Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Final Lab Report Group 1 Flow Through Fluidized Beds
Caricato da
Charlie CB PortnerTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Final Lab Report Group 1 Flow Through Fluidized Beds
Caricato da
Charlie CB PortnerCopyright:
Formati disponibili
Flow Through Fluidized Beds University of Illinois
Flow Through Fluidized Beds
Basic diagram of a fluidized bed reactor showing the how the gas entering through the distributor
at the base bubbles up through the packed bed and causes it to become fluidized. The solid
particles are the catalyst for the reaction, fluidization allows for more effective use of the surface
area of the particles as well as achieving more uniform temperature gradient and degree of
mixing while running the reactor in a continuous state.
http://en.wikipedia.org/wiki/File:Fluidized_Bed_Reactor_Graphic.JPG
Unit Operations Lab 3
October 22, 2009
Group 1:
Michael Czepizak
Krista Sutton
Jake Biberstein
Stanley Das
Russell Boyer
Jeff Umbach
Unit Operations ChE-381 Group No. 1 p. 1 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
0. Table Of Contents
0. Table Of Contents...................................................................................................2
1. WP&C.....................................................................................................................3
2. Abstract...................................................................................................................4
3. Introduction.............................................................................................................5
4. Theory.....................................................................................................................6
5. Apparatus..............................................................................................................12
6. Materials and Supplies..........................................................................................17
7. Procedure..............................................................................................................18
8. Data Tabulation.....................................................................................................24
9. Results...................................................................................................................30
10. Discussion...........................................................................................................33
11. Error Analysis.....................................................................................................36
12. Conclusion..........................................................................................................37
13. References...........................................................................................................38
14. Appendix I..........................................................................................................40
15. Appendix II.........................................................................................................40
Unit Operations ChE-381 Group No. 1 p. 2 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
1. WP&C
What is the purpose of this experiment?
The purpose of Flow Through Fluidized Beds is to measure the effects of particle size,
packing amount, packing materials, and heated packing materials on flow through a packed
column. We will be working with high-pressure air streams and a heater. Inhalation hazards are
unlikely as there is insufficient airflow for packing material to escape from the column.
What are the hazards associated with the experiment?
Eye and ear damage can result from compressed air streams. Spilled sand or silica can
present a slipping hazard. There is a potential burning hazard from the heater in the silica packed
column. Be prepared to clean up any broken glass.
How will the experiment be conducted in a safe manner?
All valves will remain closed until the apparatus has been checked for leaks, buildup of
materials, and stoppages. The air inlet valve will be opened and the pressure checked before the
valve into the apparatus is opened.
What safety controls are in place?
The high-pressure air first enters the apparatus through a pressure valve that should be set
to approximately 40.0 PSI, not to exceed 100.0 PSI. Each column then has an independent air
flowmeter and shutoff valve.
Describe safe and unsafe ranges of operations.
The range of airflow needed to conduct the experiment is 0.0-1000.0 cc/s of air for the
sand packed column and 0.0-13.9 SCFM for the silica packed column. It is not possible to
increase the flow beyond these values; if it were possible then doing so would risk damage to
sensitive equipment. The pressure range for the apparatus is 0.0-100.0 PSI, which should not be
exceeded.
I have read the relevant background material for the Unit Operations Laboratory entitled:
Flow Through Fluidized Beds and understand the hazards associated with conducting this
experiment. I have planned out my experimental work in accordance to standards and acceptable
safety practices and will conduct all of my experimental work in a careful and safe manner. I
will also be aware of my surroundings, my group members, and other lab students, and will look
out for their safety as well.
Signatures: _Jeff Umbach_______________________________________
_Russell Boyer_______________________________________
_Michael Czepizak___________________________________
_Krista Sutton_______________________________________
_Jake Biberstein______________________________________
_Stanley Das_________________________________________
Unit Operations ChE-381 Group No. 1 p. 3 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
2. Abstract
In this experiment, we measured pressure drop vs. flow rate in two different columns
packed with sand or silica at different heights and temperatures. Theoretically, the superficial
velocity Vs should equal the air velocity at minimum fluidization Vf. For the sand trials, we
found that an average Vs of 0.045 +/- 0.011 meters/second for the large grain sand. The small
grain sand had an average Vs of 0.034 +/- 0.0083 meters/sec. For silica, the Vs values were 0.440
+
/- 0.109 meters/second and 0.321 +/- 0.080 meters/second. The Vf values for the small grain
sand were very close to the theoretical values with an average of 0.035 +/- 0.0082 meters/sec.
The Vf values for the large grain sand did not fit the theoretical values. The Vf values for the
large grain sand averaged out to 0.110 +/- 0.027 meters/second, which is more than twice the Vs
value. The Vf values for silica were 0.50 +/- 0.124 meters/second and 0.48 +/- 0.119
meters/second for unheated and heated silica respectively. These values are close to the Vs
values, but they are not as accurate as the values obtained for the smaller grain size of sand. In
general, the pressure drop in the column was greater when more material was added to the
column bed. Also, the heated silica fluidized much faster than the room temperature, which
would be expected due to lower densities of air particles at higher temperatures.
Unit Operations ChE-381 Group No. 1 p. 4 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
3. Introduction
Fluidized bed reactors are a relatively new tool in the chemical engineering field. Fritz
Winkler developed the first fluidized bed gas generator in Germany in the 1920s. One of the
first United States fluidized bed reactors used was the Catalytic Cracking Unit, created in Baton
Rouge, LA in 1942 by the Standard Oil Company (Exxon Mobil). A fluidized bed is a packed
bed through which fluid flows at such a high velocity that the particles in the bed are loosened
such that the bed behaves as though it is a liquid.
Fluidized beds provide a large surface area for contact between solids and a liquid or a
gas that is conducive for heat and mass transfer. In this environment, nearly uniform
temperatures can be maintained in the reactor even with highly exothermic reactions. This is
important because a temperature gradient can form in a poorly mixed bed, leading to equipment
failure, product degradation, and decreased efficacy of the reaction. A fluidized bed also provides
uniform mixing, which is important for product quality and efficiency. Fluidized bed reactors
are often a continuous process, meaning they are also very efficient compared to batch processes.
However, there are some disadvantages to fluidized beds. One disadvantage is that the
cost of a fluidized bed reactor is usually high because the vessels are typically larger than batch
or other processes. Another disadvantage is that sometimes particles may become entrained, or
blown along with the flow, which can be costly and problematic to repair. There is also an extra
power input that is required for the pump to moderate the pressure drop. Finally, the fluid-like
behavior of these fine particles may eventually cause erosion issues.
Fluidized beds can be stimulated by either gas or liquid flows. In either case, the process
of fluidization is a competition between the force of gravity pointing downwards and the upward
pointing drag force caused by friction between the flowing fluid and the individual particles that
Unit Operations ChE-381 Group No. 1 p. 5 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
make up the fluidized bed. As the flow rate of the working fluid is increased, it flows faster
across the individual particles, increasing the magnitude of the drag force. Eventually, at a
certain velocity (called the minimum fluidization velocity, Vf), the drag and gravitational forces
will be in balance, and the bed will begin to fluidize and bubble. As the velocity is further
increased, the drag force becomes more and more dominant over gravity, and the bed bubbles
more furiously. The individual particles are not carried away with the flow, because their settling
velocities are far larger than the minimum fluidization velocity, perhaps 50-75 times larger.
In this experiment, minimum fluidization velocity will be found for several different
types of fluidized beds. The effects of pressure drop, bed height, bed type, grain size, and
temperature will be investigated.
4. Theory
In packed beds the Ergun equation is a very important relation. The Ergun equation
relates the friction factor to numerical constants and the Reynolds Number (Re).
(1)
Where:
fp = friction factor of bed (dimensionless)
Re = Reynolds number (dimensionless)
The Reynolds number for this scenario is defined as follows:
(2)
Where:
Re = Reynolds number (dimensionless)
Unit Operations ChE-381 Group No. 1 p. 6 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
Dp = Equivalent spherical diameter of the particle (m)
Vs = Superficial velocity (m/s)
= Density of the fluid (kg/m3)
= Void fraction of the bed (dimensionless)
= Dynamic viscosity of the fluid (Pa-s)
The equivalent spherical diameter of the particle is defined by:
D p 6 * SAp
Vp
(3)
Where:
Dp = Equivalent spherical diameter of the particle (m)
Vp = Volume of the particle (m3)
SAp = Surface area of the particle (m2)
Notice that the Reynolds number depends on the void fraction (). The void fraction is
the ratio of the void volume to the total volume of the bed. Common values for the void fraction
range between 0.4-0.45.
If the flow is very viscous and Re 1 then the Ergun equation (1) can be approximated to
the Kozeny-Carman Equation:
(4)
Where:
fp = friction factor of bed (dimensionless)
Re = Reynolds number (dimensionless)
Unit Operations ChE-381 Group No. 1 p. 7 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
The form of the Ergun equation given in equation (1), while true, is not in terms of
variables that are easily measured. We must derive a relation between the fundamental force
balance of fluidization and the minimum fluidization velocity. Using the force balance, and
starting with the drag force on the bed from the fluid:
(5)
Where:
F = Drag force exerted by fluid on the bed (N)
p = Change in pressure across the bed (N/m2)
A = Cross-sectional area of the bed (m2)
This gives us the upward pointing drag force acting on the bed. In fluidization, this force
is balanced with the downward force of gravity, a volumetric force. We must first get the volume
of the particles in the bed:
(6)
Where:
Vp = Volume of the bed (m3)
= Void fraction of the bed (dimensionless)
A = Cross-sectional area of the bed (m2)
L = Height of bed (m)
Knowing that the force of gravity is volume multiplied by density and the gravitational
constant, we can turn equation 6 into:
(7)
Unit Operations ChE-381 Group No. 1 p. 8 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
Where:
F = Gravitational force on the bed (N)
= Density of the bed (kg/m3)
= Density of the fluid (kg/m3)
= Void fraction of the bed (dimensionless)
A = Cross-sectional area of the bed (m2)
L = Height of bed (m)
g = gravitational constant (9.8 m/s2)
Knowing that, at minimum fluidization velocity, the drag and gravitational forces are
equal, we can set equations 5 and 7 equal to one another, and rearrange for the pressure drop,
which is something the apparatus can measure:
(8)
Where:
p = Change in pressure across the bed (N/m2)
= Density of the bed (kg/m3)
= Density of the fluid (kg/m3)
= Void fraction of the bed (dimensionless)
L = Height of bed (m)
g = gravitational constant (9.8 m/s2)
Unit Operations ChE-381 Group No. 1 p. 9 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
At minimum fluidization velocity, viscous forces dominate, so the Kozeny-Carman
equation (4) can be used to get a useful approximation for the minimum fluidization velocity.
However, we need an expression relating the friction factor to the pressure drop:
p D p
fp
3
L Vs2 ( 1 ) (9)
Where:
fp = friction factor of bed (dimensionless)
p = Change in pressure across the bed (N/m2)
L = Height of bed (m)
Dp = Equivalent spherical diameter of the particle (m)
= Density of the fluid (kg/m3)
Vs = Superficial velocity (m/s)
= Void fraction of the bed (dimensionless)
Substituting equation 8 into equation 9 (for the delta-p term), then using equation 4 along with
equation 2 yields, after rearrangement:
(10)
Where:
Vf = Minimum fluidization velocity (m/s)
= Density of the particles (kg/m3)
= Density of the fluid (kg/m3)
Unit Operations ChE-381 Group No. 1 p. 10 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
g = gravitational constant (9.8 m/s2)
Dp = Equivalent spherical diameter of the particle (m)
= Dynamic viscosity of the fluid (Pa-s)
= Void fraction of the bed (dimensionless)
Our goal of determining a way to find the minimum fluidization velocity in terms of
variables that can be measured is complete. If we wish not to entrain our particles into the
flowing fluid stream, its useful to know the settling velocity of the particles involved.
(11)
Where:
Vsettling = Settling velocity of the bed (m/s)
= Density of the bed (kg/m3)
= Density of the fluid (kg/m3)
g = gravitational constant (9.8 m/s2)
Dp = Equivalent spherical diameter of the particle (m)
= Dynamic viscosity of the fluid (Pa-s)
Its also useful to know the maximum value the velocity can be increased to without
entraining particles. Expressed as a multiple of the minimum fluidization velocity:
(12)
Where:
Unit Operations ChE-381 Group No. 1 p. 11 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
Vsettling
Vf = The ratio of settling velocity to the minimum fluid fluidization velocity
Vsetting = Settling velocity (m/s)
Vf = Minimum fluidization velocity (m/s)
= Void fraction of the bed (dimensionless)
In this experiment, we are indirectly finding the minimum fluidization velocity, Vf, by
increasing the gas flow rate until the pressure drop no longer increases, then decreasing the flow
rate until the pressure drop returns. From this information, as well as recording the pressure drop,
grain size, and void fraction of the bed used, we can analytically calculate the minimum
fluidization velocity necessary to fluidize the bed, and can draw out any correlations between
this velocity, grain size of the bed used, and temperature of the bed.
5. Apparatus
The Fluidized Bed Apparatus consists of two columns each with a wood packed bottom
(6) and a funnel (9). One is packed with sand (5) and the other with silica pellets (14). Each
Column has an air rotameter (3,18), which monitors the flow rate of the air entering the column.
The air enters though the bottom of the columns and flows upward through the wood beads,
which distribute the airflow evenly throughout the width of the column. The air then flows
through the packing where the air manometer (8) measures the pressure drop of the air stream.
The sand column is used for experiments in which the variable if interest is the size of the
packing material or the amount of packing material. The sand is sieved using different grates to
separate the particles by size. A meter stick is used to measure the height of the packed sand in
the column when varying the amount of packing material.
Unit Operations ChE-381 Group No. 1 p. 12 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
The silica column is used in addition to the sand column to test different packing
materials. It is also used in varying the temperature of the packing media. The peanut heater (20)
is connected to the silica packed portion of the column (16) and is used to heat the packed silica
to different temperatures. The thermocouples (16) and the thermometer (15) are used to make
sure the entire packed portion is uniform in temperature.
Figure 1. The figure above shows a flow diagram of the Flow through Packed Beds and
Fluidized Beds Apparatus. The general streams pictured in black represent the air streams. The
yellow stream at the top left is the sieved sand of specific diameter. The grey stream at the top
right is the silica pellets.
Unit Operations ChE-381 Group No. 1 p. 13 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
9 Funnel 9 Funnel
3 Air
Rotameter
8 Air
Mamometer
4 Sand Packed 18 Air
Column Rotameter
14 Silica Packed
Portion
2 Pressure
Gauge
6 Wood Packed
5 Sand Packed Portion
Portion
17 Air inlet
Valve
7 Flask
6 Wood Packed
Portion
1 Air Inlet
Valve
Figure 2. The picture above shows the Flow through Fluidized Bed apparatus. Note: there have
been changes made to the apparatus since captured. The top rim on the silica column was loose
and needed to be repaired. http://images.google.com/imgres?
imgurl=http://www.uic.edu/depts/chme/UnitOps/Humidification.jpg
Unit Operations ChE-381 Group No. 1 p. 14 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
Table 1. Apparatus
No. Equipment Manufacturer Description
1 Air Inlet Valve Crane Co. CAT No. 7 Allows air into the sand packed column
Pressure
2 N/A Monitors the pressure of the incoming air
Gauge
Monitors the incoming air flow rate for the sand
3 Air Rotameter Gilmont D_6626
packed column
Uses sand as a packing material. The amount of
Sand Packed
4 N/A sand and the diameter of the sand particles can be
Column
varied in this column
Sand Portion This is the portion where the packing material
5 N/A
of Column settles
Allows incoming air into the column and disperses
Bead Packed
the air stream using packing to create a relatively
6 Base of N/A
consistent stream though out the diameter of the
Column
sand portion
Connected to the packing material portions of the
7 Flask N/A columns this filters out excess packing material
from the air
Meriam Instrument,
8 Air Manometer Cleavland OH Mo: RC- Measures the pressure loss of the air stream
4615
The top of the column. Facilitates adding packing
9 Funnel N/A material to the column and prevents spilling
caused by high pressure air streams
Excess Air The excess air from the column comes out the top
10 N/A
Stream into the surroundings
Dual Manufacturing Co.
Used to filter sand using different grates so that a
11 Sieve Chicago IL (63-4760
certain range in diameters can be obtained
Microns)
The sand will get sieved then funneled into the
12 Sand Entrance N/A
column from the top
the silica packing with be funneled through the top
13 Silica Entrance N/A
of the column
Silica Portion this is where the silica will reside while the
14 N/A
of Column experiment is running
Fluke. Omega Measures the temperature through the packed
Digital
15 Engineering Inc: Stanford portions of the column to make sure temperature
Thermometer
CT. Mo:2166A remains the same
Measure the temperature in the silica packed
16 Thermocouples N/A
column. Connected to the digital thermometer.
17 Air Inlet Valve Crane Co. CAT No. 7 Allows air into the silica packed column
18 Air Rotameter F&P Co. Precision Bore Monitors the incoming air flow rate for the silica
Flowrator Tube No: FP packed column
Unit Operations ChE-381 Group No. 1 p. 15 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
3/4-21-G-10/83
Pressure
19 N/A Monitors the pressure of the incoming air
Gauge
Superior Electric Co:
Used to heat the air and packing material in the
Bristol, Conn. Powerstat
20 Peanut Heater silica column in order to measure the effects of
Variable transformer
temperature on airflow rate
3PN116B 120 V, 10 Amp
Small Air Located after the Pressure Gauge to protect the air
21 Crane Co. CAT No. 7
Valve rotameter from harmful air flows.
Uses silica pellets as a packing material. The
Silica Packed
22 N/A temperature of the packing material can be varied
Column
in this column
Silica Column
23 N/A Allows are to flow into the packed column.
Valve
Sand Column
24 N/A Allows are to flow into the packed column.
Valve
6. Materials and Supplies
Table 2. Materials and Supplies
Material
Manufacturer Description Safety/Comments
Name
The pressure should be
Provides a constant stream of
Compressed UIC regulated as high pressure
air which flows through the
Air Compressors streams are hazardous both to
packed column
participants and equipment
Packing material used to
Silica measure affects of temperature Avoid spilling to prevent a
N/A
Pellets of stream on air flow through tripping hazard
packed beds
Fisher Packing material used to
Avoid spilling to prevent a
Sand Chemical Lot measure affects of particle size
tripping hazard
No. 080318 on air flow though packed beds
Dual
Manufacturing These cylinders with different Do not leave out as excess
Sieves Co: 63 size grates sift sand according sand may spill creating a
Microns-4760 to range in diameter tripping hazard
Microns
The heater for the air stream in Circuits should be avoided if
Electricity ComEd the silica bed needs electricity there is a problem seek an
to heat the air stream electrician
Meter Stick N/A Measures the amount of Should be put back properly
material in the sand packed as is could fall and cause
Unit Operations ChE-381 Group No. 1 p. 16 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
column injury
Used to sweep up any spilled
Broom N/A Prevents tripping hazards
sand or silica
The vacuum is used to clean
Dayton: Mo. Spilling may occur while
Vacuum the columns in order to vary
4YE74 vacuuming
the size or amount of the sand.
7. Procedure
Things to Know Before You Start
1. The entire lab shares the air supply. If other experiments requiring the air supply are
being run at the same time as yours then you will have issues with maintaining steady air
pressure. Keep an eye on the flowmeter at all times. You may need to coordinate with
other lab groups in order to secure the amount of airflow that you need.
2. There is insufficient pressure to operate both columns simultaneously, so perform the
experiment on only one column at a time.
a. As it can take a long time for the heater (20) in the right column (22) to achieve
steady state, we recommend first performing the silica experiment at room
temperature and then performing the sand experiments in the left column (4)
while the right column (22) heats up. Make sure that someone keeps an eye on the
temperature of the right column.
Start Up
1. Check all connections for air and electrical supply.
2. Check that all apparatus and materials in the cabinet are accounted for and that nothing is
broken. Make sure that the temperature probes are connected to the digital thermometer
(15). Make sure that you have a Shop-Vac (vacuum cleaner) for cleanup later.
Unit Operations ChE-381 Group No. 1 p. 17 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
3. Clean out the left (4) and right (22) columns if they have not been already.
4. After making sure that all other airflow valves (1, 17, 21, 23, 24) on the apparatus are
closed, turn on the main air supply valve.
Packing Material Preparation
1. Using the sieve trays (11), prepare separate samples of sand and silica that are of the
approximate diameter required for the experiment. The sand will be placed in the left
column (4) while the silica will be placed in the right column (22).
2. You will prepare two samples of sand that are of two different particle diameters and one
sample of silica. One sand sample should be between 63-297 microns and the other
should be between 297-595 microns. The silica sample should have a grain size ranging
from 500-841 microns. Record the diameter range for each sample in your data.
3. To perform the sifting, choose a two sieve trays (in order from smallest to largest with the
largest on top) and one catch tray. These trays stack atop each other with the bottom sieve
being the of the lowest grain diameter that you need, the top sieve is of the highest grain
diameter that you need. You will use the sifted material that is captured in the bottom
sieve tray (not the catch tray) for your sample. Repeat this step for each sample using the
appropriate sieve trays.
a. Note: You will at least 2.5 to 3 times as much silica than sand, as you will need to
fill the entire heater assembly (14) in the right column (22) before you will be
able to see enough of the silica bed to visually measure its height.
b. Note: Save at least 5ml of each sample (using a beaker or graduated cylinder to
measure) for the determination of the void fraction.
Unit Operations ChE-381 Group No. 1 p. 18 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
Determining Void Fraction
1. For each sample, fill a graduated cylinder with up to 5ml of the sample material.
2. Measure separately 5ml of water. Add the water to the graduated cylinder and allow a few
minutes for the water to completely soak into the material.
3. Measure the total volume in the graduated cylinder.
4. Use the dry volume of the packing material, the volume of the water that was added to
the packing material, and the total volume measured after the material has soaked
thoroughly to calculate the void fraction of the packing material.
5. Repeat for all samples.
Experimental Procedure for Sand
1. Load one sample of the sand packing material into the left column (4) using the funnel at
the top to keep from spilling.
a. The height of the packing material in the column should be between 6 to 10 cm.
Accurately measure this height from the base of the bed, just above the gas
distributor (6) at the bottom of the column.
b. While pouring the packing material into the column, tap the side of the column to
make sure that the material does not stick to the side. This will also allow for the
packing material to settle more evenly at the base of the column.
2. Open the airflow valve (23) at the base of the left column (4) and make sure that the
airflow valve (24) at the base of the right column (22) is turned off.
Unit Operations ChE-381 Group No. 1 p. 19 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
3. Using the flowmeter (3) on the left side of the apparatus, slowly open the larger valve (1)
below the flowmeter and then gently turn the smaller valve (21) at the bottom of the flow
meter to increase the flow in increments of 5%. (Note: On this meter, each increment is a
percentage of the max flow rate of 2.2 L/s.)
c. Note: The flow meter adjustments can be tricky, if you go past the change you
were attempting for do not go back down or you will skew your results.
4. For each change in airflow rate record: the air flow rate, the pressure drop on the left side
of the manometer (8), the height of the packed bed (5) using the meter Stick, and your
visual observations.
5. Keep increasing the airflow rate until you reach the maximum for this bed of material.
You will know that this has been achieved when the bed is completely fluidized and
further increases in airflow rate do not cause further significant drops in pressure on the
manometer (8). Record the maximum flow rate, bed height, and pressure drop.
6. Decrease the airflow rate in increments of 5 on the flowmeter (3). Record the airflow
rate, bed height, and pressure drop for each change in airflow rate.
7. Once the airflow is turned off, add more of the sand sample that you were testing to the
left column (4) and increase the height of the bed by 3 to 4 cm. Repeat steps 2 through 6.
8. Once finished with this sample, remove the funnel and use the Shop-Vac to remove all of
the sand from the left column (4). Then perform steps 1 through 7 using the second sand
sample.
Experimental Procedure for Silica
Unit Operations ChE-381 Group No. 1 p. 20 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
1. Load one sample of the silica packing material into the right column (22) using the funnel
at the top to keep from spilling.
d. The height of the packing material in the column should be between 6-10 cm
above the top of the heater unit at the base of the column, at least 2-3 cm above
the highest thermal sensor. Accurately measure this height from the base of the
bed, just above the gas distributor (6) at the bottom of the column. This includes
the heater area (14).
e. While pouring the packing material into the column, tap the side of the column to
make sure that the material does not stick to the side. This will also allow for the
packing material to settle more evenly at the base of the column.
2. Open the airflow valve (24) at the base of the right column (22) and make sure that the
airflow valve (4) at the base of the left column (23) is turned off. Make sure that the
electrical heater (20) is turned off.
3. Use the digital thermometer (15) to record the temperatures of the right column (22).
There are temperature probes (16) mounted at multiple positions in the silica bed.
4. Using the flowmeter (18) on the right side of the apparatus, slowly open the valve (17)
below the flowmeter 5%. (Note: On this meter, each increment is a percentage of the max
flow rate of 139.0 SCFM.)
f. Note: The flow meter adjustments can be tricky, if you go past the change you
were attempting for do not go back down or you will skew your results.
5. For each change in airflow rate record: the air flow rate, the pressure drop on the right
side of the manometer (8), the height of the packed bed using the meter Stick, the
temperatures of the column, and your visual observations.
Unit Operations ChE-381 Group No. 1 p. 21 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
6. Keep increasing the airflow rate until you reach the maximum for this bed of material.
You will know that this has been achieved when the bed is completely fluidized and
further increases in airflow rate do not cause further significant drops in pressure on the
manometer (8). Record the maximum flow rate, the temperatures of the column, bed
height, and pressure drop.
7. Decrease the airflow rate in increments of 5 on the flowmeter (18). Record the airflow
rate, the temperatures of the column, bed height, and pressure drop for each change in
airflow rate.
8. Once the air flow is turned off, turn on the heater (20) and allow the silica bed to reach a
steady state at a temperature about 30 to 40 degrees higher than in the previous test. Use
the digital thermometer (15) to monitor the temperatures in the silica bed. (Note: Higher
temperatures than this are more difficult to maintain at a steady state.) Repeat steps 4
through 8.
9. Optional: If you have time to allow the column to cool back down to room temperature,
add more of the silica sample that you were testing to the right column (22) and increase
the height of the bed by 3 to 4 cm. Repeat steps 2 through 8.
10. Optional: Once finished with this sample, remove the funnel and use the Shop-Vac to
remove all of the silica from the right column (22). Then perform steps 1 through 9 using
the second silica sample.
11. You will not likely have time for steps 9 and 10.
Shut Down and Clean Up
Unit Operations ChE-381 Group No. 1 p. 22 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
1. Turn off the air supply. Make sure that the heater (20) is turned off. Turn off the digital
thermometer (15).
2. Remove the funnel from the top of each column. Use the Shop-Vac to vacuum out all
packing material from inside each column. Put the funnel back in place when done.
3. Clean each sieve tray. Do not use water as the trays may corrode!
4. Place all materials in the cabinet below the apparatus and close the door.
8. Data Tabulation
The data presented in Table 3 corresponds to a large sand particle diameter in the range of 297-
595 microns and an initial bed height of 7.31 cm.
Table 3. Pressure Drop across Large Sand Particles Trial 1
Flow Rate (% of Pressure Drop Sand Height
Observations
2.2 L/s, +/- 1%) (+/- 0.01 in H2O) (+/- 0.25 cm)
0 -0.59 47.13 Stable, surface not bubbling at all
3.8 -0.46 47.15 Stable, surface not bubbling at all
7 -0.31 47.15 Stable, surface not bubbling at all
14.9 -0.09 47.15 Stable, surface not bubbling at all
19 0.01 47.15 Stable, surface not bubbling at all
24.5 0.19 47.16 Stable, surface not bubbling at all
34.1 0.12 47.36 Slight bubbling
45 0.15 47.44 Near even mixing
52.7 0.18 47.82 Slightly higher than even mixing
60.2 0.22 48.27 Slightly higher than even mixing
69.7 0.28 48.47 Low turbulent mixing
76.9 0.29 48.57 Turbulent mixing
83.3 0.31 48.68 High turbulent mixing
91.8 0.31 48.92 High turbulent mixing
96.9 0.31 49.05 Very high turbulent mixing
89.2 0.31 48.87 High turbulent mixing
82.6 0.31 48.76 High turbulent mixing
66.8 0.27 48.18 Just above even mixing
58.1 0.23 48.12 Even mixing
Unit Operations ChE-381 Group No. 1 p. 23 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
49.7 0.2 48.15 Just less than even mixing
40 0.11 47.87 Just above minimum fluidization
32.5 0 47.8 Stable, surface not bubbling at all
28.1 -0.02 47.71 Stable, surface not bubbling at all
25.5 -0.02 47.55 Stable, surface not bubbling at all
12 -0.08 47.4 Stable, surface not bubbling at all
2.9 -0.43 47.4 Stable, surface not bubbling at all
0 -0.43 47.23 Stable, surface not bubbling at all
The data presented in Table 4 corresponds to a large sand particle diameter in the range of 297-
595 microns and an initial bed height of 10.19 cm.
Table 4. Pressure Drop across Large Sand Particles Trial 2
Air Flow Rate (% Pressure Drop Sand Height
Observations
of 2.2 L/s, +/- 1%) (+/- 0.01 in H2O) (+/- 0.25 cm)
0 -0.62 50.01 Stable, surface not bubbling at all
5 -0.25 50.05 Stable, surface not bubbling at all
10.8 0.06 50.06 Stable, surface not bubbling at all
24.6 0.9 50.17 Stable, surface not bubbling at all
34 1.01 50.2 Slight bubbling
44.9 1.01 50.65 Slightly more bubbling
52.2 1.01 50.87 Almost even mixing
63 1.06 51.21 Slightly turbulent mixing
73 1.11 51.68 Turbulent mixing
85.3 1.2 52.15 High turbulent mixing
93.1 1.2 52.38 Violent mixing
99 1.21 52.48 Violent mixing
85.11 1.2 52.25 Violent mixing
78.8 1.2 52.11 High turbulent mixing
73.8 1.19 51.98 Turbulent mixing
66 1.19 51.83 Just above even mixing
51.2 1.01 51.8 Very slight bubbling
44 0.8 51.31 Very slight bubbling
34 0.63 51.18 Stable, surface not bubbling at all
5.9 -0.31 51.05 Stable, surface not bubbling at all
2.1 -0.58 50.85 Stable, surface not bubbling at all
0 -0.59 50.45 Stable, surface not bubbling at all
The data in Table 5 corresponds to a silica particle diameter in the range of 63 to 297 microns, a
range in temperature from 69F to 78F, and an initial bed height of 6.63 cm.
Unit Operations ChE-381 Group No. 1 p. 24 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
Table 5. Pressure Drop across Small Sand Particles Trial 1
Air Flow Rate (% Pressure Drop Sand Height
Observations
of 2.2 L/s, +/- 1%) (+/- 0.01 in H2O) (+/- 0.25 cm)
0 -0.6 46.45 Stable, surface not bubbling at all
4.3 -0.45 46.46 Stable, surface not bubbling at all
11.2 -0.28 46.46 Stable, surface not bubbling at all
14.2 -0.2 46.48 Stable, surface not bubbling at all
19.4 -0.08 46.48 Stable, surface not bubbling at all
32 0.01 47.21 Even mixing
36.9 0.01 47.28 Slightly more than even mixing
42.3 0.01 47.32 Slightly more than even mixing
50.3 0.02 47.42 Fairly turbulent mixing
61.8 0.08 47.49 High turbulent mixing
68.8 0.1 47.95 Violent mixing
65.9 0.09 47.74 High turbulent mixing
57 0.08 47.5 Fairly turbulent mixing
50 0.04 47.41 Slightly more than even mixing
38.2 0.01 47.35 Even mixing
27 -0.01 47.2 Slight bubbling
21.8 -0.1 46.95 Very little bubbling
6.7 -0.4 46.6 Stable, surface not bubbling at all
0 -0.58 46.46 Stable, surface not bubbling at all
The data in Table 6 corresponds to a silica particle diameter in the range of 500 to 841 microns, a
range in temperature from 69F to 78F, and an initial bed height of 19.15 cm.
Table 6. Pressure Drop across Small Sand Particles Trial 2
Air Flow Rate (% Pressure Drop Sand Height
Observations
of 2.2 L/s, +/- 1%) (+/- 0.01 in H2O) (+/- 0.25 cm)
0 -0.59 50.92 Stable, surface not bubbling at all
6 0.31 50.98 Stable, surface not bubbling at all
14 1 51.08 Stable, surface not bubbling at all
20 1.51 51.22 Slight bubbling
28.1 1.22 52.05 Slightly more bubbling
35 1.31 52.45 Almost even mixing
43.2 1.33 52.53 Just above even mixing
53.4 1.4 53.1 Turbulent mixing
65 1.51 53.56 Very turbulent mixing
75.6 1.51 53.98 Very turbulent mixing
70.9 1.52 53.52 Very turbulent mixing
66.1 1.51 53.42 Turbulent mixing
Unit Operations ChE-381 Group No. 1 p. 25 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
57.8 1.49 53.38 Turbulent mixing
51.7 1.49 53.19 Slightly turbulent mixing
47.2 1.46 53.05 Above even mixing
40.9 1.41 52.85 Even mixing
33.2 1.21 52.72 Slight bubbling
23.3 0.72 52.6 Stable, surface not bubbling at all
11 0.5 51.83 Stable, surface not bubbling at all
7.6 0.2 51.3 Stable, surface not bubbling at all
3.9 -0.07 50.97 Stable, surface not bubbling at all
0 -0.6 50.92 Stable, surface not bubbling at all
The data in Table 7 corresponds to a silica particle diameter in the range of 500 to 841 microns, a
range in temperature from 69F to 78F, and an initial bed height of 19.15 cm.
Table 7. Pressure Drop across Silica in the Temperature Range 69F to 78F
Air Flow Rate (%
Pressure Drop Silica Height (+/-
of 139 CFM, +/- Observations
(+/- 0.01 in H2O) 0.25 cm)
1%)
0 -0.62 59.13 Stable, surface not bubbling at all
7.2 1.63 59.14 Stable, surface not bubbling at all
9 1.8 59.14 Stable, surface not bubbling at all
10 1.9 59.14 Stable, surface not bubbling at all
Very slight movement on silica
12.1 2.32 59.14
surface
More noticeable movement on
14 3.05 59.14
surface
More noticeable movement on
15.1 3.3 59.14
surface
More noticeable movement on
16.4 3.7 59.14
surface
Near even mixing; more bubbling
19.2 4.04 59.17
than before
21.2 4.04 59.43 Slow bubbling but large bubbles
23 4.06 60.01 Even mixing
27 4.1 60.42 Turbulent mixing
31.8 4.1 61.23 Violent mixing
35.9 4.18 62.65 Extremely violent mixing
31.5 4.14 61.45 Violent mixing
29.8 4.11 60.94 Highly turbulent mixing
26.9 4.1 60.45 Turbulent mixing
25.2 4.09 60.31 Slight turbulent mixing
24 4.08 60.14 Just above even mixing
Unit Operations ChE-381 Group No. 1 p. 26 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
21.8 4.02 59.87 Even mixing
21 3.95 59.72 Just above minimum fluidization
19.1 3.98 59.19 Minimum fluidization
17.9 3.91 59.13 Slight movement
14.6 3.4 58.6 Slight movement
12 2.87 58.6 Slight movement
9.3 2.2 58.57 Very minimal bubbling
6.8 1.1 58.57 Stable, surface not bubbling at all
0 -0.6 58.57 Stable, surface not bubbling at all
The data presented in Table 8, corresponds to a silica particle diameter in the range of 500-841
microns and an initial bed height of 18.34 cm.
Table 8. Pressure Drop across Silica in the Temperature Range 88F-108F
Air Flow Rate (% Silica
Pressure Drop
of 139 CFM, +/- Height (+/- Observations T (F)
(+/- 0.01 in H2O)
1%) 0.25 cm)
Stable, surface not bubbling at
0 -0.6 58.32 100
all
Stable, surface not bubbling at
7 3.1 58.42 99
all
9.3 3.89 58.84 Slight bubbling 97
11.1 3.98 59.31 Bubbling 94
13.5 4.04 59.71 Fairly turbulent bubbling 88
16.3 4.1 60.45 Turbulent bubbling 108
19.7 4.11 61.02 Highly turbulent bubbling 105
17.2 4.11 60.61 Highly turbulent bubbling 105
14.4 4.11 60.15 Turbulent bubbling 104
12.2 4.03 59.57 Just above even mixing 104
10.1 3.97 58.72 Bubbling 103
8.7 3.9 58.46 Slight bubbling 98
Stable, surface not bubbling at
7.1 3.5 58.32 94
all
Stable, surface not bubbling at
0 -0.62 58.32 94
all
Table 9. Density of Air Calculation
Pressure Density of Air
R constant (kPa*m3/kmol*K) Temperature (+/- 0.5 K) rho (kmol/m^3)
(kPa) (kg/m^3)
101.325 8.314 298 0.0409 1.19
101.325 8.314 296 0.0411 1.19
Unit Operations ChE-381 Group No. 1 p. 27 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
101.325 8.314 311 0.0392 1.14
Table 10. Void Fraction Calculation
Dry Volume (+/- Water Volume(+/- Combined Void Fraction
Substance
0.1 mL) 0.1 mL) Volume(+/- 0.1 mL) (dimensionless)
Large Sand 5 15 18 0.40
Small Sand 5.5 16 18.9 0.47
Silica 8.8 15.5 20.4 0.44
Table 11. Superficial Velocity Calculation
Superficial
Flowrate (L/s, +/-
Substance Flowrate (m^3/s) Area (m^2) Velocity
1%)
(m/s)
Large Sand/Trial 1 1.83 0.00183 0.0408 0.045
Large Sand/Trial 2 1.88 0.00188 0.0408 0.046
Small Sand/Trial 1 1.36 0.00136 0.0408 0.033
Small Sand/Trial 2 1.43 0.00143 0.0408 0.035
Flowrate (ft^3/min,
Substance Flowrate (m^3/s) Area (m^2) Vs (m/s)
+/- 1%)
Silica / Unheated 37.5 0.0177 0.0403 0.440
Silica / Heated 27.4 0.0129 0.0403 0.321
Table 12. Calculation Summary
Density Density Particle Fluidization Superficial
Void Fraction
Substance of Bed of Air Diameter Velocity Velocity
(Pa*s) (dimensionless)
(kg/m^3) (kg/m^3) (m) (m/s) (m/s)
Large
Sand/Trial 1600 1.19 4.46E-04 1.96E-05 0.40 0.11 0.045
1
Large
Sand/Trial 1600 1.19 4.46E-04 1.96E-05 0.40 0.11 0.046
2
Small
Sand/Trial 1600 1.19 1.80E-04 1.96E-05 0.47 0.035 0.0333
1
Small
Sand/Trial 1600 1.19 1.80E-04 1.96E-05 0.47 0.035 0.0350
2
Silica /
2100 1.19 6.71E-04 1.94E-05 0.44 0.50 0.440
Unheated
Silica /
2100 1.14 6.71E-04 2.00E-05 0.44 0.48 0.321
Heated
Unit Operations ChE-381 Group No. 1 p. 28 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
9. Results
For this lab, trials were run at two different starting bed heights of sand, each tested
against two ranges of grain size, and two different temperatures of silica at equal starting bed
heights and one range of grain sizes. The grain size ranges of sand were 63-297 microns and
297-595 microns. The range of particle sizes for the silica was 500-841 micrometers. For each
trial, the data collected included bed height, pressure drop, airflow rate, and temperature for the
silica trials. The data collected is best represented in figures 3, 4, and 5.
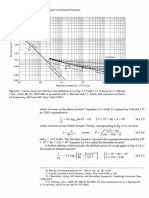
Figure 3 shows the relation between airflow rate and the overall pressure drop in the
column for a bed height of approximately 10.5 cm, and both sand sieve ranges. The portion
where pressure drop starts levels off is the point of minimum fluidization. We can see that for the
large sieve size, minimum fluidization occurs at a flow rate of approximately 85% of 2.2L/s.
Similarly, for the small sieve size, minimum fluidization occurs at a flow rate of approximately
65% of 2.2L/s. Fluidization occurs when the superficial velocity equals the minimum fluidization
velocity, and superficial velocity is directly proportional to the flowmeter reading. Tables 3 and 5
show that fluidization for the smaller bed height occurs at 83.3% and 61.8% of 2.2L/s, for large
and small sieve sizes, respectively.
Unit Operations ChE-381 Group No. 1 p. 29 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
Figure 3. Pressure drop vs flowmeter reading for large bed heights of sand. Superficial velocity
increases as flowmeter reading increases. Fluidization occurs when the slope of the graph levels
off.
Figure 4 shows the same correlation of air flow rate to pressure drop for the silica column
at both room temperature and the increased temperature average of about 103 F. The data
displayed below shows this correlation for both increasing and decreasing flow rates. For cool
silica, fluidization occurred at 27% of 139 CFM, and for hot silica, fluidization occurred at
16.3% of 139 CFM.
Unit Operations ChE-381 Group No. 1 p. 30 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
Figure 4. Pressure drop vs flowmeter reading for silica. Silica bed was heating to approximately
103F. The pressure drop was the same magnitude for both cases.
Figure 5 illustrates the correlation between airflow rate and bed height of the larger sand
particles from both increasing and decreasing flow rates. When the experiment began, the bed of
sand was 47.13 centimeters above the workbench that apparatus was mounted on. Increases in
flow rate lead to an increase of the bed height and a decrease in the flow rate leads to a decrease
in bed height. This makes sense since the air flowing through the bed applies a force in the
vertical direction causing the bed height to change. This graph is representative for all tested
scenarios.
Unit Operations ChE-381 Group No. 1 p. 31 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
Figure 5. Bed height vs flowmeter reading for large sand size at the small bed height. Bed
height increases with increasing flow rate. This figure is representative for all tested scenarios.
10. Discussion
A fluidized bed can be created by a gas flowing through a bed of solid particles that are in
some kind of vessel, for our experiment inside a column. Under the appropriate conditions the
gas will cause the solid to begin to behave as a fluid and when this occurs the bed is fluidized.
Fluidized beds are useful because they create a large contact surface area between the solid and
gas, which allows for an increase in the overall heat or mass transfer. Some of uses of this
technique in chemical engineering are in the processes of fluid catalytic cracking and fluidized
bed combustion, both of which are widely used in the energy industries today.
For this experiment we had two columns, one filled with sand and the other with silica.
For the silica we only used one height and grain size range, 500-841 microns, but varied the
Unit Operations ChE-381 Group No. 1 p. 32 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
temperature to determine any effect this has on rate of fluidization. For sand we used two grain
size ranges, 63-297and 297-595 microns, at two different bed heights. We then measured the
change in pressure as the flow rate of the air was increased until fluidization occurred, and then
decreased back down to zero. For the silica column, in addition to pressure drop, the
temperature was also recorded at every flow rate. We also took note of any observations
regarding the surface of the silica or sand bed as the flow rate of air was changed. The void
fraction was also calculated for silica and both grain size ranges of sand. For every tested
scenario, as the flow rate was increased, the height of the bed also increased, because the air
flowing through the bed forced the solid to move vertically.
With respect to the silica column, we can see that an increased temperature decreases the
flow rate required to cause fluidization, and, by extension, the minimum fluidization velocity. At
high temperatures, because of this decrease in flow rate, the pressure changes at a faster rate for
hot silica than it changes for cooler silica. This temperature effect on minimum fluidization
velocity cannot be explained as a viscosity effect, as minimum fluidization velocity is inversely
proportional to viscosity, and the viscosity of air increases with increasing temperature, implying
that minimum fluidization velocity should increase with increasing temperature, not the opposite
as shown in the experiment. The temperature effect on minimum fluidization velocity could be
due to convective heat transfer between the air and heated silica: the hot silica at the top of the
bed transfers heat by convection to the air around it, which rises due to its own buoyancy. If the
velocity of this convective hot air is non-negligible with respect to the forced airflow through the
bed, it could be a sizable effect on minimum fluidization velocity.
With respect to the sand column, using smaller grain sizes of sand increased the
magnitude of the pressure drop at fluidization, as well as decreasing the necessary flow rate
Unit Operations ChE-381 Group No. 1 p. 33 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
required to fluidize the bed. The rise in the magnitude of the pressure drop is unexpected, as the
pressure drop is directly proportional to the amount of material that is not void space. It was
shown that the smaller grains of sand had more void, implying less non-void space, implying a
lesser pressure drop. For this increased pressure drop to occur, that would mean the bed of
smaller sand grains must have had a higher density. The decrease in the minimum fluidization
velocity is expected however, as minimum fluidization velocity is directly proportional to the
square of the equivalent spherical diameter of the bed grains, and, as such, smaller grains
decrease the minimum fluidization velocity.
The major reason for errors in this experiment derives from a lack of true knowledge of
the equivalent spherical diameter of the bed grains. Minimum fluidization velocity is directly
proportional to the square of the equivalent spherical diameter of the bed grains. In this
experiment, it is assumed that the simple average of the sieve size is a good approximation for
this equivalent spherical diameter, but this could be problematic, as having more small grains
than large grains would mean the true equivalent diameter would be less than the simple average.
Similarly, having more large grains than small grains would mean the true equivalent diameter
would be more than the simple average.
Other reasons for error in our data and calculations came from the fact that the flow rate
of the air fluctuates, due to the source being shared, so we had to take approximate values for the
flow rates in our calculations. The air flow meters were extremely sensitive so we could not
change our flow rates at a constant interval, which would have helped the usefulness of the
calculated values. Another factor that contributed to the error was the heights of the beds of sand
and silica, which were measured with a meter stick, from the outside of the column. A more
accurate way of measuring the heights of the beds would include graduations being marked on
Unit Operations ChE-381 Group No. 1 p. 34 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
the column, perhaps with a black marker. There was also some error associated with the beds
themselves. Since we had air flowing through the beds the heights changed and while the change
was only slight with low flow rates at higher flow rates the height changed dramatically, making
it difficult to get an accurate value for the height of the bed. The turbulent mixing associated with
fluidization also changed the distribution of mass between any two points in the column.
11. Error Analysis
Each piece of equipment contributes to uncertainties in the measured data. These
uncertainties affect the calculations in such a way that causes uncertainties in the results
produced. The uncertainty in the results is calculated by propagation of errors from the measured
uncertainties. The uncertainties associated with each piece of equipment are as follows:
The airflow used for silica is measured with a flow meter that reads in percentage of
139.0 SCFM with markings at each 1% interval. Since the flow meter level remained constant
throughout the experiment, one extra significant digit could be approximated which would create
an uncertainty of 0.1%. The air flow meter used for sand reads in a percentage of 2.2 liters per
second in intervals of 1%. As with the silica side air flow meter, one extra significant digit could
also be approximated to an uncertainty of 0.1% of max flow. The manometer used for measuring
the difference in pressure reads in intervals of 0.1 inches of water. Since the level of water in the
manometer remained level at each flow rate, the last digit can accurately be measured to an
uncertainty of 0.01 inches of water. The digital thermometer used to measure the temperature in
the silica column only reads in integer values. We read the values in Fahrenheit because the
scale is more sensitive than the Celsius scale; however, since the first decimal is rounded off,
each reading has an uncertainty of 0.5 F. The height of material in each column was measured
using a meter stick, which reads in increments of 1 mm. The height of the column could not be
read as accurately due to bubbling and mixing of material at higher flow rates which caused the
uncertainty of each measurement to be 0.25 cm at high flow rates.
Unit Operations ChE-381 Group No. 1 p. 35 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
Table. 13 - Error Analysis
Equipment Units Uncertainty Reason
SCFM (Standard
Air Rotameter 0.1 % of Lowest readable increment is
Cubic Feet per
(Silica) 139 scfm 1%. Last digit approximated.
Minute)
L/s (cubic
Air Rotameter 0.1% of Lowest readable increment is
centimeters per
(Sand) 2.2 L/s 1%. Last digit approximated.
second)
Lowest readable increment is
0.01
Monometer Inches of Water 0.1 inch. Last digit
inches H2O
approximated.
Only reads integer values
Digital 0.5 F or
F or C which are rounded off to the
Thermometer C
nearest value
Bed height
Height of material fluctuates at
measurements cm (centimeters) 0.25 cm
higher flow rates
with meter stick
12. Conclusion
Fluidized beds provide a large surface area for contact between solids and a liquid or a
gas that is conducive for heat and mass transfer providing for an environment where nearly
uniform temperatures can be maintained in the reactor even with highly exothermic reactions.
This is important because hot or cold spots may develop in beds where there is a temperature
gradient or the temperature is not uniform. These hot spots can cause equipment failure and
product degradation while the cold spots decrease the efficiency of the reaction. A fluidized bed
also provides uniform mixing, which is important for product quality and efficiency.
Consequently, the study of fluidized beds is valuable to the chemical engineering field.
In this experiment we analyzed the effects of grain size and temperature to determine the
minimum fluidization velocity (Vf ). The trials were run at two different starting bed heights of
sand each for two ranges of grain size, and two different temperatures of silica at equal starting
bed heights and one range of grain sizes. The grain size ranges used for sand were 63-297 m
Unit Operations ChE-381 Group No. 1 p. 36 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
and 297-595 m. For the silica the particle size ranged from 500-841 m. For the 297-595
micrometer range sand the bed heights were 7.31 cm and 10.19 cm and for the 63-297
micrometer range the bed heights were 6.63 cm and 11.10 cm. Finally for the silica we used bed
heights of 19.15 cm and 18.34 cm. The temperature range for the silica trials was 69-78F for
the colder trial and 88-100F for the warmer trial. The void fraction was also calculated for the
large sand (0.40), small sand (0.47), and silica (0.44). For each trial, the data collected included
bed height, pressure drop, airflow rate, and temperature for the silica trials.
As expected when the flow rate increased the bed height also increased. As the flow rate
increased the sand and silica exhibited different behaviors. At very low flow rates there was no
appreciable impact on the silica and sand. As the flow rate gradually increased the bed began to
bubble and eventually reached the minimum fluidization velocity. Beyond this point the beds
began to exhibit turbulent behavior with severe bubbling and mixing.
The minimum fluidization velocity was experimentally determined to be 0.45 and 0.46
m/s for the large sand trials, 0.0333 and 0.0350 m/s for the small sand trials, and 0.44 and 0.321
m/s for the silica trials. These values are determined by identifying the point in the flow rate vs
pressure drop curves where the graph first flattens out; this is the point where the derivative is
equal to zero. Therefore as grain size decreases, the minimum fluidization velocity also
decreases. This result makes sense because a fluid is essentially composed of several tiny
particles condensed together. From the silica trials it can be inferred that as bed temperature
increases the minimum fluidization velocity decreases; that is hotter beds fluidize faster.
13. References
1. Flow Through Fluidized Bed Lab Manual.
2. Fluidized Bed Reactor. http://en.wikipedia.org/wiki/Fluidized_bed_reactor
Unit Operations ChE-381 Group No. 1 p. 37 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
3. Material Safety Data Sheet: Sand. Fisher Scientific.
http://fscimage.fishersci.com/msds/09890.htm
4. Material Safety Data Sheet: Silica Gel Desiccant. Fisher Scientific.
http://www.atmos.umd.edu/~russ/MSDS/silicagel28200.html
5. Fogler, Scott H. Elements of Chemical Reaction Engineering. 4th Ed. Boston: Pearson.
2006.
Unit Operations ChE-381 Group No. 1 p. 38 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
14. Appendix I
In this appendix, it will be shown how the minimum fluidization velocity and interstitial
velocity for the first trial of the large sand size was calculated. Starting from the minimum
fluidization velocity equation:
Where:
Vf = Minimum fluidization velocity (m/s)
= Density of the bed (kg/m3)
= Density of the fluid, air (kg/m3)
g = gravitational constant (9.81 m/s2)
Dp = Equivalent spherical diameter of the bed particles (m)
= Dynamic viscosity of the fluid, air (Pa-s)
= Void fraction of the bed (dimensionless)
, , Dp, , and are our unknowns of interest. We will start with void fraction . Void fraction
is defined as:
Void Volume
Dry Volume
From our preliminary data for the large sand size, 5 mL of sand were mixed with 15 mL of water
in a graduated cylinder, yielding an 18 mL solution. Thusly, two mL of the water added filled the
void space in the sand, so:
( 20 18)
0.4
5
Unit Operations ChE-381 Group No. 1 p. 39 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
From the void fraction, we can obtain the density of the bed. We postulate that sand is simply
made up of silicon dioxide (SiO2) particles and void space, so:
p s (1 )
Where:
= Density of the bed (kg/m3)
s = Density of pure SiO2 (kg/m3)
= Void fraction of the bed (dimensionless)
Knowing that pure SiO2 has a density of 2634 kg/m3, and using our previously found void
fraction:
p 2634 * (1 .4)
p 2634 * 0.6
kg
p 1580.4 1600
m3
, the density of the air, can be easily obtained from the ideal gas law:
P f RT
Where:
P = Ambient Pressure (101.325 kPa)
= Density of the air (kmol/m3)
R = Gas constant (8.314 kPa*m^3/kmol*K)
T = Absolute Temperature (298 K)
Solving for :
(101.325) 0.0409 kmol
f
(8.314)(298) m3
0.0409 kmol 29 kg 1.19 kg
*
m3 kmol m3
Dp is taken as the simple average of the size of the sieve used. This trial corresponded to a sieve
range of 297-595 microns. Thusly:
Unit Operations ChE-381 Group No. 1 p. 40 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
(595 297)
Dp * 10 6 m
2
D p 4.46 *10 4 m
, the viscosity of the air, is linearly interpolated between two known values of at 250K and
300K. for a temperature of 298K is:
1.9632 *10 5 Pa * s
We have solved for all of our unknowns and can find Vf, the minimum fluidization velocity:
(1600 1.19) * (9.81) * (4.46 * 104 ) 2 0.43
Vf ( )
150(1.9632 * 10 5 ) 1 0.4
0.11 m
Vf
s
This will be compared to the interstitial velocity, calculated using the flow rate, Q. Minimum
fluidization occurs when the pressure drop across the fluidized bed stops increasing. In this trial,
the flowmeter read 83.3% of 2.2 L/s when the pressure drop stabilized. As such:
83.3
Q * 2.2
100
1.83 L 1 m3 0.00183 m 3
Q *
s 1000 L s
From this flow rate, we can calculate the interstitial velocity by dividing the flow rate, Q, by the
cross sectional area, A. Knowing the diameter of the column is 0.114 m:
A 0.0408 m 2
Q 0.00183 0.045 m
Vs
A 0.0408 s
Unit Operations ChE-381 Group No. 1 p. 41 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
15. Appendix II
The lab group members and their contributions are presented below
Krista Sutton 13 hrs 45 min
Time Spent in Lab 4 hrs
Section Time Spent Comments
1 WP&C 15 min Review
2 Abstract 15 min Review
3 Introduction 30 min Review
4 Theory 45 min Review
5 Apparatus 4 hrs Writing initial Drat
6 Materials and Supplies 30 min Writing initial Draft
7 Procedure 30 min Review
8 Data Tabulation/Graphs 1 hr 45 min Reformatting Tables
9 Results 30 min Review
10 Discussion 15 min Review
11 Error Analysis 15 min Review
12 Conclusion 15 min Review
13 References 0 hrs
14 Appendix I 0 hrs
Unit Operations ChE-381 Group No. 1 p. 42 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
Jake Biberstein 16 hrs 0 min
Time Spent in Lab 4 hrs
Section Time Spent Comments
1 WP&C 0 hrs
2 Abstract 15 min Group Section
3 Introduction 1 hr Edited Mikes First Version
4 Theory 2 hrs Edited Mikes First Version
5 Apparatus 0 hrs
6 Materials and Supplies 0 hrs
7 Procedure 0 hrs
Did the master Excel file which
8 Data Tabulation/Graphs 5 hrs
contained all graphs, calcs, tables
9 Results 1 hr Edited Russs First Version
10 Discussion 30 min Edited Stans First Version
11 Error Analysis 0 hrs
12 Conclusion 15 min Group Section
13 References 15 min Provided the things I used
14 Appendix I 1 hr 45 min Wrote the section
Unit Operations ChE-381 Group No. 1 p. 43 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
Stanley Das 10 hrs 0 min
Time Spent in Lab 4 hrs
Section Time Spent Comments
1 WP&C 0 hrs N/A
Helped write this section with the rest
2 Abstract 30 min
of the group
3 Introduction 0 hrs N/A
4 Theory 0 hrs N/A
5 Apparatus 0 hrs N/A
Reviewed this section for errors and
6 Materials and Supplies 30 min
typos
7 Procedure 0 hrs N/A
Input all data collected into excel
8 Data Tabulation/Graphs 1 hr spreadsheets, increased font size of
axes, legend. Collected data.
9 Results 0 hrs N/A
10 Discussion 3 hrs Wrote first draft of this section
11 Error Analysis 30 min Wrote first draft of this section
Reviewed this section for errors and
12 Conclusion 30 min
typos
13 References 0 hrs N/A
14 Appendix I 0 hrs N/A
Unit Operations ChE-381 Group No. 1 p. 44 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
Michael Czepizak 13 hrs 0 min Total Time Spend on this Lab
Time Spent in Lab 4 hrs
Section Time Spent Comments
1 WP&C 10 min Reviewed the section
2 Abstract 10 min Reviewed
3 Introduction 2 hrs 30 min Wrote this section
4 Theory 2 hrs 30 min Wrote this section
5 Apparatus 0
6 Materials and Supplies 0
7 Procedure 0
8 Data Tabulation/Graphs 0
9 Results 10 min Reviewed this section
10 Discussion 10 min Reviewed this section
11 Error Analysis 0
12 Conclusion 3 hrs Wrote this section
13 References 10 min Add references
14 Appendix I 10 min Reviewed section
Unit Operations ChE-381 Group No. 1 p. 45 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
Jeff Umbach 13 hrs 10 min Total Time Spend on this Lab
Time Spent in Lab 4 hrs
Section Time Spent Comments
1 WP&C 45 min Revised and proofread.
2 Abstract 10 minutes Formatted and proofread
3 Introduction 30 min Revised and proofread.
4 Theory 1 hr Edited, revised, and proofread.
5 Apparatus 30 min Revised and proofread.
6 Materials and Supplies 20 min Revised and proofread.
Wrote original and revised as issues
7 Procedure 4 hrs
were found during the lab experiment.
8 Data Tabulation/Graphs 10 min Proofread
9 Results 15 min Proofread and fixed grammar.
10 Discussion 15 min Proofread and fixed grammar.
11 Error Analysis 30 min Revised and proofread. Fixed grammar.
12 Conclusion 10 min Formatted, revised, and proofread.
13 References 20 min Tracked down some references.
14 Appendix I 15 min Proofread
Unit Operations ChE-381 Group No. 1 p. 46 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Flow Through Fluidized Beds University of Illinois
Russ Boyer 10 hrs 55 minutes
Time Spent in Lab 4 hrs
Section Time Spent Comments
1 WP&C 30 min Writing and revisions
2 Abstract 20 min Writing and revisions
3 Introduction 30 min Proofreading and editing
4 Theory 30 min Proofreading and editing
5 Apparatus 15 min Gathering apparatus specifications
6 Materials and Supplies 15 min Gathering specifications
7 Procedure 30 min Editing and revisions
8 Data Tabulation/Graphs 45 min Data gathering and reduction
9 Results 1 hr 30 min Wrote initial draft
10 Discussion 15 min Proofreading and editing
11 Error Analysis 1 hr 15 min Writing and proofreading
12 Conclusion 10 min Proofreading and editing
13 References 10 min Gathering references
14 Appendix I 0 hrs
Unit Operations ChE-381 Group No. 1 p. 47 Fall 2009 10/22/2009
Biberstein, Boyer, Czepizak, Das, Sutton, Umbach
Potrebbero piacerti anche
- Fluidized Bed Pressure Drop ExperimentDocumento8 pagineFluidized Bed Pressure Drop Experimentlovelygirl_256Nessuna valutazione finora
- Tray DryerDocumento16 pagineTray Dryermirdza94Nessuna valutazione finora
- Lab Report - Distillation of Bubble CapDocumento21 pagineLab Report - Distillation of Bubble Capratish100% (1)
- Plug Flow Reactor Experiment ReportDocumento25 paginePlug Flow Reactor Experiment ReportCesarah Cabungcal100% (1)
- Postlab 2 Gas AbsorptionDocumento7 paginePostlab 2 Gas AbsorptionDean Joyce AlborotoNessuna valutazione finora
- Exp - 2 Bubble Cap Distillation ColumnDocumento13 pagineExp - 2 Bubble Cap Distillation ColumnAdawiyah Al-jufri100% (1)
- Continuous Stirred Tank Reactor (40 L)Documento16 pagineContinuous Stirred Tank Reactor (40 L)Mohd Zhariff75% (4)
- FIXED AND FLUIDIZED BED EXPERIMENTDocumento6 pagineFIXED AND FLUIDIZED BED EXPERIMENTTuğbaNessuna valutazione finora
- Lab Report TPP Experiment 3Documento10 pagineLab Report TPP Experiment 3Nurul Najwa100% (1)
- Packed Bed Distillation Column Lab ReportDocumento13 paginePacked Bed Distillation Column Lab ReportShamini Sathivel100% (6)
- Experiment 1B - Tubular ReactorDocumento14 pagineExperiment 1B - Tubular ReactorNajmul Puda PappadamNessuna valutazione finora
- CHE504 - Lab Report On Distillation ColuDocumento27 pagineCHE504 - Lab Report On Distillation ColuMuhammad Irfan MalikNessuna valutazione finora
- Fixed and Fluidized Bed ExperimentDocumento9 pagineFixed and Fluidized Bed Experimentsunlias50% (2)
- Climbing FilmDocumento34 pagineClimbing FilmTunji Aminu100% (1)
- LAB REPORT-Gas AbsorptionDocumento16 pagineLAB REPORT-Gas Absorptionmizizasbonkure90100% (1)
- Gas Absorption: Determining Drag and Flooding FlowsDocumento5 pagineGas Absorption: Determining Drag and Flooding FlowsDean Joyce AlborotoNessuna valutazione finora
- Experiment 7 - Batch ReactorDocumento5 pagineExperiment 7 - Batch Reactormythili83% (6)
- Fluidisation ReportDocumento29 pagineFluidisation ReportBenjamin Jie100% (2)
- Final Report Expt10 (CHELAB1)Documento8 pagineFinal Report Expt10 (CHELAB1)PatrickGara100% (1)
- Oil Distillation ReportDocumento10 pagineOil Distillation ReportnisasoberiNessuna valutazione finora
- Distillation Column Pressure Drop & Refractive IndexDocumento18 pagineDistillation Column Pressure Drop & Refractive IndexAmir Al-AimanNessuna valutazione finora
- Batch Distillation of Methanol-Isopropanol MixtureDocumento20 pagineBatch Distillation of Methanol-Isopropanol Mixturecsgo accountNessuna valutazione finora
- Batch Distillation Laboratory ReportDocumento17 pagineBatch Distillation Laboratory ReportNayantara Soni100% (1)
- Report Distillation ColumnDocumento20 pagineReport Distillation ColumnAzam Najmi33% (3)
- Lab Report Batch Reactor GGDocumento25 pagineLab Report Batch Reactor GGFrost Orchid100% (1)
- Optimized Solids Suspension: Achieving Uniform Dispersion is Critical to Product QualityDocumento7 pagineOptimized Solids Suspension: Achieving Uniform Dispersion is Critical to Product QualitymichsantosNessuna valutazione finora
- Final Report DistillationDocumento8 pagineFinal Report DistillationAlice ToNessuna valutazione finora
- Tray Drying Experiment: Effects of Air Velocity on Drying RateDocumento13 pagineTray Drying Experiment: Effects of Air Velocity on Drying RateSrinyanavel ஸ்ரீஞானவேல்75% (4)
- Lab 10-Batch ReactorDocumento22 pagineLab 10-Batch Reactorniraj_bairagiNessuna valutazione finora
- Distillation Column EfficiencyDocumento33 pagineDistillation Column EfficiencyAldi StefanusNessuna valutazione finora
- Exp1 - Fixed and Fluidized BedDocumento3 pagineExp1 - Fixed and Fluidized BedAhmedNessuna valutazione finora
- CLB11003 - Exp 4Documento6 pagineCLB11003 - Exp 4Nur DiyanahNessuna valutazione finora
- Investigation of Liquid-Solid and Gas-Solid Fluidized BedDocumento18 pagineInvestigation of Liquid-Solid and Gas-Solid Fluidized Bedmahbub1332100% (1)
- Continuous Stirred Tank Reactor: CHEN-410 Unit Operation LabDocumento34 pagineContinuous Stirred Tank Reactor: CHEN-410 Unit Operation LabMohamad Abou DaherNessuna valutazione finora
- CSTR 40L LAB EXPERIMENTDocumento18 pagineCSTR 40L LAB EXPERIMENTSaber Minato Azrul100% (2)
- Gaseous Diffusion CoefficientDocumento17 pagineGaseous Diffusion CoefficientAddiaAzizan93% (14)
- Lab 2 Full Report PDFDocumento20 pagineLab 2 Full Report PDFmuhammad ilyas100% (1)
- Fluidization Post Laboratory ReportDocumento25 pagineFluidization Post Laboratory ReportGail100% (1)
- Experiment 3 - Fixed and Fluidized BedDocumento12 pagineExperiment 3 - Fixed and Fluidized BedNajmul Puda PappadamNessuna valutazione finora
- Batch Reactor (Saponification) ExptDocumento3 pagineBatch Reactor (Saponification) ExptVijay Prasad0% (1)
- Results and Discussion of CSTR in SeriesDocumento3 pagineResults and Discussion of CSTR in SeriesleenzalalNessuna valutazione finora
- Effects of Particle Size On DryingDocumento4 pagineEffects of Particle Size On DryingAshley Perida50% (2)
- Drying of Solids (Sand)Documento15 pagineDrying of Solids (Sand)Mahe Rukh75% (4)
- Lab 3 Plug FlowDocumento29 pagineLab 3 Plug FlowHikaru MokaNessuna valutazione finora
- CKB 20104 Reaction Engineering UniKL MICET Mini Project (Stage 1) : Production of Acetylene 80 Metric Ton/year PFRDocumento22 pagineCKB 20104 Reaction Engineering UniKL MICET Mini Project (Stage 1) : Production of Acetylene 80 Metric Ton/year PFRSiti Hajar Mohamed100% (6)
- PFR Lab ReportDocumento16 paginePFR Lab Reportcog0812Nessuna valutazione finora
- Fluidization: Chemical Engneering PracticeDocumento24 pagineFluidization: Chemical Engneering PracticeFarhan M JafrI100% (1)
- Acetone DiffusionDocumento15 pagineAcetone DiffusionArmaan Hussain40% (5)
- Sedimentation Studies Apparatus DesignDocumento7 pagineSedimentation Studies Apparatus Designgrkhari1100% (2)
- Batch Sedimentation Lab ReportDocumento16 pagineBatch Sedimentation Lab Reportlucas67% (3)
- Batch Reactor ExpDocumento12 pagineBatch Reactor ExpJack AndreasNessuna valutazione finora
- Properties of Gas and Liquid Experiment ReportDocumento18 pagineProperties of Gas and Liquid Experiment ReportFadh At-Tarf67% (3)
- Apparatus, Procedure, Recommendation Tray DryerDocumento4 pagineApparatus, Procedure, Recommendation Tray DryerillyzlNessuna valutazione finora
- Diffusivity of Liquid Into LiquidDocumento8 pagineDiffusivity of Liquid Into LiquidZahraa GhanemNessuna valutazione finora
- Energy BalanceDocumento16 pagineEnergy BalancewizlanNessuna valutazione finora
- Report Tray DryerDocumento15 pagineReport Tray DryerSharing Caring75% (4)
- Group 4 - Experiment 2 ReportDocumento13 pagineGroup 4 - Experiment 2 Reportwasit.mNessuna valutazione finora
- Exhaust Housing - 1992 Johnson Outboards 9.9 J10renaDocumento7 pagineExhaust Housing - 1992 Johnson Outboards 9.9 J10renaCharlie CB PortnerNessuna valutazione finora
- CARBURETOR - 1992 Johnson Outboards 9.9 J10RENA Crowley MarineDocumento8 pagineCARBURETOR - 1992 Johnson Outboards 9.9 J10RENA Crowley MarineCharlie CB PortnerNessuna valutazione finora
- 1992 Johnson Outboards 9.9 - J10RENA Motor Cable Parts ListDocumento3 pagine1992 Johnson Outboards 9.9 - J10RENA Motor Cable Parts ListCharlie CB PortnerNessuna valutazione finora
- Steering Handle & Throttle Control - 1992 Johnson Outboards 9.9 J10rena Crowley MarineDocumento7 pagineSteering Handle & Throttle Control - 1992 Johnson Outboards 9.9 J10rena Crowley MarineCharlie CB PortnerNessuna valutazione finora
- Seborg, Mellichamp, Edgar, Doyle: Process Dynamics and Control, 3 Edition Edgar, Himmelblau, Lasdon: Optimization of Chemical Processes, 2 EditionDocumento21 pagineSeborg, Mellichamp, Edgar, Doyle: Process Dynamics and Control, 3 Edition Edgar, Himmelblau, Lasdon: Optimization of Chemical Processes, 2 EditionCharlie CB PortnerNessuna valutazione finora
- CYLINDER & CRANKCASE - 1992 Johnson Outboards 9.9 J10RENA Crowley MarineDocumento18 pagineCYLINDER & CRANKCASE - 1992 Johnson Outboards 9.9 J10RENA Crowley MarineCharlie CB PortnerNessuna valutazione finora
- GEARCASE - 1992 Johnson Outboards 9.9 J10RENA Crowley MarineDocumento22 pagineGEARCASE - 1992 Johnson Outboards 9.9 J10RENA Crowley MarineCharlie CB PortnerNessuna valutazione finora
- CYLINDER & CRANKCASE - 1992 Johnson Outboards 9.9 J10RENA Crowley MarineDocumento18 pagineCYLINDER & CRANKCASE - 1992 Johnson Outboards 9.9 J10RENA Crowley MarineCharlie CB PortnerNessuna valutazione finora
- GEARCASE - 1992 Johnson Outboards 9.9 J10RENA Crowley MarineDocumento22 pagineGEARCASE - 1992 Johnson Outboards 9.9 J10RENA Crowley MarineCharlie CB PortnerNessuna valutazione finora
- ELECTRIC PRIMER SYSTEM - 1992 Johnson Outboards 9.9 J10RENA Crowley MarineDocumento7 pagineELECTRIC PRIMER SYSTEM - 1992 Johnson Outboards 9.9 J10RENA Crowley MarineCharlie CB PortnerNessuna valutazione finora
- MIDSECTION (10SEL ONLY) - 1992 Johnson Outboards 9.9 J10RENA Crowley MarineDocumento19 pagineMIDSECTION (10SEL ONLY) - 1992 Johnson Outboards 9.9 J10RENA Crowley MarineCharlie CB PortnerNessuna valutazione finora
- Seborg, Mellichamp, Edgar, Doyle: Process Dynamics and Control, 3 Edition Edgar, Himmelblau, Lasdon: Optimization of Chemical Processes, 2 EditionDocumento17 pagineSeborg, Mellichamp, Edgar, Doyle: Process Dynamics and Control, 3 Edition Edgar, Himmelblau, Lasdon: Optimization of Chemical Processes, 2 EditionCharlie CB PortnerNessuna valutazione finora
- 1992 Johnson Outboards 9.9 Ignition PartsDocumento9 pagine1992 Johnson Outboards 9.9 Ignition PartsCharlie CB PortnerNessuna valutazione finora
- CARBURETOR - 1992 Johnson Outboards 9.9 J10RENA Crowley MarineDocumento8 pagineCARBURETOR - 1992 Johnson Outboards 9.9 J10RENA Crowley MarineCharlie CB PortnerNessuna valutazione finora
- ENGINE COVER - JOHNSON - 1992 Johnson Outboards 9.9 J10RENA Crowley MarineDocumento5 pagineENGINE COVER - JOHNSON - 1992 Johnson Outboards 9.9 J10RENA Crowley MarineCharlie CB PortnerNessuna valutazione finora
- CRANKSHAFT & PISTON - 1992 Johnson Outboards 9.9 J10RENA Crowley MarineDocumento5 pagineCRANKSHAFT & PISTON - 1992 Johnson Outboards 9.9 J10RENA Crowley MarineCharlie CB PortnerNessuna valutazione finora
- Polymers Plus User Guide Volume 1Documento452 paginePolymers Plus User Guide Volume 1Adnaloh KistortNessuna valutazione finora
- ENCH 442 Lecture 1 Process Control IntroductionDocumento16 pagineENCH 442 Lecture 1 Process Control IntroductionCharlie CB PortnerNessuna valutazione finora
- DiffEq PDFDocumento6 pagineDiffEq PDFCharlie CB PortnerNessuna valutazione finora
- PJN3-1000 Assy8Documento12 paginePJN3-1000 Assy8Charlie CB PortnerNessuna valutazione finora
- HW4 PDEsDocumento10 pagineHW4 PDEsCharlie CB PortnerNessuna valutazione finora
- Environmental Organic ChemistryDocumento16 pagineEnvironmental Organic ChemistryCharlie CB PortnerNessuna valutazione finora
- DiffEq PDFDocumento6 pagineDiffEq PDFCharlie CB PortnerNessuna valutazione finora
- Chemistry and Technology of Rubber PDFDocumento215 pagineChemistry and Technology of Rubber PDFCharlie CB Portner100% (1)
- GPSS3Documento82 pagineGPSS3Charlie CB PortnerNessuna valutazione finora
- Baltimore Water and Wastewater Bureau OverviewDocumento29 pagineBaltimore Water and Wastewater Bureau OverviewCharlie CB PortnerNessuna valutazione finora
- ENCH 474 / ENCE 660 - Air Pollution The Big PictureDocumento18 pagineENCH 474 / ENCE 660 - Air Pollution The Big PictureCharlie CB PortnerNessuna valutazione finora
- Lab Guide 2017Documento48 pagineLab Guide 2017Charlie CB PortnerNessuna valutazione finora
- Lecture 2 AirPollution PDFDocumento31 pagineLecture 2 AirPollution PDFCharlie CB PortnerNessuna valutazione finora
- Heat Load CalculationDocumento6 pagineHeat Load Calculationavj278631Nessuna valutazione finora
- BLACK GOLD DISSOLVABLE FRAC PLUG Technical Sales Brochure PDFDocumento3 pagineBLACK GOLD DISSOLVABLE FRAC PLUG Technical Sales Brochure PDFImadNessuna valutazione finora
- Samsung DvmsDocumento88 pagineSamsung DvmsGingubaNessuna valutazione finora
- Control of Foaming in Amine Systems - Brimstone 07 Rev A-2-2 PDFDocumento13 pagineControl of Foaming in Amine Systems - Brimstone 07 Rev A-2-2 PDFscm996100% (1)
- MFDG Low-Res Proof Part2Documento154 pagineMFDG Low-Res Proof Part2Philip BgNessuna valutazione finora
- 03SA0S04Documento5 pagine03SA0S04Jhonny RinconesNessuna valutazione finora
- Chemical Reactor Design-CHEM-E7135: Yongdan LiDocumento56 pagineChemical Reactor Design-CHEM-E7135: Yongdan LiVaseline RobinsonNessuna valutazione finora
- Plug Flow Reactor NotesDocumento4 paginePlug Flow Reactor Notesahmad pidotNessuna valutazione finora
- General Corrosion Survey of Tr 1A Glycol PipingDocumento11 pagineGeneral Corrosion Survey of Tr 1A Glycol PipingShamsul AffendiNessuna valutazione finora
- Entropy Generation Minimization: The New Thermodynamics of Finitesize Devices and Finitetime ProcessesDocumento29 pagineEntropy Generation Minimization: The New Thermodynamics of Finitesize Devices and Finitetime ProcessesSouadHadjadjNessuna valutazione finora
- FLARESIM Industry Standard Flare Radiation Analysis SoftwareDocumento2 pagineFLARESIM Industry Standard Flare Radiation Analysis SoftwareerjainrachitNessuna valutazione finora
- 40 KLD MBR QuoteDocumento15 pagine40 KLD MBR QuoteV Narasimha RajuNessuna valutazione finora
- Bulk Properties of PowdersDocumento15 pagineBulk Properties of Powderslhphong021191Nessuna valutazione finora
- Introduction To Fluid Mechanics - Ch14Documento11 pagineIntroduction To Fluid Mechanics - Ch14Nguyễn Hồng Quân100% (5)
- Class IX Chemistry Chapter 06Documento4 pagineClass IX Chemistry Chapter 06Sam FisherNessuna valutazione finora
- 11th National Combustion Meeting - 2019Documento25 pagine11th National Combustion Meeting - 2019Bikram Basu RoychowdhuryNessuna valutazione finora
- Overall Heat Transfer Coefficient and Pipe Length CalculationDocumento2 pagineOverall Heat Transfer Coefficient and Pipe Length CalculationCaleb FalcoteloNessuna valutazione finora
- (BMPS) : Bapco Modernization Program SpecificationsDocumento20 pagine(BMPS) : Bapco Modernization Program SpecificationschaitanyaNessuna valutazione finora
- LESER at A Glance enDocumento12 pagineLESER at A Glance enRADPROCESSSYTEMSNessuna valutazione finora
- - =-3.6 log ф ^ - J J (: 182 Chapter 6 Interphase Transport in Isothermal SystemsDocumento3 pagine- =-3.6 log ф ^ - J J (: 182 Chapter 6 Interphase Transport in Isothermal SystemsAndrianPratamaNessuna valutazione finora
- SP100B PDFDocumento8 pagineSP100B PDFNicolas AguilarNessuna valutazione finora
- Sandwich Type Butterfly Valve: I ApplicationDocumento2 pagineSandwich Type Butterfly Valve: I ApplicationFery FebryantoNessuna valutazione finora
- Abb RRTC PaperDocumento24 pagineAbb RRTC PapersyaplinNessuna valutazione finora
- Netzsch-ARC en Web PDFDocumento20 pagineNetzsch-ARC en Web PDFdarksideministryNessuna valutazione finora
- Lecture 1 SeriesDocumento43 pagineLecture 1 SerieschetsNessuna valutazione finora
- FLNG Training Module 1.1 Introduction To FLNG Rev 1Documento34 pagineFLNG Training Module 1.1 Introduction To FLNG Rev 1Jay Kanes100% (3)
- Introducing Rheology - Sep 2017Documento34 pagineIntroducing Rheology - Sep 2017Daniel Perez ColmenaresNessuna valutazione finora
- MarineDocumento16 pagineMarineNguyễn Thị Kim PhượngNessuna valutazione finora
- Aerodynamics 2Documento30 pagineAerodynamics 2rahulbqaNessuna valutazione finora
- PS0121-DWG-B-003 Sheet 5 Jalur Pemipaan Dari WDO Fase 1 & 2 Menuju Drain Tank CCDS - Isometric Sect. 4 R.5Documento1 paginaPS0121-DWG-B-003 Sheet 5 Jalur Pemipaan Dari WDO Fase 1 & 2 Menuju Drain Tank CCDS - Isometric Sect. 4 R.5yusak adi setiawanNessuna valutazione finora
- Power of Habit: The Ultimate Guide to Forming Positive Daily Habits, Learn How to Effectively Break Your Bad Habits For Good and Start Creating Good OnesDa EverandPower of Habit: The Ultimate Guide to Forming Positive Daily Habits, Learn How to Effectively Break Your Bad Habits For Good and Start Creating Good OnesValutazione: 4.5 su 5 stelle4.5/5 (21)
- ISO 50001: A strategic guide to establishing an energy management systemDa EverandISO 50001: A strategic guide to establishing an energy management systemNessuna valutazione finora
- Introduction to Power System ProtectionDa EverandIntroduction to Power System ProtectionValutazione: 5 su 5 stelle5/5 (1)
- Operational Amplifier Circuits: Analysis and DesignDa EverandOperational Amplifier Circuits: Analysis and DesignValutazione: 4.5 su 5 stelle4.5/5 (2)
- The Way Home: Tales from a life without technologyDa EverandThe Way Home: Tales from a life without technologyValutazione: 4 su 5 stelle4/5 (45)
- Formulas and Calculations for Drilling, Production, and Workover: All the Formulas You Need to Solve Drilling and Production ProblemsDa EverandFormulas and Calculations for Drilling, Production, and Workover: All the Formulas You Need to Solve Drilling and Production ProblemsNessuna valutazione finora
- The Grid: The Fraying Wires Between Americans and Our Energy FutureDa EverandThe Grid: The Fraying Wires Between Americans and Our Energy FutureValutazione: 3.5 su 5 stelle3.5/5 (48)
- Idaho Falls: The Untold Story of America's First Nuclear AccidentDa EverandIdaho Falls: The Untold Story of America's First Nuclear AccidentValutazione: 4.5 su 5 stelle4.5/5 (21)
- Renewable Energy: A Very Short IntroductionDa EverandRenewable Energy: A Very Short IntroductionValutazione: 4.5 su 5 stelle4.5/5 (12)
- Asset Integrity Management for Offshore and Onshore StructuresDa EverandAsset Integrity Management for Offshore and Onshore StructuresNessuna valutazione finora
- The Rare Metals War: the dark side of clean energy and digital technologiesDa EverandThe Rare Metals War: the dark side of clean energy and digital technologiesValutazione: 5 su 5 stelle5/5 (2)
- Build Your Own Electric Vehicle, Third EditionDa EverandBuild Your Own Electric Vehicle, Third EditionValutazione: 4.5 su 5 stelle4.5/5 (3)
- Shorting the Grid: The Hidden Fragility of Our Electric GridDa EverandShorting the Grid: The Hidden Fragility of Our Electric GridValutazione: 4.5 su 5 stelle4.5/5 (2)
- OFF-GRID PROJECTS: A Comprehensive Beginner's Guide to Learn All about OffGrid Living from A-Z and Live a Life of Self-SufficiencyDa EverandOFF-GRID PROJECTS: A Comprehensive Beginner's Guide to Learn All about OffGrid Living from A-Z and Live a Life of Self-SufficiencyNessuna valutazione finora
- Nuclear Energy in the 21st Century: World Nuclear University PressDa EverandNuclear Energy in the 21st Century: World Nuclear University PressValutazione: 4.5 su 5 stelle4.5/5 (3)
- Industrial Piping and Equipment Estimating ManualDa EverandIndustrial Piping and Equipment Estimating ManualValutazione: 5 su 5 stelle5/5 (7)
- Handbook on Battery Energy Storage SystemDa EverandHandbook on Battery Energy Storage SystemValutazione: 4.5 su 5 stelle4.5/5 (2)
- VSC-FACTS-HVDC: Analysis, Modelling and Simulation in Power GridsDa EverandVSC-FACTS-HVDC: Analysis, Modelling and Simulation in Power GridsNessuna valutazione finora
- Energy, Light and Electricity - Introduction to Physics - Physics Book for 12 Year Old | Children's Physics BooksDa EverandEnergy, Light and Electricity - Introduction to Physics - Physics Book for 12 Year Old | Children's Physics BooksNessuna valutazione finora
- Well Control for Completions and InterventionsDa EverandWell Control for Completions and InterventionsValutazione: 4 su 5 stelle4/5 (10)
- Air-Cooled Condenser Fundamentals: Design, Operations, Troubleshooting, Maintenance, and Q&ADa EverandAir-Cooled Condenser Fundamentals: Design, Operations, Troubleshooting, Maintenance, and Q&AValutazione: 5 su 5 stelle5/5 (1)
- The Complete HVAC BIBLE for Beginners: The Most Practical & Updated Guide to Heating, Ventilation, and Air Conditioning Systems | Installation, Troubleshooting and Repair | Residential & CommercialDa EverandThe Complete HVAC BIBLE for Beginners: The Most Practical & Updated Guide to Heating, Ventilation, and Air Conditioning Systems | Installation, Troubleshooting and Repair | Residential & CommercialNessuna valutazione finora
- Introduction to Power System ProtectionDa EverandIntroduction to Power System ProtectionNessuna valutazione finora