Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Biochemical Engineering Final Requirement
Caricato da
LeeGonzaLgo0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
25 visualizzazioni5 pagineBiochemical engineering
Titolo originale
Biochem.docx
Copyright
© © All Rights Reserved
Formati disponibili
PDF, TXT o leggi online da Scribd
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoBiochemical engineering
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PDF, TXT o leggi online su Scribd
0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
25 visualizzazioni5 pagineBiochemical Engineering Final Requirement
Caricato da
LeeGonzaLgoBiochemical engineering
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PDF, TXT o leggi online su Scribd
Sei sulla pagina 1di 5
ADAMSON UNIVERSITY
COLLEGE OF ENGINEERING
CHEMICAL ENGINEERING DEPARTMENT
BIOCHEMICAL ENGINEERING
FINAL REQUIREMENT
Submitted By:
Bernales, Candice Gareth P.
Gabinete, Aljomarie
Gonzalgo, Leerra Maurreen Z.
Humarang, Jelain A.
Mateo, Mizzrah
Submitted To:
Engr. Rainier G. Gomez
Date Submitted:
October 21, 2016
1. An enzyme is assayed at an initial substrate concentration of 2 x 10-5 M. In 6 minutes,
half of the substrate is used. The Km for the substrate is 2 x 10-3 M. The value of k in
minute is:
a. 0.42
b. 0.093
c. 0.115
d. 6.693
2. Given the enzyme with a Km = 5m M and Vmax = 15 m mol/min. If [S] = 20 m M,
which of the following will be true?
a. A 100% increase of Vmax would increase velocity by 100%
b. A 10% decrease in Km would increase velocity
c. Both (a) and (b)
d. A 10 % increase in Vmax would decrease velocity 20 fold
3. Which of the following statements is true for enzymatically catalyzed reaction?
a. The activation energy of the reaction is lowered so that a fewer substrate
molecules can overcome it
b. Additional substrate molecules are energized to overcome the activation energy of
the reaction
c. The activation energy of the reaction is increased, thus decreasing the likelihood
that any substrate molecules will overcome it
d. The activation energy of the reaction is lowered so that a larger proportion of
the substrate qualifies to overcome it
4. Non-competitive inhibitor of an enzyme catalyzed reaction
a. can increase reaction velocity in rare cases
b. binds to Michaelis complex
c. decreases Vmax
d. Both(b) and (c)
5. The plot commonly used for determining the value of Vmax is
a. Lineweaver Burk Plot
b. Langmuir plot
c. Eadie-Hofstee plot
d. All of the above
6. A noncompetitive inhibitor of an enzyme-catalyzed reaction
a. increases KM and increases Vmax
b. increases KM and reduces Vmax
c. reduces KM and increases Vmax
d. and reduces Vmax
reduces KM
[S] V, no inhibitor V, with Inhibitor
(mM) (mmol/min) (mmol/min)
4.5 6.78 4.58
6.0 9.72 7.5
7.5 11.5 8.56
9.0 12.23 9.12
10.5 15.94 9.88
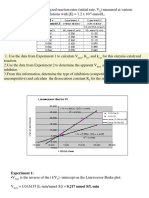
7. Draw a Lineweaver-Burk Plots for an enzyme with the following data:
What are the Km and Vmax values for the inhibited and uninhibited enzymes? Is the
inhibitor competitive, non-competitive or uncompetitive?
The growth rate of
E. coli
can be expressed by monod kinetics with the parameters of
µmax = 0.935 hr
-1
and k
s
= 0.71 g/L. Assume that the cell yield Y
x/s
is 0.6 g dry cells per g
substrate. If Cx
0
is 1 g/L and Cs = 10 g/L when the cells start to grow exponentially, at t
0
=
0, show how ln Cx, Cx, Cs, dln Cx/dt and dCx/dt, change with respect to time.
The growth rate of
E. coli
can be expressed by monod kinetics with the parameters of
µmax = 0.935 hr
-1
and k
s
= 0.71 g/L. Assume that the cell yield Y
x/s
is 0.6 g dry cells per g
substrate. If Cx
0
is 1 g/L and Cs = 10 g/L when the cells start to grow exponentially, at t
0
=
0, show how ln Cx, Cx, Cs, dln Cx/dt and dCx/dt, change with respect to time.
The growth rate of
E. coli
can be expressed by monod kinetics with the parameters of
µmax = 0.935 hr
-1
and k
s
= 0.71 g/L. Assume that the cell yield Y
x/s
is 0.6 g dry cells per g
substrate. If Cx
0
is 1 g/L and Cs = 10 g/L when the cells start to grow exponentially, at t
0
=
0, show how ln Cx, Cx, Cs, dln Cx/dt and dCx/dt, change with respect to time.
8. The growth rate of S. cerevisiae is expressed in monod kinetics with the parameters of
µmax = 0.894 hr-1 and ks = 0.65 g/L. Assume that the cell yield Yx/s is 0.6 g dry cells
per g substrate. If Cx0 is 1 g/L and Cs = 10 g/L when the cells start to grow exponentially,
at t0 = 0, show how ln Cx, Cx, Cs, d(ln Cx)/dt and d(Cx)/dt, change with respect to time.
Potrebbero piacerti anche
- Physical Biochemistry Dr. NabilDocumento22 paginePhysical Biochemistry Dr. NabilKhaled M FawzyNessuna valutazione finora
- Enzymes Prob SetDocumento2 pagineEnzymes Prob SetrxpturousNessuna valutazione finora
- Enzyme KineticsDocumento3 pagineEnzyme KineticsZeny Naranjo100% (2)
- 3Documento4 pagine3biotech_vidhya100% (1)
- Problem Set MidtermDocumento34 pagineProblem Set MidtermRaymond YabutNessuna valutazione finora
- 395 GKinetics HWDocumento3 pagine395 GKinetics HWKeinth JosephNessuna valutazione finora
- Haramaya University Haramaya Institute of Technology Department of Chemical Engineering Introduction To Biochemical Engineering Worksheet 2Documento3 pagineHaramaya University Haramaya Institute of Technology Department of Chemical Engineering Introduction To Biochemical Engineering Worksheet 2workisaNessuna valutazione finora
- Enzyme Biochemistry PracticeDocumento6 pagineEnzyme Biochemistry PracticealirezamdfNessuna valutazione finora
- Biochem Problem SolvingDocumento53 pagineBiochem Problem SolvingNasser Gemina PantaoNessuna valutazione finora
- The Answers Are Posted On The Last PageDocumento6 pagineThe Answers Are Posted On The Last Pagearkangel97Nessuna valutazione finora
- CH 600 LC Name: Problem Set Yr. & Sec: Enzymes ANSWER THE FOLLOWING QUESTIONS. Use Yellow Pad For Your AnswersDocumento2 pagineCH 600 LC Name: Problem Set Yr. & Sec: Enzymes ANSWER THE FOLLOWING QUESTIONS. Use Yellow Pad For Your AnswersLinette GuillermoNessuna valutazione finora
- Enzym ESDocumento21 pagineEnzym ESpoopnoodlemanNessuna valutazione finora
- Enzyme KineticsDocumento39 pagineEnzyme Kineticsjpm smurfNessuna valutazione finora
- Enzmology RevisionDocumento8 pagineEnzmology RevisionRyan Fortune AludaNessuna valutazione finora
- Biochemistry 13 Class Notes Saakaar 20 Batch For IIT JAM 2Documento48 pagineBiochemistry 13 Class Notes Saakaar 20 Batch For IIT JAM 2Adeeti RaiNessuna valutazione finora
- A. CompetitiveDocumento3 pagineA. CompetitiveworkisaNessuna valutazione finora
- Biochem Enzyme KineticsDocumento53 pagineBiochem Enzyme KineticsJayvee Francisco67% (3)
- Cell Kinetics and Fermenter Design 2Documento17 pagineCell Kinetics and Fermenter Design 2rhia81% (16)
- 5 Enzyme KineticsDocumento39 pagine5 Enzyme KineticsEbook Download100% (1)
- Problem Set - Enzymes From LehningerDocumento11 pagineProblem Set - Enzymes From LehningervioletbrownNessuna valutazione finora
- Midnight HWDocumento3 pagineMidnight HWDont MeNessuna valutazione finora
- GATE HELPLINE Bioprocess Engineering MCQ IDocumento4 pagineGATE HELPLINE Bioprocess Engineering MCQ ISanthosh Kalash100% (8)
- Exam II - Review QuestionsDocumento9 pagineExam II - Review QuestionsEmmanuel JoyNessuna valutazione finora
- Assignment 1 BRD BT5071-2022Documento3 pagineAssignment 1 BRD BT5071-2022Subhrojyoti GhoshNessuna valutazione finora
- Biochem Eng ProblemsDocumento23 pagineBiochem Eng ProblemsAdu GilbertNessuna valutazione finora
- CH 369 Practice Exam 2Documento11 pagineCH 369 Practice Exam 2Tracy NwanneNessuna valutazione finora
- Modul 2Documento2 pagineModul 2Aditya Whisnu Heryudhanto0% (1)
- Problems in Biochemical EngineeringDocumento22 pagineProblems in Biochemical EngineeringArrianne Jaye Mata50% (4)
- Enzyme Questions 2Documento6 pagineEnzyme Questions 2Edvair FilhoNessuna valutazione finora
- Miscellaneous Biochemistry QuestionsDocumento8 pagineMiscellaneous Biochemistry QuestionsphoenixscarNessuna valutazione finora
- Vet 115 Quiz 4Documento14 pagineVet 115 Quiz 4Chiku MteghaNessuna valutazione finora
- Set8ans 10Documento5 pagineSet8ans 10Agustina Evania DewiNessuna valutazione finora
- Worked ExamplesDocumento9 pagineWorked ExamplesDzung Pham0% (1)
- PROBLEM SET March 4.2021Documento4 paginePROBLEM SET March 4.2021Thalia RodriguezNessuna valutazione finora
- Biochemical AssDocumento1 paginaBiochemical AssbezNessuna valutazione finora
- Practical QuestionsDocumento6 paginePractical QuestionsLidiya MitikuNessuna valutazione finora
- List of Problems-Lec28Documento2 pagineList of Problems-Lec28Sachin MogerNessuna valutazione finora
- GATE HELPLINE Bioprocess Engineering MCQ IIDocumento3 pagineGATE HELPLINE Bioprocess Engineering MCQ IISanthosh Kalash100% (5)
- 2018 QuizDocumento16 pagine2018 QuizrobsNessuna valutazione finora
- Department of Chemical Engineering & Technology (PICS) Bio Chemical Engineering 8 Semester (CHE 485A)Documento6 pagineDepartment of Chemical Engineering & Technology (PICS) Bio Chemical Engineering 8 Semester (CHE 485A)Abubakr KhanNessuna valutazione finora
- Balmes, Patricia R. - Enzyme KineticsDocumento11 pagineBalmes, Patricia R. - Enzyme KineticsGlecie RasNessuna valutazione finora
- Biochemsitry 3304 Midterm 3 - KeyDocumento12 pagineBiochemsitry 3304 Midterm 3 - Keyabelopez12Nessuna valutazione finora
- 19-20 Spring PS12Documento2 pagine19-20 Spring PS12GG MMNessuna valutazione finora
- Question No 1.: CHE3165 Practical Problems Semester 1Documento2 pagineQuestion No 1.: CHE3165 Practical Problems Semester 1Khalid HassanNessuna valutazione finora
- First Year Medics TestDocumento15 pagineFirst Year Medics TestJosephNessuna valutazione finora
- Problems CSTRsDocumento5 pagineProblems CSTRsJasonBrody67% (3)
- BT 302: Biochemical Engineering Tutorial-1Documento1 paginaBT 302: Biochemical Engineering Tutorial-1Brenda BanderadoNessuna valutazione finora
- Enzyme KineticsDocumento17 pagineEnzyme KineticsIsuru JayalathNessuna valutazione finora
- Assignment - 1 Chemical Process Technology: 1. Petrochemical's End Product: Polymers & Polymerization TechniquesDocumento16 pagineAssignment - 1 Chemical Process Technology: 1. Petrochemical's End Product: Polymers & Polymerization TechniquesAnik MondalNessuna valutazione finora
- Ch06 Homework ProblemsDocumento6 pagineCh06 Homework Problemsheyyyale100% (1)
- ChE 197 Enzyme Biotechnology Practice Prob 1Documento2 pagineChE 197 Enzyme Biotechnology Practice Prob 1datUPstudentdoeNessuna valutazione finora
- Bioprocesos Exámenes Actualizado Abril 2018 TareaDocumento26 pagineBioprocesos Exámenes Actualizado Abril 2018 TareaRuben MarquezNessuna valutazione finora
- Lecture 7 (MT Resistances in Immobilized Enzyme)Documento21 pagineLecture 7 (MT Resistances in Immobilized Enzyme)sanyukta sinha100% (1)
- Biochemical Engineering Enzyme KineticsDocumento5 pagineBiochemical Engineering Enzyme KineticsLin Xian Xing33% (3)
- Lecture 8 Sample Problem AnswersDocumento5 pagineLecture 8 Sample Problem Answerssonicdragon100% (1)
- 6Documento9 pagine6Andreah BaylonNessuna valutazione finora
- Tutorial 2 CLL271 Please DO NOT Plagiarise. Severe Penalty For Non-Compliance. ReviewDocumento3 pagineTutorial 2 CLL271 Please DO NOT Plagiarise. Severe Penalty For Non-Compliance. ReviewCuriousNessuna valutazione finora
- Analytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportDa EverandAnalytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportNessuna valutazione finora
- Biofilms in Bioelectrochemical Systems: From Laboratory Practice to Data InterpretationDa EverandBiofilms in Bioelectrochemical Systems: From Laboratory Practice to Data InterpretationNessuna valutazione finora
- O Level Biology Practice Questions And Answers EnzymesDa EverandO Level Biology Practice Questions And Answers EnzymesValutazione: 5 su 5 stelle5/5 (1)
- Unloading of Goods ActivitiesDocumento3 pagineUnloading of Goods ActivitiesLeeGonzaLgoNessuna valutazione finora
- Resume KoDocumento3 pagineResume KoLeeGonzaLgoNessuna valutazione finora
- Ber 60-6Documento3 pagineBer 60-6LeeGonzaLgoNessuna valutazione finora
- What You Should Know About The Clean Water ActDocumento4 pagineWhat You Should Know About The Clean Water ActJazzel Avila ParadiseNessuna valutazione finora
- CV-Kirk Martin GonzagloDocumento2 pagineCV-Kirk Martin GonzagloLeeGonzaLgoNessuna valutazione finora
- Jethro Jones AquinoDocumento2 pagineJethro Jones AquinoLeeGonzaLgoNessuna valutazione finora
- Multiple Choice Questions in Engineering Mathematics by Diego Inocencio T. GillesaniaDocumento216 pagineMultiple Choice Questions in Engineering Mathematics by Diego Inocencio T. GillesaniaAko Si Karla90% (10)
- 40 Ways To Leave A LessonDocumento5 pagine40 Ways To Leave A Lessonliyahgotiza100% (2)
- 40 Ways To Leave A LessonDocumento5 pagine40 Ways To Leave A Lessonliyahgotiza100% (2)
- Final Chap 7Documento56 pagineFinal Chap 7LeeGonzaLgoNessuna valutazione finora
- Earthquake Preparation FinalDocumento13 pagineEarthquake Preparation FinalLeeGonzaLgoNessuna valutazione finora
- 9032 08 0Documento3 pagine9032 08 0LeeGonzaLgoNessuna valutazione finora
- Lecture Filtration 1Documento20 pagineLecture Filtration 1LeeGonzaLgo0% (2)
- Bioreactor: Chemical Reaction Engineering (CRE) Is TheDocumento25 pagineBioreactor: Chemical Reaction Engineering (CRE) Is ThekhunafaNessuna valutazione finora
- Biomimetic ChemistryDocumento29 pagineBiomimetic ChemistryPrabhatNessuna valutazione finora
- Cell KineticsDocumento56 pagineCell KineticsXy karNessuna valutazione finora
- Enzyme Assays A Practical Approach by Robert Eisenthal, Michael DansonDocumento304 pagineEnzyme Assays A Practical Approach by Robert Eisenthal, Michael DansonSara OchoaNessuna valutazione finora
- Enzyme KineticsDocumento72 pagineEnzyme Kineticsitokki otoya100% (1)
- Biochemistry DKA NOTESDocumento278 pagineBiochemistry DKA NOTESTrisNessuna valutazione finora
- Sas4 Bio024Documento24 pagineSas4 Bio024Merlyn Limbaga CastroverdeNessuna valutazione finora
- M.tech (Chem) R13Documento54 pagineM.tech (Chem) R13Siva Jagadish Kumar MNessuna valutazione finora
- Luff SchoorlDocumento13 pagineLuff Schoorlmarijuana.hesNessuna valutazione finora
- Enzyme InhibitionDocumento7 pagineEnzyme InhibitionJane Docdoc100% (1)
- Bachelor of Biomedical Science (Hons) Programme Metabolic Biochemistry BBS2121Documento6 pagineBachelor of Biomedical Science (Hons) Programme Metabolic Biochemistry BBS2121limNessuna valutazione finora
- Enzyme Inhibition/Enzyme InhibitorsDocumento5 pagineEnzyme Inhibition/Enzyme InhibitorsFaria bukhariNessuna valutazione finora
- Lesson 6 InhibitorsDocumento12 pagineLesson 6 Inhibitorstbrook2017Nessuna valutazione finora
- Assignmnet 6 - SolutionDocumento3 pagineAssignmnet 6 - SolutionRita100% (1)
- Biochemistry PRC 2 Data Analysis EnzymeDocumento4 pagineBiochemistry PRC 2 Data Analysis EnzymeSARAH SAFIAH TAJUL ARIFFINNessuna valutazione finora
- Lecture 1 - Enzyme & Kinetics PDFDocumento33 pagineLecture 1 - Enzyme & Kinetics PDFRooth AdajetNessuna valutazione finora
- Dynamic Model For Nutrient Uptake by Tomato Plant in HydroponicsDocumento46 pagineDynamic Model For Nutrient Uptake by Tomato Plant in HydroponicsAmmarsalNessuna valutazione finora
- Week 2 - Enzyme Kinetics All - v2 - 3slide HandoutsDocumento14 pagineWeek 2 - Enzyme Kinetics All - v2 - 3slide HandoutsFizza HussainNessuna valutazione finora
- Lineweaver Burk PlotDocumento2 pagineLineweaver Burk PlotTAN YEE LINGNessuna valutazione finora
- Targeting Root Ion Uptake Kinetics To Increase Plant Productivity and Nutrient Use Efficiency (Plant Physiology Article)Documento15 pagineTargeting Root Ion Uptake Kinetics To Increase Plant Productivity and Nutrient Use Efficiency (Plant Physiology Article)Noel CambayaNessuna valutazione finora
- Cell Lab ReportDocumento10 pagineCell Lab ReportWilliamNessuna valutazione finora
- Lecture 26-Problems On Cell Growth KineticsDocumento15 pagineLecture 26-Problems On Cell Growth KineticsHemanth Peddavenkatappa GariNessuna valutazione finora
- EnzymeDocumento3 pagineEnzymeCZARYL HANNAH GLORIANessuna valutazione finora
- Lecture Notes in Medical Technology - Lecture #11 - EnzymologyDocumento22 pagineLecture Notes in Medical Technology - Lecture #11 - EnzymologyKat JornadalNessuna valutazione finora
- Anaerobic Degradation of Textile Dye Bath Effluent Using Halomonas SPDocumento5 pagineAnaerobic Degradation of Textile Dye Bath Effluent Using Halomonas SPFlor GonzalezNessuna valutazione finora
- Problems in Biochemical EngineeringDocumento22 pagineProblems in Biochemical EngineeringAdu GilbertNessuna valutazione finora
- SyllabusDocumento22 pagineSyllabusSohael AftabNessuna valutazione finora
- Optimization of Extracellular Keratinase Production by Aspergillus Terreus Isolated From Chicken's LitterDocumento7 pagineOptimization of Extracellular Keratinase Production by Aspergillus Terreus Isolated From Chicken's LitterKanhiya MahourNessuna valutazione finora
- Bioprocess Technology Kinetics and Reactors by Professor Dr. Anton MoserDocumento479 pagineBioprocess Technology Kinetics and Reactors by Professor Dr. Anton MoserNguyễn Ánh100% (3)
- Chapter 5 Cell Kinetics and Fermenter Design - PPT (Update) - 1 Bio GamalDocumento101 pagineChapter 5 Cell Kinetics and Fermenter Design - PPT (Update) - 1 Bio GamalMatewos Sada100% (1)