticlesDocumento8 pagineSilicon Solar Cell Enhancement by Using Au NanoparticlesInternational Journal of Application or Innovation in Engineering & ManagementNessuna valutazione finora
- Science Direct Photoluminescence From Colloidal Silver NanoparticlesDocumento6 pagineScience Direct Photoluminescence From Colloidal Silver NanoparticlesYu Shu HearnNessuna valutazione finora
- Practice Problem Set 4 Atomic Absorption SpectrosDocumento14 paginePractice Problem Set 4 Atomic Absorption SpectrosKassimNessuna valutazione finora
- 1 - Journal of Electroanalytical Chemistry 2008Documento8 pagine1 - Journal of Electroanalytical Chemistry 2008Érico Teixeira NetoNessuna valutazione finora
- 1985 Woste 强簇源+三重四级杆制造和研究金属团簇Documento6 pagine1985 Woste 强簇源+三重四级杆制造和研究金属团簇1592162022Nessuna valutazione finora
- 2008 - An Optical, EPR and Electrical Conductivity Study of Blue Barium TitanateDocumento8 pagine2008 - An Optical, EPR and Electrical Conductivity Study of Blue Barium TitanateBeh NaatNessuna valutazione finora
- Diffusion and Reactions in Gold Films: John M. PoateDocumento10 pagineDiffusion and Reactions in Gold Films: John M. PoateHiden HidenNessuna valutazione finora
- Simulation FeCr Using Neutron, Heavy-Ion or Electron With Diff FluxesDocumento16 pagineSimulation FeCr Using Neutron, Heavy-Ion or Electron With Diff FluxesLe HoangNessuna valutazione finora
- XRD and Electron Microscope Study of An As-Cast 26.6% Chromium White Iron MicrostructureDocumento9 pagineXRD and Electron Microscope Study of An As-Cast 26.6% Chromium White Iron MicrostructureJMarce16Nessuna valutazione finora
- A Surface-Enhanced Raman Scattering Study of CTAB Adsorption On CopperDocumento14 pagineA Surface-Enhanced Raman Scattering Study of CTAB Adsorption On CopperlinuspaulingNessuna valutazione finora
- Puga Lambers1998Documento10 paginePuga Lambers1998nevinkoshyNessuna valutazione finora
- Kanungo 2003Documento4 pagineKanungo 2003Đức PhạmNessuna valutazione finora
- Spontaneous Coalescence in Ultrafine Metal Particle AggregatesDocumento4 pagineSpontaneous Coalescence in Ultrafine Metal Particle AggregatesYassine GouzzaliNessuna valutazione finora
- Nanoparticle Plasma Ejected Directly From Solid Copper by Localized MicrowavesDocumento4 pagineNanoparticle Plasma Ejected Directly From Solid Copper by Localized MicrowavesKholmahmad KholovNessuna valutazione finora
- 1997 Goldby AIP 气体凝聚源用于团簇的制造和沉积Documento9 pagine1997 Goldby AIP 气体凝聚源用于团簇的制造和沉积1592162022Nessuna valutazione finora
- X-Ray Diffraction Studies of Catalysts: It Is Now PossibleDocumento6 pagineX-Ray Diffraction Studies of Catalysts: It Is Now PossibleIsadora PereiraNessuna valutazione finora
- PAPER CLAIM TO BE FIRST IN DETECT ALPHA PRIME IN FECR BY ION IRRAD - Fe Irrad at 300CDocumento8 paginePAPER CLAIM TO BE FIRST IN DETECT ALPHA PRIME IN FECR BY ION IRRAD - Fe Irrad at 300CLe HoangNessuna valutazione finora
- Full TextDocumento9 pagineFull TextAlvaro Torres AravenaNessuna valutazione finora
- The Electrooxidation of Carbon Monoxide On Unsupported PT AgglomeratesDocumento12 pagineThe Electrooxidation of Carbon Monoxide On Unsupported PT Agglomeratesmirybendecida91Nessuna valutazione finora
- 1 s2.0 S1572665717301819 MainDocumento10 pagine1 s2.0 S1572665717301819 MainMuhammad ImranNessuna valutazione finora
- Effect of Carbon Concentration On Changing The Morphology of Titanium Carbide Nanoparticles From Cubic To CuboctahedronDocumento6 pagineEffect of Carbon Concentration On Changing The Morphology of Titanium Carbide Nanoparticles From Cubic To CuboctahedronRabia Farman 3428Nessuna valutazione finora
- 350.nature.454 MeyerDocumento4 pagine350.nature.454 MeyeryaxooNessuna valutazione finora
- Hoshi p1073-1078 97Documento6 pagineHoshi p1073-1078 97mauricio rojas alvarezNessuna valutazione finora
- Nature08859 s1Documento10 pagineNature08859 s1Dejan DjokićNessuna valutazione finora
- Feature Article: Electronic Wave Functions in Semiconductor Clusters: Experiment and TheoryDocumento6 pagineFeature Article: Electronic Wave Functions in Semiconductor Clusters: Experiment and TheoryNitika GuptaNessuna valutazione finora
- Time-Resolved Reflectivity TechniqueDocumento6 pagineTime-Resolved Reflectivity TechniqueYeixon Quintero MartinezNessuna valutazione finora
- Electrodes For The Measurement of Oxygen and Carbon Dioxide TensionsDocumento11 pagineElectrodes For The Measurement of Oxygen and Carbon Dioxide TensionsMujeeb JavedNessuna valutazione finora
- Strain-Induced Quantum Confinement of Carriers Due To Extended Defects in SiliconDocumento5 pagineStrain-Induced Quantum Confinement of Carriers Due To Extended Defects in SiliconManju ManoharNessuna valutazione finora
- 1 s2.0 0022311587900705 MainDocumento19 pagine1 s2.0 0022311587900705 MainMy Tech WorldNessuna valutazione finora
- 1 s2.0 S0169433209001457 MainDocumento8 pagine1 s2.0 S0169433209001457 Mainranim najibNessuna valutazione finora
- Optical Properties of ALON Aluminum OxynitrideDocumento9 pagineOptical Properties of ALON Aluminum OxynitrideAlex FaudoaNessuna valutazione finora
- Olsson 1995Documento13 pagineOlsson 1995Steve OoiNessuna valutazione finora
- Linear and Nonlinear Optical Studies in Photonic Crystal AlloysDocumento3 pagineLinear and Nonlinear Optical Studies in Photonic Crystal AlloysVaswati BiswasNessuna valutazione finora
- 1996 J Chem Soc Ag RougheningDocumento7 pagine1996 J Chem Soc Ag RougheningSara AldabeNessuna valutazione finora
- Tin Whiskers Formation in SN Cu Ni Bi Under Electro-Migration StressingDocumento4 pagineTin Whiskers Formation in SN Cu Ni Bi Under Electro-Migration StressingNOOR ZAIMAH BINTI MOHD MOKHTAR STUDENTNessuna valutazione finora
- The Oxidation of Tantalum at 50-3oo°cDocumento6 pagineThe Oxidation of Tantalum at 50-3oo°cGiselle GalloNessuna valutazione finora
- Crystalline Orientation of Bimno Thin Films Grown by Rf-SputteringDocumento4 pagineCrystalline Orientation of Bimno Thin Films Grown by Rf-SputteringPugazh VadivuNessuna valutazione finora
- Influence of Titanium Oxide Films On Copper Nucleation During ElectrodepositionDocumento12 pagineInfluence of Titanium Oxide Films On Copper Nucleation During Electrodepositionapi-19973331Nessuna valutazione finora
- Saban 1992Documento9 pagineSaban 1992NidhiNessuna valutazione finora
- Journal Club 23 de Agosto 2019Documento10 pagineJournal Club 23 de Agosto 2019Eduardo CamachoNessuna valutazione finora
- X-Ray Line Profile Analysis in Alkali-Treated Ramie: FiberDocumento4 pagineX-Ray Line Profile Analysis in Alkali-Treated Ramie: Fiberapi-3733260Nessuna valutazione finora
- Substituted Cobalt Ferrites For Sensors ApplicationsDocumento5 pagineSubstituted Cobalt Ferrites For Sensors ApplicationsAlin DrucNessuna valutazione finora
- UAS Korosi Resume PDFDocumento8 pagineUAS Korosi Resume PDFMochamad FijayNessuna valutazione finora
- Thin FilmDocumento5 pagineThin FilmDipender SinghNessuna valutazione finora
- EXPERIMENT 8. Monolayer Characterization: Contact Angles, Reflection Infrared Spectroscopy, and EllipsometryDocumento9 pagineEXPERIMENT 8. Monolayer Characterization: Contact Angles, Reflection Infrared Spectroscopy, and EllipsometryavniNessuna valutazione finora
- CBSE Class 12 Chemistry Paper Sample Paper Solution Set 4Documento14 pagineCBSE Class 12 Chemistry Paper Sample Paper Solution Set 4Gamer ChannelNessuna valutazione finora
- 1991.02.01 - PARKS - JPC - Ni ClustersDocumento22 pagine1991.02.01 - PARKS - JPC - Ni ClustersAlejandra AwimbaweNessuna valutazione finora
- Liquid Hydrogen Gas h2 Safety Data Sheet Sds p4603Documento9 pagineLiquid Hydrogen Gas h2 Safety Data Sheet Sds p4603joseramonfachadoNessuna valutazione finora
- Msds h2Documento9 pagineMsds h2Ammar ZakyNessuna valutazione finora
- Advances in ASME Section VIII Division 2 Pressure Vessel Design and Analysis PDFDocumento10 pagineAdvances in ASME Section VIII Division 2 Pressure Vessel Design and Analysis PDFjoseramonfachadoNessuna valutazione finora
- (Phillip C. Wankat) Instructor's Solution ManualDocumento478 pagine(Phillip C. Wankat) Instructor's Solution ManualFranco Gates90% (39)
- (Phillip C. Wankat) Instructor's Solution ManualDocumento478 pagine(Phillip C. Wankat) Instructor's Solution ManualFranco Gates90% (39)
- Banksy Responses Done With A Partner 655512Documento122 pagineBanksy Responses Done With A Partner 655512api-569248887Nessuna valutazione finora
- 10th Syllbus PDFDocumento104 pagine10th Syllbus PDFGagandeep KaurNessuna valutazione finora
- PDF EnglishDocumento36 paginePDF EnglishSanti CheewabantherngNessuna valutazione finora
- Alonex Special Amp Industrial Electronic Equipment PDFDocumento342 pagineAlonex Special Amp Industrial Electronic Equipment PDFthanh vanNessuna valutazione finora
- Week 1 - Intrduction To Nursing Research - StudentDocumento24 pagineWeek 1 - Intrduction To Nursing Research - StudentWani GhootenNessuna valutazione finora
- What Is Situational Coaching and When To Use ItDocumento3 pagineWhat Is Situational Coaching and When To Use ItBrian KamoedNessuna valutazione finora
- Ug Vol1Documento433 pagineUg Vol1Justin JohnsonNessuna valutazione finora
- Planmeca Promax 3D Max CBVT Product PresentationDocumento36 paginePlanmeca Promax 3D Max CBVT Product PresentationAD TwentyOne DentalNessuna valutazione finora
- Technical Seminar Report CV94Documento62 pagineTechnical Seminar Report CV941MS19CV053 KARTHIK B SNessuna valutazione finora
- V Packing SealDocumento10 pagineV Packing SealBobby ArbianugrohoNessuna valutazione finora
- NCERT Exemplar Class 7 Maths IntegersDocumento661 pagineNCERT Exemplar Class 7 Maths IntegersRohiniNessuna valutazione finora
- TRANSLATIONDocumento4 pagineTRANSLATIONGarren Jude Aquino100% (1)
- AEF0 - File 1,2,3Documento3 pagineAEF0 - File 1,2,3Nayib Bucarin CarlNessuna valutazione finora
- Modernization and Crumbling ValuesDocumento3 pagineModernization and Crumbling ValuesZeeyah Khan100% (3)
- Giamarie Marbella - SP Essay Rough DraftDocumento11 pagineGiamarie Marbella - SP Essay Rough Draftapi-667933283Nessuna valutazione finora
- 2.talent Management New ChallengesDocumento17 pagine2.talent Management New ChallengesAlejandra AGNessuna valutazione finora
- Resume FixedDocumento2 pagineResume Fixedapi-356691606Nessuna valutazione finora
- Appendix in Research PaperDocumento6 pagineAppendix in Research Papergvzfmq91100% (1)
- Current Office Phone Number Vijayawada, Andhra Pradesh (A.p.)Documento11 pagineCurrent Office Phone Number Vijayawada, Andhra Pradesh (A.p.)Manoj Digi Loans100% (1)
- EER Model: Enhance Entity Relationship ModelDocumento12 pagineEER Model: Enhance Entity Relationship ModelHaroon KhalidNessuna valutazione finora
- Guidelines For The Oral Presentation Bands Singers e MusicDocumento2 pagineGuidelines For The Oral Presentation Bands Singers e Musicjuliusdelazare100% (1)
- AWS Lecture NotesDocumento4 pagineAWS Lecture NotesAsad Bin Ala Qatari0% (1)
- AVT 2217 Module 5 Aiport Lighting SystemDocumento15 pagineAVT 2217 Module 5 Aiport Lighting SystemJane PazNessuna valutazione finora
- (Type The Documen T Title) : (Year)Documento18 pagine(Type The Documen T Title) : (Year)goodluck788Nessuna valutazione finora
- B1+ Exam MappingDocumento3 pagineB1+ Exam Mappingmonika krajewskaNessuna valutazione finora
- Pure Theory of Law Hans Kelson-Ltp..Documento45 paginePure Theory of Law Hans Kelson-Ltp..ShabnamNessuna valutazione finora
- Respons 910 Analyzer: Operator's ManualDocumento246 pagineRespons 910 Analyzer: Operator's ManualUmashankar LoganathanNessuna valutazione finora
- B737 SRM 51 - 40 - 08 Rep - Fiberglass OverlaysDocumento6 pagineB737 SRM 51 - 40 - 08 Rep - Fiberglass OverlaysAlex CanizalezNessuna valutazione finora
- FAUDI Aviation Diesel - Company Profile-ENDocumento6 pagineFAUDI Aviation Diesel - Company Profile-ENAttila HontváriNessuna valutazione finora
- Social Gaming Merchant AccountDocumento2 pagineSocial Gaming Merchant AccountstarprocessingusNessuna valutazione finora