Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Haluros de Alquilo
Caricato da
Fiama BonomiCopyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Haluros de Alquilo
Caricato da
Fiama BonomiCopyright:
Formati disponibili
EXPERIMENT 8
RELATIVE RATES OF NUCLEOPHILIC
SUBSTITUTION REACTIONS
Reading Assignment: Smith, Chapter 7
Pre-lab Questions:
1) What determines whether 2-bromobutane undergoes an SN1 or an SN2 reaction?
How would you favor SN2 formation?
2) Benzyl bromide reacts rapidly with sodium iodide in acetone, and also reacts
rapidly with ethanol and silver acetate. Bromobenzene doesnt react under
either of these conditions. Explain.
3) Allyl bromide is a primary alkyl halide, yet it reacts rapidly with silver nitrate in
ethanol. Explain.
There are two mechanisms for nucleophilic substitution. The SN2 reaction involves a one
step concerted displacement of the leaving group by a nucleophile with inversion of
configuration. The SN1 reaction is a 2-step reaction involving loss of the leaving group to form
of a carbocation intermediate, followed by attack by a weak nucleophile with loss of
stereochemistry.
In Part 1 of this experiment, we will examine the factors that affect the relative rate of
the SN2 reaction for a series of alkyl chloride and alkyl bromides. We will see how alkyl halide
structure and the nature of the leaving group effects the rate of an SN2 reaction, and deduce the
rate law by varying the initial concentrations of the nucleophile and alkyl halide, and observing
the effect this has on the rate of the reaction. We will use reaction conditions that favor an SN2
reaction: a solution of sodium iodide in acetone.
acetone ! !
R Cl + NaI I R X I R + NaCl
Iodide ion is an excellent nucleophile, and SN2 reactions are favored in polar aprotic solvents
such as acetone. Because acetone cannot sufficiently stabilize a carbocation intermediate, the
competing SN1 reaction is suppressed. Sodium iodide is soluble in acetone, but when bromide or
chloride is the leaving group in this reaction, sodium bromide or sodium chloride precipitates as
an insoluble salt. This precipitation drives the equilibrium to the right, and allows easy
CHEM M52LA Experiment 8 Page 1
monitoring of the rate of the reaction.
In Part 2 of this experiment, we will examine the factors that affect the relative rate of
the SN1 reaction for the same series of alkyl chloride and alkyl bromides. We will see how alkyl
halide structure and the nature of the leaving group affects the rate of an SN1 reaction, and
deduce the rate law by varying the initial concentrations of the nucleophile and alkyl halide, and
observing the effect this has on the rate of the reaction. We will use reaction conditions that
favor an SN1 reaction: a solution of silver nitrate in ethanol.
AgNO3 ! ! !
R Cl R Cl Ag R + AgCl + NO3
R + CH3 CH2 OH ROCH2 CH3
Ethanol is a polar protic solvent, and a poor nucleophile. The silver ion coordinates with the
halide ion in the alkyl halide and enhances carbocation formation. The SN2 reaction is suppressed
because of the absence of a good nucleophile. When bromide or chloride is the leaving group in
this reaction, silver bromide or silver chloride precipitates as an insoluble salt. This precipitation
drives the equilibrium to the right, and allows easy monitoring of the rate of the reaction.
CAUTION
All alkyl halides are harmful if inhaled, ingested , or absorbed through the skin. Wear
gloves, and do all work in an efficient fume hood.
Silver nitrate solutions are toxic and will discolor the skin.
Part 1. Factors that affect the Relative Rate of the SN2 Reaction
Make sure all test tubes for Part 1 are clean and dry! Obtain 11 test tubes with stoppers (do
not use rubber stoppers) for part 1.
Alkyl halide Structure: Measure 2 mL of 15% sodium iodide in acetone into each of three test
tubes. Add 2 drops of 1-bromobutane (butyl bromide) to tube 1, 2 drops 2-bromobutane (sec-
butyl bromide) to tube 2, and 2 drops 2-bromo-2-methylpropane (tert-butyl bromide) to tube 3,
recording the time of each addition. Stopper and shake each. Note the time at which the first
signs of cloudiness or precipitate appears. Closely observe for the first 15-20 minutes, and then
at intervals throughout the lab period.
CHEM M52LA Experiment 8 Page 2
Steric Effects: Measure 1 mL of 15% sodium iodide in acetone into each of two test tubes. Add
2 drops 1-bromobutane (butyl bromide) into tube 1, and 2 drops of 1-bromo-2,2-
dimethylpropane (neopentyl bromide) into tube 2, recording the time of each addition. Stopper
and shake each and observe closely. Note the time at which the first signs of cloudiness or
precipitate appears.
Leaving Group Effects: Measure 1 mL of 15% sodium iodide in acetone into each of two test
tubes. Add 2 drops 1-bromobutane (butyl bromide) into tube 1, and 2 drops of 1-chlorobutane
(butyl chloride) into tube 2, recording the time of each addition. Stopper and shake each and
observe closely. Note the time at which the first signs of cloudiness or precipitate appears.
Rate Law: Measure 1.0 mL of 15% sodium iodide in acetone into each of two test tubes. At the
same time, (or do one at a time and mark each time carefully), add 0.1 mL of 1.0 M 1-
bromobutane in acetone to tube 1, and 0.1 mL of 2.0 M 1-bromobutane in acetone to tube 2.
Record your observations.
Measure 1.0 mL of 1.0 M 1-bromobutane in acetone into each of two test tubes. At the
same time, (or do one at a time and mark each time carefully), add 0.1 mL of 7.5% sodium
iodide in acetone to tube 1, and 0.1 mL of 15% sodium iodide in acetone to tube 2. Record your
observations.
Results and Conclusions for Part 1:
1. Which alkyl bromide reacts fastest with sodium iodide in acetone: butyl bromide, sec-
butyl bromide, or tert-butyl bromide? Which reacts slowest? Explain how structure
affects the rate in the SN2 reaction.
2. Which alkyl bromide reacts fastest with sodium iodide in acetone: butyl bromide or
neopentyl bromide? Both of these are primary alkyl bromides. Explain the difference in
reactivity.
3. Which alkyl halide reacted fastest with sodium iodide in acetone: butyl bromide or butyl
chloride? Explain how the nature of the leaving group affects the rate in the SN2 reaction.
4. How did the following changes affect the rate of reaction of 1-bromobutane with NaI:
doubling the concentration of 1-bromobutane? Doubling the concentration of NaI?
5. Write a generalized rate expression for an SN2 reaction.
Part 2. Factors that affect the Relative Rate of the SN1 Reaction
Make sure all test tubes for Part 2 are clean and dry! Obtain 11 test tubes with stoppers (do
not use rubber stoppers) for part 2.
Alkyl halide Structure: Measure 2 mL of a 0.1 M solution of silver nitrate in absolute ethanol
into each of three test tubes. Add 1 drop of 1-bromobutane (butyl bromide) to tube 1, 1 drop 2-
bromobutane (sec-butyl bromide) to tube 2, and 1 drop 2-bromo-2-methylpropane (tert-butyl
bromide) to tube 3, recording the time of each addition. Stopper and shake tubes continuously.
Watch carefully for the first signs of cloudiness or precipitate formation.
CHEM M52LA Experiment 8 Page 3
Leaving Group Effects: Measure 2 mL of a 0.1 M solution of silver nitrate in absolute ethanol
into each of two test tubes. At the same time (or do one at a time and mark each time carefully),
add 1 drop 2-bromo-2-methylpropane (tert-butyl bromide) into tube 1, and add 1 drop 2-chloro-
2-methylpropane (tert-butyl chloride) into tube 2. Stopper and shake each and observe closely.
Note the time at which the first signs of cloudiness or precipitate appears.
Solvent Polarity Effects: Measure 2 mL of a 0.1 M solution of silver nitrate in absolute ethanol
into one test tube. Into a second test tube, measure 2 mL of a 0.1 M solution of silver nitrate in
5% absolute ethanol/ 95% acetone. At the exact same time (get a partner to help), add 1 drop 2-
chloro-2-methylpropane (tert-butyl chloride) into each tube. Stopper and shake each and
observe closely. Record observations.
Rate Law: Measure 0.5 mL of a 0.1 M 2-chloro-2-methylpropane (tert-butyl chloride) in
absolute ethanol solution into one test tube. Into a second test tube, measure 0.5 mL of a 0.2 M
2-chloro-2-methylpropane (tert-butyl chloride) in abasolute ethanol solution. At the same time
(or do one at a time and mark each time carefully), add 1.0 mL of of a 0.1 M solution of silver
nitrate in absolute ethanol to each. Record your observations.
Measure 1.0 mL of a 0.1 M solution of silver nitrate in absolute ethanol into one test tube.
Into a second test tube, measure 0.5 mL of of a 0.1 M solution of silver nitrate in absolute ethanol
AND 0.5 mL absolute ethanol (to ensure volumes are the same). At the same time (or do one at a
time and mark each time carefully), add 1.0 mL of 0.1M 2-chloro-2-methylpropane (tert-butyl
chloride) in absolute ethanol solution to each. Record your observations.
Results and Conclusions for Part 2:
1. Which alkyl bromide reacts fastest with silver nitrate in ethanol: butyl bromide, sec-butyl
bromide, or tert-butyl bromide? Which reacts slowest? Explain how structure affects the
rate in the SN1 reaction.
2. Which alkyl halide reacted fastest with silver nitrate in ethanol: tert-butyl bromide or
tert-butyl chloride? Explain how the nature of the leaving group affects the rate in the
SN1 reaction.
3. Which solvent gave the fastest reaction; ethanol, or the ethanol/acetone mixture? Explain
how the solvent affects the rate in the SN1 reaction.
4. How did changing the concentration of tert-butyl chloride affect the rate of the reaction?
In part 1, we varied the concentration of the nucleophile in order to help determine the
rate law. Why didnt we vary the concentration of nucleophile in Part 2?
5. Write a generalized rate expression for an SN1 reaction.
Summarize your conclusions for Part 1 and Part 2 in your final lab report.
Post-lab Questions:
CHEM M52LA Experiment 8 Page 4
1) Secondary alkyl halides can undergo SN2 and SN1 reactions. Comparing results
for Part 1 and Part 2, which is a faster reaction for secondary alkyl halides, an
SN2 or an SN1 reaction? Explain.
2) 1-Bromoadamantane is a tertiary halide, yet it is 10,000 times slower than tert-
butyl bromide when allowed to react with silver nitrate in ethanol. Explain.
3) Are alkyl fluorides good substrates in SN2 and SN1 reactions? Explain.
Reference:
1. This procedure is adapted from: A.M. Schoffstall, B.A. Gaddis, M. L. Druelinger,
Microscale and Miniscale Organic Chemistry Laboratory Experiments, McGraw-Hill,
2000, p. 253, and J.R. Mohrig, C.N. Hammond, P.F. Schatz, T.C. Morrill, Modern
Projects and Experiments in Organic Chemistry: Miniscale & Standard Taper
Microscale, 2nd Ed., W.H. Freeman and Company, 2003, p. 67.
CHEM M52LA Experiment 8 Page 5
Potrebbero piacerti anche
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (895)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (400)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (588)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (74)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (344)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (121)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- Research Paper in PH IndicatorDocumento11 pagineResearch Paper in PH IndicatorKhristine Khate Odiaman MendezNessuna valutazione finora
- Mock 5 Unit 4 QP PDFDocumento27 pagineMock 5 Unit 4 QP PDFMohibur Rahman AbirNessuna valutazione finora
- DLP ForDocumento22 pagineDLP ForFegy MabuhisanNessuna valutazione finora
- Presentation 9Documento8 paginePresentation 9sun shineNessuna valutazione finora
- Lab Report Experiment 1 Chm624Documento11 pagineLab Report Experiment 1 Chm624Hazwan HamimNessuna valutazione finora
- 12th Chemistry Unit 8 Study Material English MediumDocumento14 pagine12th Chemistry Unit 8 Study Material English Mediumanu.pavansuresh30Nessuna valutazione finora
- Water For InjectionsDocumento4 pagineWater For InjectionsAlvina Arum PuspitasariNessuna valutazione finora
- The Diagram Shows Part of The Structures of Sodium Bromide and SulfurDocumento3 pagineThe Diagram Shows Part of The Structures of Sodium Bromide and SulfurHashimNessuna valutazione finora
- Factors Affecting SolubilityDocumento3 pagineFactors Affecting SolubilityJason Raquin RoqueNessuna valutazione finora
- Production of Detergent From Castor OilDocumento8 pagineProduction of Detergent From Castor OilLenh DavidleNessuna valutazione finora
- AK - Practice Problems Chapter 15 Organic ChemistryDocumento10 pagineAK - Practice Problems Chapter 15 Organic ChemistryangelineNessuna valutazione finora
- Alkalinity Relationships in Water ChemistryDocumento3 pagineAlkalinity Relationships in Water ChemistrytinuvalsapaulNessuna valutazione finora
- The Logic of Chemical Synthesis Corey E J Amp Cheng X M 271 330Documento60 pagineThe Logic of Chemical Synthesis Corey E J Amp Cheng X M 271 330bann tvNessuna valutazione finora
- Study Material For Practical - RVCEDocumento25 pagineStudy Material For Practical - RVCEMalavika UnnikrishnanNessuna valutazione finora
- 2.review of LiteratureDocumento20 pagine2.review of LiteratureMjd ObiedNessuna valutazione finora
- Trends in Group 2 Elements Alkaline Earth MetalsDocumento52 pagineTrends in Group 2 Elements Alkaline Earth MetalsKemoy FrancisNessuna valutazione finora
- NMR Chemical Shifts of Common Laboratory SolventsDocumento4 pagineNMR Chemical Shifts of Common Laboratory Solventspharmacysmile8049Nessuna valutazione finora
- 15 Chapter 5Documento48 pagine15 Chapter 5Hiren MoghariyaNessuna valutazione finora
- Jee Main 2019 Jan ChemDocumento84 pagineJee Main 2019 Jan ChemBhavesh KriplaniNessuna valutazione finora
- Revised Ifra Analytical Method On Peroxide ValueDocumento8 pagineRevised Ifra Analytical Method On Peroxide ValuetitrasiNessuna valutazione finora
- Telemarketing InmobiliariodaDocumento270 pagineTelemarketing Inmobiliariodaremigio cervantesNessuna valutazione finora
- Acids Bases and SaltsDocumento22 pagineAcids Bases and Saltsd anjilappaNessuna valutazione finora
- Reactions of Copper (Edited, 10 Sept 2023)Documento5 pagineReactions of Copper (Edited, 10 Sept 2023)Rose-AnnMirambilNessuna valutazione finora
- Chapter 28Documento16 pagineChapter 28Muhamad Ivan AbrorNessuna valutazione finora
- 10.0 Carboxylic Acids 2021Documento64 pagine10.0 Carboxylic Acids 2021NURUL HIDAYAH SAIFUL ANUARNessuna valutazione finora
- Australian Product HandbookDocumento88 pagineAustralian Product HandbookjarwokoesoemoNessuna valutazione finora
- Analysis of Cold DrinksDocumento18 pagineAnalysis of Cold DrinksSuman Das0% (1)
- IIT Chemistry: EnthuseDocumento15 pagineIIT Chemistry: EnthuseArjun SabnisNessuna valutazione finora
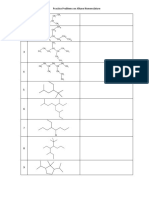
- Practice Problems On Alkane Nomenclature: CH CHDocumento2 paginePractice Problems On Alkane Nomenclature: CH CHRishav Sasmal100% (2)
- Miscibility ChartDocumento2 pagineMiscibility ChartEdward PittsNessuna valutazione finora