Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
P-V Diagram Into T-S Diagram
Caricato da
sxcastro0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
54 visualizzazioni5 pagineThermodynamics, pv diagram to ts diagram
Titolo originale
P-V Diagram Into T-s Diagram
Copyright
© © All Rights Reserved
Formati disponibili
PDF o leggi online da Scribd
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoThermodynamics, pv diagram to ts diagram
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PDF o leggi online su Scribd
0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
54 visualizzazioni5 pagineP-V Diagram Into T-S Diagram
Caricato da
sxcastroThermodynamics, pv diagram to ts diagram
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PDF o leggi online su Scribd
Sei sulla pagina 1di 5
Skip to content
Exergic.in
Online courses for GATE
Home
Online Content
TE18 Test Series
Revision & Crash Course
Question Bank
Shortcuts to convert P-v diagram into T-s
diagram
1 Comment / THERMO / By Exergic
Most of you might have faced problems while converting P-v diagram into T-s
diagram. In this post I will be sharing some shortcuts using which you can easily
convert any P-v diagram into T-s diagram.
Tam starting with basic process and then discuss polytropic process in the end.
isothermal proces:
Note that constant temperature line is drawn such that entropy has decreased.
Why? Why not along increasing entropy? Keep this funda in mind that
increase/decrease in entropy in T-s diagram is determined only by a single
phenomenon : Heat addition/rejection
Ifheat is being added, entropy will increase. If heat is being rejected, entropy will
decrease. | -2 is compression still temperature is same. Using first law, this is
possible only if the system is rejecting heat, so entropy should also decrease.
2s
2. Isentropic process:
Note that constant entropy (rev adiabatic) line is drawn such that temperature has
increased. Why? Why not along decreasing temperature? You have to see this from
the process on P-v. It is an adiabatic compression, so T will increase. If it was
adiabatic expansion, T would have decreased.
P
2, ' 7
S96
ee 1
Vv s
3. Constant volume process:
Slope of constant volume line on T-s diagram = T / Cv.
This knowledge will be all to convert isometric (v=c) process from P-v into T-s.
See Image.
2
1
v
. oa
« Slepe>
ste Th Sipe f
& Soper > SHOL cuQr¢
4. Constant pressure process:
Slope of constant volume line on T-s diagram = T/Cp
This knowledge will be all to convert isometric (p=c) process from P-v into T-s.
See Image.
Note that on T-s diagram, slope of constant volume line is more than slope of
constant pressure since T/Cv > T/Cp
e* = x 2
3 clepe e fo
ote TH Sipe f
%, Slobe @ 2 ? slepe@ 1 shpe@} L
5. Polytropic process:
Use the funda of polytropic specific heat in determining this.
Polytropic Specific Heat
n
mee
n
C potyropic =
- fr 1 Y, polytropic specific heat will be +ve
From the relation Q= meAT, if ¢ is positive, increase in T will increase Q (so
increase entropy) and decrease in T will decrease Q (so decrease entropy).
It means if n>gamma, then T and $ will have same characteristic.
Learn more such shortcuts which are not taught in any coaching:
Enrolled students of Exergic rated their overall experience 4.9 out
of 5.0 in previously conducted survey. (learn.exergic.in
Post navigation
<= Previous Post
Next Post >
| thought on “Shortcuts to convert P-v diagram into T-s diagram”
Kumar Rajesh
March 25, 2017 at 6:02 pm
thank you very much sir.
Potrebbero piacerti anche
- Introduction To Matlab For Experimental PhysicsDocumento34 pagineIntroduction To Matlab For Experimental PhysicssxcastroNessuna valutazione finora
- Accretion DisksDocumento23 pagineAccretion DiskssxcastroNessuna valutazione finora
- Intro Programming Using MatlabDocumento5 pagineIntro Programming Using MatlabsxcastroNessuna valutazione finora
- A Review of Redshift and Its Interpretation in Cosmology and AstrophysicsDocumento16 pagineA Review of Redshift and Its Interpretation in Cosmology and AstrophysicssxcastroNessuna valutazione finora
- X-Winds Theory and ObservationsDocumento27 pagineX-Winds Theory and ObservationssxcastroNessuna valutazione finora
- X Spec ManualDocumento427 pagineX Spec ManualsxcastroNessuna valutazione finora
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (400)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (74)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (345)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- Helipad DesignDocumento61 pagineHelipad DesignOkie RezkianNessuna valutazione finora
- Bolt Preload Calculation PDFDocumento2 pagineBolt Preload Calculation PDFTimon2005Nessuna valutazione finora
- Bell Helicopter Stabilizer BarDocumento6 pagineBell Helicopter Stabilizer Barjorge paez100% (1)
- Performance-Based Design in Earthquake Engineering: A Multidisciplinary ReviewDocumento19 paginePerformance-Based Design in Earthquake Engineering: A Multidisciplinary ReviewBrowinMethownaDavidNessuna valutazione finora
- Chapter 4Documento26 pagineChapter 4gilbert850507Nessuna valutazione finora
- Chicago Undergraduate Physics BibliographyDocumento12 pagineChicago Undergraduate Physics Bibliographyyouth4everNessuna valutazione finora
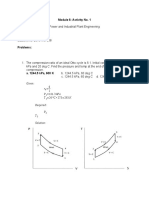
- Module 6 Exercises Problem No. 1Documento4 pagineModule 6 Exercises Problem No. 1Ariel GamboaNessuna valutazione finora
- Force, Torque and StrainDocumento22 pagineForce, Torque and StrainLungie MbathaNessuna valutazione finora
- FCGuide-pre4 2 0Documento124 pagineFCGuide-pre4 2 0José MiguelNessuna valutazione finora
- 12VCE Physics Notes 2014Documento135 pagine12VCE Physics Notes 2014zachbeefNessuna valutazione finora
- 0300708: Advanced Heat ConductionDocumento34 pagine0300708: Advanced Heat Conductionvanchai sapaNessuna valutazione finora
- Hacon TMDocumento68 pagineHacon TMTiago CoutoNessuna valutazione finora
- Magnitude IntensityDocumento15 pagineMagnitude IntensitymamandaweNessuna valutazione finora
- Lesson 4 - Heat TransferDocumento16 pagineLesson 4 - Heat TransferJoanna Ruth SeproNessuna valutazione finora
- Thermodynamic Properties of Superheated and Supercritical Steam PDFDocumento7 pagineThermodynamic Properties of Superheated and Supercritical Steam PDFRoberto ErazoNessuna valutazione finora
- Transmission MTDocumento29 pagineTransmission MTDidier ÁlvarezNessuna valutazione finora
- API Calcs Rev1 (Version 2)Documento112 pagineAPI Calcs Rev1 (Version 2)Jake Sparrow100% (1)
- Heat and Mass Transfer Through A Thick Bed of Cocoa Beans During DryingDocumento9 pagineHeat and Mass Transfer Through A Thick Bed of Cocoa Beans During DryingFadli Ryan ArikundoNessuna valutazione finora
- Vortex Methods Theory and Practice by Georges Henri Cottet PDFDocumento4 pagineVortex Methods Theory and Practice by Georges Henri Cottet PDFLam Trinh NguyenNessuna valutazione finora
- Simplified Response Analysis of Earth Dams To Spatially Varying Earthquake Ground MotionDocumento8 pagineSimplified Response Analysis of Earth Dams To Spatially Varying Earthquake Ground Motionlphuong_20Nessuna valutazione finora
- "Chapter 7 - Deflection of Beams - Geometric Methods" in "Structural Analysis" On Manifold @tupressDocumento35 pagine"Chapter 7 - Deflection of Beams - Geometric Methods" in "Structural Analysis" On Manifold @tupressrpsirNessuna valutazione finora
- Engine Performance and ModelingDocumento20 pagineEngine Performance and ModelingsathishskymechNessuna valutazione finora
- LandingString, 80%, 5.875 OD, 0.750 Wall, IEU, S-135.. XT57 (7.250 X 3.500)Documento3 pagineLandingString, 80%, 5.875 OD, 0.750 Wall, IEU, S-135.. XT57 (7.250 X 3.500)Garcia C L AlbertoNessuna valutazione finora
- 4.state of Matter - Gases and Liquids - 72-95Documento8 pagine4.state of Matter - Gases and Liquids - 72-95eamcetmaterialsNessuna valutazione finora
- Torsional Stability CheckingDocumento3 pagineTorsional Stability Checkingpile raftNessuna valutazione finora
- Gec 223 AssignmentDocumento6 pagineGec 223 AssignmentDaniel AgbajeNessuna valutazione finora
- Plano Explosivo & Partes y Piezas Wmf-m160gbDocumento7 paginePlano Explosivo & Partes y Piezas Wmf-m160gbCatalina GonzalezNessuna valutazione finora
- DCS800 Winder DescriptionDocumento53 pagineDCS800 Winder DescriptionAndré Carlos CorenzanNessuna valutazione finora
- Shear and Moment Diagrams For Frames Shear and Moment Diagrams For FramesDocumento5 pagineShear and Moment Diagrams For Frames Shear and Moment Diagrams For FramesYurene HornaNessuna valutazione finora
- Understanding Choked FlowDocumento2 pagineUnderstanding Choked FlowDaniel DuongNessuna valutazione finora