Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Egg Osmosis Lab
Caricato da
api-3915406710 valutazioniIl 0% ha trovato utile questo documento (0 voti)
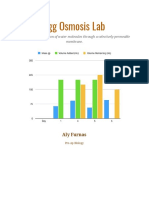
86 visualizzazioni2 pagineThis lab experiment examines osmosis through observing eggs in different solutions. An egg was placed in vinegar, which is hypotonic, causing the egg to absorb water and increase in mass from 61.5g to 85.9g. The egg was then placed in Karo syrup, which is hypertonic, causing it to lose water and decrease in mass to 50.8g. Finally, the egg was returned to water, becoming hypotonic, increasing its mass again to 90.3g. The student concluded that osmosis occurred based on changes in the egg's mass and the solution volumes.
Descrizione originale:
Titolo originale
egg lab
Copyright
© © All Rights Reserved
Formati disponibili
PDF, TXT o leggi online da Scribd
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoThis lab experiment examines osmosis through observing eggs in different solutions. An egg was placed in vinegar, which is hypotonic, causing the egg to absorb water and increase in mass from 61.5g to 85.9g. The egg was then placed in Karo syrup, which is hypertonic, causing it to lose water and decrease in mass to 50.8g. Finally, the egg was returned to water, becoming hypotonic, increasing its mass again to 90.3g. The student concluded that osmosis occurred based on changes in the egg's mass and the solution volumes.
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PDF, TXT o leggi online su Scribd
0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
86 visualizzazioni2 pagineEgg Osmosis Lab
Caricato da
api-391540671This lab experiment examines osmosis through observing eggs in different solutions. An egg was placed in vinegar, which is hypotonic, causing the egg to absorb water and increase in mass from 61.5g to 85.9g. The egg was then placed in Karo syrup, which is hypertonic, causing it to lose water and decrease in mass to 50.8g. Finally, the egg was returned to water, becoming hypotonic, increasing its mass again to 90.3g. The student concluded that osmosis occurred based on changes in the egg's mass and the solution volumes.
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PDF, TXT o leggi online su Scribd
Sei sulla pagina 1di 2
Egg Osmosis Lab
If one observes an egg in different solutions, then they can see how a cell goes through osmosis to
react to hypotonic and hypertonic solutions.
Sophie Wickersham - Ms. Jeter Biology Pre- AP Period
The lab started when a single cell (chickens
egg) was placed in 200 mL of vinegar.
Vinegar is a hypotonic solution, meaning
there is more solutes inside of the cell than
outside. Thus, water passes through the
membrane and into the cell. The starting
mass of the egg was 61.5 g, after soaking in
vinegar for 3 days the mass of the egg was
85.9 g. To further prove that osmosis
occurred, the starting amount of vinegar was
200 mL, after 3 days it dropped to 175 mL.
Day 1 Day 4
This is a 12.5 % decrease from the starting
amount of vinegar. The water molecules in
the vinegar were able to pass through the
membrane, therefore decreasing the volume
of liquid in the beaker and making the egg
larger
Karo syrup is a hypertonic solution, meaning there are
more solutes outside of the egg than the inside and more
water inside the egg than outside. Thus, water passes
through the membrane into the liquid surrounding the egg.
This makes the egg look small and shrunken. The mass of
the egg on day 3 was 85.9 g, after soaking in the syrup the
mass of the egg was 50.8 g. This is a 40.8614% decrease of
the eggs mass. Also, the beginning amount of liquid in the
beaker was 200 mL , after the egg soaked in the liquid there
was 235 mL of liquid in the beaker. The amount of liquid
increased by 17.5%.This shows that water passed through
the membrane and into the syrup.
EGG OSMOSIS LAB- SOPHIE WICKERSHAM 1
How can you explain the amount of liquid remaining when the egg was removed from the syrup?
Syrup is a hypertonic solution, meaning there was less water (more solvents) in the
syrup than there was in the egg. This caused water from inside the cell to pass through
membrane into the syrup. The liquid amount would increase due to the loss of water from
the egg through osmosis. At the start of the day, the egg was put in 200 mL of syrup, by the
next day there was 235 mL of liquid in the beaker. This is a 17.5 % increase of liquid in the
beaker due to osmosis.
Water is a hypotonic solution, meaning there was less solutes
outside of the egg than inside of the egg. This caused water to
pass through the membrane and into the egg. Before soaking
in water, the eggs mass was 50.8 grams, after soaking in water
the mass was 90.3 grams. This is a 77.75590% increase of the
mass. The staring measurement of water in the beaker was
200 mL, after the egg soaked in the beaker for a day, the
volume remaining 155 mL. This is a 22.5% decrease in the
liquid remaining in the beaker, thus osmosis occurred and
water passed through the membrane and into the cell.
Was your hypothesis validated?
Yes, the egg was placed in different hypotonic and hypertonic solutions causing
solvents to pass through the cell membrane (osmosis did occur). Thus, causing the
inflation and deflation of the egg. One can prove osmosis by looking at the mass of
the egg. If the mass has increased, then the egg was placed in a hypotonic solution
and took in water (causing the egg to have a greater mass). If the mass of the egg
decreased, then the egg was placed in hypertonic solution and expelled water
(causing the egg to have a lower mass). One can also tell if osmosis has occurred
by looking at the remaining liquid in the beaker when you remove the egg. If the
liquid level has increased, then the egg was placed in a hypertonic solution
causing it to lose/expel water. If the liquid level has decreased, then the egg was
placed in a hypotonic solution causing the egg to take in water.
EGG OSMOSIS LAB- SOPHIE WICKERSHAM 2
Potrebbero piacerti anche
- MEIOSIS WorksheetDocumento8 pagineMEIOSIS WorksheetEliseo PamandananNessuna valutazione finora
- AABB Sample New Program Business PlanDocumento14 pagineAABB Sample New Program Business Planbiospacecowboy100% (1)
- Topic 1-Osmosity and Tonicity - EditedDocumento6 pagineTopic 1-Osmosity and Tonicity - Editedsalve joy villanuevaNessuna valutazione finora
- Fermentation Lab Report Example With Guidelines To Write Lab ReportsDocumento6 pagineFermentation Lab Report Example With Guidelines To Write Lab ReportsbellasyazanaNessuna valutazione finora
- Osmosis Practice ProblemsDocumento3 pagineOsmosis Practice ProblemsOlinese AugustinNessuna valutazione finora
- Diffusion and Osmosis WorksheetDocumento2 pagineDiffusion and Osmosis WorksheetDesmond simpsonNessuna valutazione finora
- Lab Report 1Documento14 pagineLab Report 1api-340424634100% (5)
- Egg Osmosis LabDocumento11 pagineEgg Osmosis Labchizzy gNessuna valutazione finora
- Math Internal Assessment:: Investigating Parametric Equations in Motion ProblemsDocumento16 pagineMath Internal Assessment:: Investigating Parametric Equations in Motion ProblemsByron JimenezNessuna valutazione finora
- Properties of LiquidsDocumento24 pagineProperties of LiquidsRogelyn Mejia BarbocoNessuna valutazione finora
- Osmosis, Diffusion, Active TransportDocumento21 pagineOsmosis, Diffusion, Active TransportPrince SanjiNessuna valutazione finora
- Egg Osmosis LabDocumento4 pagineEgg Osmosis Labapi-371081506Nessuna valutazione finora
- Photosynthesis Activity Sheet Group6Documento5 paginePhotosynthesis Activity Sheet Group6Angelica TejadaNessuna valutazione finora
- Transport Mechanism Quiz-1Documento2 pagineTransport Mechanism Quiz-1KLARK ANDREW MASAQUEL MARQUEZNessuna valutazione finora
- Plant Science NotesDocumento14 paginePlant Science NotesMerve ÖzkayaNessuna valutazione finora
- 01 Macromolecules Study Guide ANSWERSDocumento5 pagine01 Macromolecules Study Guide ANSWERSkaycelyn jimenezNessuna valutazione finora
- Enzymes Lab Report - Activty 8Documento6 pagineEnzymes Lab Report - Activty 8JengNessuna valutazione finora
- Diffusion LabDocumento8 pagineDiffusion LabGioVanna GVNessuna valutazione finora
- 2015 Incomplete, Codominance, Multiple Allele Notes and PracticeDocumento7 pagine2015 Incomplete, Codominance, Multiple Allele Notes and Practiceapi-264255406100% (1)
- Onion Root Mitosis LabDocumento6 pagineOnion Root Mitosis Labapi-2469736100% (1)
- Diffusion and Osmosis Worksheet: The AnswersDocumento15 pagineDiffusion and Osmosis Worksheet: The AnswersMr GreyNessuna valutazione finora
- Diffusion and OsmosisDocumento3 pagineDiffusion and OsmosisNURUL AZZAHNessuna valutazione finora
- Lab Report: Cell TransportDocumento4 pagineLab Report: Cell Transportmaieunice75% (4)
- Scientific Method and Experimental DesignDocumento40 pagineScientific Method and Experimental Designsheila may valiao-de asisNessuna valutazione finora
- AP Biology Lab Two: Enzyme CatalysisDocumento4 pagineAP Biology Lab Two: Enzyme CatalysisCoolAsianDude95% (37)
- SBI4U Unit 5 Practice Test AnswersDocumento6 pagineSBI4U Unit 5 Practice Test AnswersAreebaNessuna valutazione finora
- Biology1125-Egg Osmosis Lab ReportDocumento8 pagineBiology1125-Egg Osmosis Lab ReportJueNessuna valutazione finora
- Egg Osmosis Lab ReportDocumento5 pagineEgg Osmosis Lab Reportapi-39326299925% (4)
- Pre Test Yeast and Glucose ExperimentDocumento4 paginePre Test Yeast and Glucose ExperimentAisya Omeira100% (1)
- Onion Root Tip MitosisDocumento5 pagineOnion Root Tip MitosisNor Ashikin IsmailNessuna valutazione finora
- Egg Osmosis Activity ObservationDocumento1 paginaEgg Osmosis Activity ObservationEllen Joy Velasco BrilloNessuna valutazione finora
- Modeling Osmosis LabDocumento4 pagineModeling Osmosis Labapi-208317373Nessuna valutazione finora
- Reduced Answer Cell Transport Level Test 2016Documento3 pagineReduced Answer Cell Transport Level Test 2016John Kevin NocheNessuna valutazione finora
- Diffusion Osmosis Lab 2Documento3 pagineDiffusion Osmosis Lab 2briangkentNessuna valutazione finora
- Properties of Liquids: General Chemistry 2 Engr. Jozel Bryan M. TerribleDocumento30 pagineProperties of Liquids: General Chemistry 2 Engr. Jozel Bryan M. TerribleJozel Bryan Mestiola TerrìbleNessuna valutazione finora
- Surface Tension Lab - ProcedureDocumento3 pagineSurface Tension Lab - Procedurekatherine corveraNessuna valutazione finora
- Chapter 4: Functional Anatomy of Prokaryotic and Eukaryotic CellsDocumento94 pagineChapter 4: Functional Anatomy of Prokaryotic and Eukaryotic CellsTrevannie EdwardsNessuna valutazione finora
- Dna Extraction From Cheek CellsDocumento9 pagineDna Extraction From Cheek CellsJohn Lhoyd TuazonNessuna valutazione finora
- Biol 240 Lab Exam Summary NotesDocumento14 pagineBiol 240 Lab Exam Summary NotesMichael BrooksNessuna valutazione finora
- Diffusion and Osmosis Lab Report-3Documento12 pagineDiffusion and Osmosis Lab Report-3api-502015003Nessuna valutazione finora
- Lab ReportDocumento24 pagineLab Reportwol aldo0% (1)
- Week 3 Lab Diffusion OsmosisDocumento8 pagineWeek 3 Lab Diffusion OsmosisoxnerdkiNessuna valutazione finora
- Osmosis Diffusion Lab-1Documento7 pagineOsmosis Diffusion Lab-1api-1658690000% (1)
- Physical or Chemical ChangeDocumento47 paginePhysical or Chemical ChangeMaicie Rose Bautista VallesterosNessuna valutazione finora
- Laboratory 1. ANALYSIS OF PLANT PIGMENTS USING PAPER CHROMATOGRAPHYDocumento8 pagineLaboratory 1. ANALYSIS OF PLANT PIGMENTS USING PAPER CHROMATOGRAPHYGualberto Tampol Jr.Nessuna valutazione finora
- Seed Germination Lab WorkDocumento3 pagineSeed Germination Lab Workashwathimahendran100% (1)
- Beano LabDocumento12 pagineBeano Labapi-284496286Nessuna valutazione finora
- AP Bio Lab1Documento10 pagineAP Bio Lab1Eva DesireeNessuna valutazione finora
- Fehling TestDocumento7 pagineFehling TestMg HNessuna valutazione finora
- POGIL Cell Size-KEYDocumento5 paginePOGIL Cell Size-KEYKali WarnkeNessuna valutazione finora
- Osmosis Lab ReportDocumento5 pagineOsmosis Lab Reportapi-281921983Nessuna valutazione finora
- Properties of WaterDocumento10 pagineProperties of WaterJohn Morrel D. MirandaNessuna valutazione finora
- Rosemarie Parse PDFDocumento4 pagineRosemarie Parse PDF李思雨Nessuna valutazione finora
- Lab Photosynthesis InquiryDocumento6 pagineLab Photosynthesis Inquirycorygunther100% (1)
- Identification of Bacteria Using Gram Staining MethodDocumento7 pagineIdentification of Bacteria Using Gram Staining MethodDani MughalNessuna valutazione finora
- 01-g Cell Fractionation - Definition, Steps MethodsDocumento6 pagine01-g Cell Fractionation - Definition, Steps MethodsCaffe AncaeusNessuna valutazione finora
- Experiment 8Documento4 pagineExperiment 8api-252952453100% (3)
- Bot 20 Exercise 8 PhotosynthesisDocumento3 pagineBot 20 Exercise 8 PhotosynthesisSam PabloNessuna valutazione finora
- Egg Osmosis LabDocumento1 paginaEgg Osmosis Labapi-391544799Nessuna valutazione finora
- Egg Lab Write - UpDocumento2 pagineEgg Lab Write - Upapi-391713335Nessuna valutazione finora
- Egg Lab: Charlotte NealDocumento4 pagineEgg Lab: Charlotte Nealapi-391552921Nessuna valutazione finora
- Egg Osmosis LabDocumento5 pagineEgg Osmosis Labapi-391626668Nessuna valutazione finora
- Đề Thi Tham Khảo (Đề thi có 5 trang) : 6: The flood victims 7: Many 8Documento7 pagineĐề Thi Tham Khảo (Đề thi có 5 trang) : 6: The flood victims 7: Many 8Quang Đặng HồngNessuna valutazione finora
- INV201 ReadParameterDocumento3 pagineINV201 ReadParameterRaul ArbaniesNessuna valutazione finora
- Manage Your Nude PhotosDocumento14 pagineManage Your Nude PhotosRick80% (5)
- Kolkata City Accident Report - 2018Documento48 pagineKolkata City Accident Report - 2018anon_109699702Nessuna valutazione finora
- Multithreading AlgorithmsDocumento36 pagineMultithreading AlgorithmsAsna TariqNessuna valutazione finora
- Numerical Methods - Fixed Point IterationDocumento4 pagineNumerical Methods - Fixed Point IterationMizanur RahmanNessuna valutazione finora
- How To Group Currency PDFDocumento3 pagineHow To Group Currency PDFanon_909438945Nessuna valutazione finora
- Marvel Science StoriesDocumento4 pagineMarvel Science StoriesPersonal trainerNessuna valutazione finora
- Project Guidelines: (A Manual For Preparation of Project Report)Documento4 pagineProject Guidelines: (A Manual For Preparation of Project Report)Shivam TiwariNessuna valutazione finora
- Curriculum Vitae: Name: Father S Name: Cell No: Email: Date of Birth: Nationality: Cnic No: Passport No: AddressDocumento2 pagineCurriculum Vitae: Name: Father S Name: Cell No: Email: Date of Birth: Nationality: Cnic No: Passport No: AddressJahanzeb JojiNessuna valutazione finora
- 27 EtdsDocumento29 pagine27 EtdsSuhag PatelNessuna valutazione finora
- Partnerships Program For Education and Training (PAEC)Documento6 paginePartnerships Program For Education and Training (PAEC)LeslieNessuna valutazione finora
- Money, Sex, and Power: Toward A Feminist Historical MaterialismDocumento2 pagineMoney, Sex, and Power: Toward A Feminist Historical MaterialismddwererNessuna valutazione finora
- Mmims5elesson BiomesDocumento3 pagineMmims5elesson Biomesapi-490524730100% (1)
- Raglio 2015 Effects of Music and Music Therapy On Mood in Neurological Patients PDFDocumento12 pagineRaglio 2015 Effects of Music and Music Therapy On Mood in Neurological Patients PDFsimaso0% (1)
- Manufacturing Technology - Metrology: Dr.B.Ramamoorthy Professor Manufacturing Engg. Section Iitmadras 600 036Documento22 pagineManufacturing Technology - Metrology: Dr.B.Ramamoorthy Professor Manufacturing Engg. Section Iitmadras 600 036Ramasubramanian KannanNessuna valutazione finora
- Kaizen Idea - Sheet: Suitable Chairs To Write Their Notes Faster & Easy Method While TrainingDocumento1 paginaKaizen Idea - Sheet: Suitable Chairs To Write Their Notes Faster & Easy Method While TrainingWahid AkramNessuna valutazione finora
- DSSSSPDocumento3 pagineDSSSSPChris BalmacedaNessuna valutazione finora
- Chapter - 5 Theory of Consumer BehaviourDocumento30 pagineChapter - 5 Theory of Consumer BehaviourpoojNessuna valutazione finora
- A Tribute To Fernando L. L. B. Carneiro (1913 - 2001)Documento2 pagineA Tribute To Fernando L. L. B. Carneiro (1913 - 2001)Roberto Urrutia100% (1)
- SugaNate 160NCDocumento5 pagineSugaNate 160NCmndmattNessuna valutazione finora
- 1300 Rev01Documento15 pagine1300 Rev01Manuel AltamiranoNessuna valutazione finora
- Saint Albert Polytechnic College, Inc. Bachelor of Science in Office AdministrationDocumento15 pagineSaint Albert Polytechnic College, Inc. Bachelor of Science in Office AdministrationAustin Grey Pineda MacoteNessuna valutazione finora
- Kina23744ens 002-Seisracks1Documento147 pagineKina23744ens 002-Seisracks1Adrian_Condrea_4281Nessuna valutazione finora
- Collaborative Planning Template W CaptionDocumento5 pagineCollaborative Planning Template W Captionapi-297728751Nessuna valutazione finora
- 01CDT00330 - Lakeshore - 330 - ManualDocumento104 pagine01CDT00330 - Lakeshore - 330 - ManualSPMS_MELECNessuna valutazione finora
- Culvert Design Write UpDocumento8 pagineCulvert Design Write UpifylasyNessuna valutazione finora
- Physics For EngineersDocumento5 paginePhysics For EngineersKonstantinos FilippakosNessuna valutazione finora