Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Sinrtesis Intestinal Iga
Caricato da
Anailil MoralesCopyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Sinrtesis Intestinal Iga
Caricato da
Anailil MoralesCopyright:
Formati disponibili
REVIEWS
INTESTINAL IgA SYNTHESIS:
REGULATION OF FRONT-LINE
BODY DEFENCES
Sidonia Fagarasan* and Tasuku Honjo*
Immunoglobulin A is the most abundant immunoglobulin isotype in mucosal secretions. In this
review, we summarize recent advances in our understanding of the sites, mechanisms and
functions of intestinal IgA synthesis in mice. On the basis of these recent findings, we propose
an updated model for the induction and regulation of IgA responses in the gut. In addition, we
discuss new insights into the role of IgA in the maintenance of gut homeostasis and into the
reciprocal interactions between gut B cells and the bacterial flora.
J CHAIN In terms of its cellular composition and architecture, its generation of IgA+ B cells8. However, despite the enor-
A polypeptide produced by exposure to antigens and its influence on the immune mous amount of knowledge that has been accumulated
immunocytes that is essential system of the whole body, the mucosal lymphoid tissue over the years, we continue to ask the same basic ques-
for the polymerization of
of the gastrointestinal tract is one of the most complex tions. What is the role of intestinal IgA? Where does IgA
immunoglobulin A and IgM,
which is required for binding to tissues of the immune system. Besides other effector class-switching take place? How are the B cells activated,
the polymeric-immunoglobulin functions, this tissue regulates the development of and what interactions regulate IgA class-switching and
receptor and transport through immunoglobulin A immune responses. IgA is the differentiation? Here, we discuss recent studies in mice
epithelia. main element of the humoral immune response that that challenge the view that Peyers patches are the main
has been selected through evolution, together with inductive site for the generation of IgA+ plasma cells,
innate mucosal defences, to provide protection against and we emphasize the importance of cellular interac-
microbial antigens at mucosal surfaces1,2. tions outside Peyers patches, in the gut lamina propria,
At least 80% of all plasma cells are located in the for the induction of mucosal immune responses. We
intestinal lamina propria, and together, they produce focus on the cellular and molecular mechanisms of IgA
more IgA (in humans, 4060 mg kg1 day1) than all predominance in the intestine, and discuss the role of
other immunoglobulin isotypes combined3,4. The IgA is IgA in the homeostasis of gut-associated lymphoid tis-
secreted mainly as dimers or larger polymers (pIgA), sue (GALT).
*Department of Medical
after incorporation of the J CHAIN and association with a
Chemistry, Graduate transmembrane epithelial glycoprotein known as the Precursors and sites for generation of gut IgA
School of Medicine, secretory component or polymeric-immunoglobulin The GALT (FIG. 1), which is the main site of the mucosal
Kyoto University, receptor (pIgR)3,5. immune system, can be divided into two functional
Yoshida, Sakyo-ku, The finding that IgA is the most abundant immuno- compartments, known as inductive and effector sites9.
Kyoto 606-8501, Japan.
RIKEN Research Center for globulin isotype in mucosal secretions6,7 led to several The primary inductive sites include the organized lym-
Allergy and Immunology, important questions regarding the origin of IgA-produc- phoid aggregates that are present in the walls of the
Tsurumi-ku, Yokohama, ing plasma-cell precursors, the sites of antigen-specific small and large intestines. In the small intestine, these
Kanagawa 230-0045, Japan. induction of IgA production and the migration of the inductive sites are represented by Peyers patches, which
Correspondence to T.H.
e-mail: honjo@mfour.med.
precursors to mucosal sites. These questions have been consist of many lymphoid follicles located on the anti-
kyoto-u.ac.jp tackled during the past four decades, giving rise to the mesenteric side of the bowel. The solitary follicles that
doi:10.1038/nri982 concept that Peyers patches are the main site for the are scattered throughout the gut lamina propria
NATURE REVIEWS | IMMUNOLOGY VOLUME 3 | JANUARY 2003 | 6 3
2002 Nature Publishing Group
REVIEWS
Gut lumen
IgA
Bacteria
FAE
M cell
SED
T
B
B Epithelial
DC DC DC
cell
GC SC Plasma B
cell
T
GC GC
Villus T
ILF B CSR/SHM
HEV
Lamina propria Peyer's patch
Figure 1 | Gut-associated lymphoid tissue. Schematic representation of gut-associated lymphoid tissue (GALT), with organized
lymphoid structures Peyers patches and isolated lymphoid follicles (ILFs) and diffuse tissue of the epithelium and the lamina
propria. Peyers patches and ILFs are composed of a specialized follicle-associated epithelium (FAE) containing M cells, a
subepithelial dome (SED) rich in dendritic cells (DCs), and B-cell follicle(s) that contain germinal centres (GCs), where follicular B cells
efficiently undergo class-switch recombination (CSR) and somatic hypermutation (SHM). Migration of B cells into the mucosa
takes place through high endothelial venules (HEVs), located in the interfollicular regions of Peyers patches, which contain mostly
T cells. The diffuse tissues of the lamina propria contain a large number of immunoglobulin A (IgA)+ plasma cells, T and B cells,
macrophages, dendritic cells (DCs) and stromal cells (SCs). Lamina-propria DCs take up antigens from the lumen and present them
directly to T cells and B cells, which can induce IgA class-switching and differentiation in situ. Secreted IgA is transported across the
epithelium, where it serves as a first line of defence against pathogens and for the maintenance of gut-flora homeostasis. IgA+ B cells
and plasma cells are shown in red, IgG+ cells in blue and IgM+ cells in pink.
B2 CELLS
known as isolated lymphoid follicles (ILFs) which could repopulate the intestine and spleen of irradiated
IgMlowIgDhiMac1B220hiCD23+
cells that are produced are architecturally similar to Peyers patches, have been recipients with IgA+ plasma cells much more efficiently
continuously in adult bone assumed for a long time to have similar functions, but than could cells isolated from lymph nodes14,15. These
marrow and secrete antibodies this has been shown only recently1012. These structures original observations were extended further in a syn-
with high affinity and fine contain a large number of B2 CELLS, which are derived geneic cell-transfer system in mice, which showed that
specificity.
from precursor cells generated in the bone marrow11,13. Peyers patch cells can effectively repopulate the gut lam-
CLASS-SWITCH The intestinal lamina propria outside Peyers patches ina propria with IgA+ plasma cells, indicating that
RECOMBINATION and ILFs is considered to be the main GALT effector site Peyers patches are an important site of IgA+ B-cell
(CSR). Alters the that is involved in the final differentiation of IgA+ B cells development16,17. IgA+ B-cell development in Peyers
immunoglobulin heavy-chain
to IgA-producing plasma cells and in the secretion of patches seems to depend on antigenic stimulation and
constant-region (CH) gene that
will be expressed from the C IgA into the gut lumen3. the induction of germinal centres1820. The specialized
region to one of the other CH However, these functional distinctions between germinal-centre microenvironment which allows
genes. This results in a switch of inductive and effector sites are not absolute, because strong interactions between B cells, antigens trapped on
immunoglobulin isotype from certain immune responses can be induced in the follicular dendritic cells and local CD4+ T cells facili-
IgM/IgD to IgG, IgA or IgE,
without altering antigen
mucosal epithelium and in the diffuse tissue of the lam- tates B-cell proliferation, CLASS-SWITCH RECOMBINATION
specificity. ina propria, and some effector functions might occur in (CSR) and SOMATIC HYPERMUTATION (SHM). Indeed, in
the Peyers patches9. For experimental reasons, it is conventionally reared mice, germinal centres are found
SOMATIC HYPERMUTATION important to point out that the cells in these compart- continuously in Peyers patches, and these structures
(SHM). Results in the
ments cannot be separated completely, and the term contain a higher proportion of actively dividing IgA+
accumulation of point
mutations in the variable-region lamina propria or lamina-propria cells is used gener- B cells than germinal centres from the spleen or periph-
genes of immunoglobulin heavy ally to refer to cells that have been isolated from the lam- eral lymph nodes18,21. This led to the proposal that
and light chains. B cells that ina propria outside Peyers patches. Peyers patch germinal centres are intrinsically different
express high-affinity The idea that intestinal IgA+ plasma-cell precursors to other germinal centres, owing to constant antigenic
immunoglobulins on their
surface are selected by limited
reside in the Peyers patches began with the cellular stimulation, as well as to the presence of special regula-
amounts of the antigens, giving studies of Craig and Cebra in a rabbit system. They tory T cells and dendritic cells (DCs) that promote
rise to high-affinity antibodies. showed that adoptively transferred Peyers patch cells preferential class-switching to IgA (reviewed in REF. 22).
64 | JANUARY 2003 | VOLUME 3 www.nature.com/reviews/immunol
2002 Nature Publishing Group
REVIEWS
B1 CELLS The potential role of ILFs in the induction of IgA expressed by blood vessels in the lamina propria form
Self-renewing immune responses was indicated only recently11. ILFs the main receptorligand pair that is required for the
IgMhiIgDlowMac1+B220loCD23 are induced to develop only after birth in parallel with homing of lymphocytes to the lamina propria3133.
cells that are dominant in the
the bacterial colonization of the gut, and the number, Although this interaction is important for mucosal
peritoneal and pleural cavities. B1
cells recognize self-components, size and cellular composition of ILFs are highly variable lymphocyte homing, as shown by the reduced size of
as well as common bacterial and dependent on the bacteria that are present11,12. Such Peyers patches and the decreased number of IgA+ plasma
antigens, and they secrete high developmental plasticity of the ILFs, together with cells in the lamina propria of 7-integrin-knockout
antibodies that tend to have low observations that germ-free mice have few ILFs that do mice34, it cannot explain the preferential homing of IgA+,
affinity and broad specificity.
not contain germinal centres or IgA+ B cells, indicates but not IgM+ or IgG+, plasma cells to the gut lamina pro-
NATURAL IgMS that ILFs might have an active role in the induction of pria. This puzzle led to the search for chemotactic factors
These antibodies normally local immune responses11,23. that are secreted by the intestine that selectively attract
circulate in the blood of non- Another source of B cells that contribute to intestinal the circulating precursors of IgA+ plasma cells. Despite
immunized mice. They are highly
IgA+ plasma cells is the peritoneal cavity. Although it progress in understanding the interactions between
cross-reactive, and bind with low
affinity to both microbial and was suggested that peritoneal B cells do not contribute chemokines and their receptors, and their importance
self-antigens. A large proportion to gut immune responses in humans24, in mice, B1 CELLS for migration across the endothelium and in lymphoid
of natural IgMs is derived from in the peritoneal cavity were found to generate large tissues35,36, it was only recently that a chemotactic factor
peritoneal B1 cells. amounts of intestinal IgA25. Unlike the B1-cell-derived specific for mouse IgA+ B cells was identified thymus-
NATURAL IgMS, which are produced even in germ-free mice, expressed chemokine (TECK; also known as CCL25)37.
the production of intestinal IgA requires the presence of Other than the thymus, CCL25 is produced mainly by
a commensal microflora, which indicates that the pro- the epithelium of the small intestine38,39. Importantly,
duction of intestinal IgA is probably induced in response in vitro studies have shown that IgA+, but not IgM+ or
to antigenic stimulation26. Moreover, unlike the IgA IgG+, plasma cells migrate in response to CCL25,
responses that are generated in the germinal centres of owing to selective expression of the CCL25 receptor
Peyers patches, which require the help of T cells, IgA (CC-chemokine receptor 9, CCR9) on IgA+ plasma-
production by B1 cells seems to be T-cell independent. cell precursors37. So, in mice, CCL25 is probably one of
This was shown in lethally irradiated T-cell receptor the chemokines that are responsible for the selective
-chain and -chain knockout mice, reconstituted with migration of circulating IgA+ B cells to the intestinal
allotype-marked peritoneal B1 cells together with host- lamina propria.
derived bone-marrow cells; most intestinal IgA+ plasma Furthermore, the presence of B cells and plasma cells
cells were derived from peritoneal B1 cells26. in the lamina propria depends crucially on lamina-
Although the relative contribution and repertoire propria stromal cells, as shown recently by studies of
specificity of B1- and B2-cell-derived IgA are not lymphotoxin- (LT)-deficient and LT receptor
known, the T-cell-independent production of intestinal (LTR)-deficient mice40,41. The deficiency of IgA+
IgA by B1 cells might be crucial for preventing systemic plasma cells in LT-deficient mice was thought initially
invasion by intestinal bacteria. This was indicated by the to be due to the absence of Peyers patches and MLNs in
following observations. First, commensal bacteria bind these mice (reviewed in REF. 42). However, when recon-
mostly B1-cell-derived intestinal IgA, and less so B2- stituted with bone marrow from normal mice, LT-
cell-derived intestinal IgA27. Second, normal mice that deficient mice developed normal levels of IgA in their
have intestinal B1-cell-derived IgA specific for commen- intestines, indicating that the presence of organized
sal bacteria do not have serum IgA or IgG with the same lymphoid structures (Peyers patches or MLNs) is not
specificities26. By contrast, mice with IgA deficiency necessary and that the lamina-propria environment
(IgA/ aly/aly mice) have serum IgG that is specific for might be sufficient for the generation of IgA+ B cells40.
commensal bacteria, and this IgG is produced by B2 Also, the absence of B cells in the lamina propria of
cells in a T-cell-dependent manner26. LT-deficient mice was not due to an intrinsic B-cell
defect, because bone-marrow cells or peritoneal-cavity
IgA+ B-cell migration to the lamina propria B cells of LT-deficient mice could migrate to the lamina
From Peyers patches, IgA+ B cells migrate to the drain- propria and differentiate into IgA-producing cells when
ing mesenteric lymph nodes (MLNs), where they prolif- injected into normal mice or recombination-activating
erate further and differentiate into plasmablasts. gene 2 (Rag2)/ mice, which are deficient in T and
Plasmablasts home preferentially to the gut lamina pro- B cells40,43. This is probably because LTR on lamina-
pria through the thoracic duct and blood28,29. The tissue propria stromal cells was activated by LT-expressing
specificity of IgA+ B-cell homing is the result of complex lymphoid or non-lymphoid resident cells in normal or
interactions between receptors that are present on the Rag2/ mice, respectively. Indeed, a functional LTR on
lymphocytes and their ligands expressed on the vascular lamina-propria stromal cells seems to be crucial for the
endothelium of the target tissues. These interactions presence of B cells in the gut lamina propria40,41. This
trigger an ordered sequence of events, which begins conclusion is based on several observations. First, LTR-
with the transient binding of lymphocytes to the deficient mice lack B-cell populations in their lamina
endothelium, followed by transmigration across the propria, which is indicated by the low amount or
vascular wall (reviewed in REF. 30). It is clear that 47 absence of IgA in their intestinal secretions41. This phe-
integrin expressed by lymphocytes and mucosal vascu- notype is similar to that of aly/aly mice44, which have
lar addressin cell adhesion molecule 1 (MADCAM1) impaired signalling through LTR because of a point
NATURE REVIEWS | IMMUNOLOGY VOLUME 3 | JANUARY 2003 | 6 5
2002 Nature Publishing Group
REVIEWS
a b
Aid +/
IgM
IgA
Aid /
IgM
B220 IgA B220
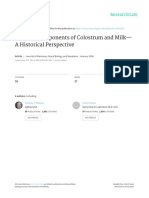
Figure 2 | Lamina-propria lymphoid cells in normal and AID-deficient mice. In the presence of activation-induced cytidine
deaminase (AID) and in the special microenvironment of the lamina propria, B220+IgM+ B cells undergo class-switch recombination
and differentiation to IgA+ plasma cells, as indicated by the presence of B220+IgA+ B cells and B220IgA+ plasma cells. AID
deficiency leads to a massive accumulation of B220+IgM+ B cells and B220IgM+ plasma cells. a | Cells isolated from the lamina
propria of Aid+/ and Aid/ mice were stained for B220 and IgM or IgA, and analysed by fluorescence-activated cell sorting (FACS).
b | The photographs show a three-colour histological comparison of wild-type (top) and AID-deficient (bottom) mouse small intestine
(IgA, green; IgM, red; nuclei, blue).
mutation in the downstream signalling molecule The migration of B1 cells out of the peritoneal cavity
nuclear factor-B-inducing kinase (Nik)45. Second, was proposed to depend on Nik, because transfer of
when injected into LTR-deficient mice, bone-marrow peritoneal cells from aly/aly mice into the peritoneal
cells from normal mice failed to restore the number of cavity of Rag2 / mice failed to generate GALT IgA+
B cells and plasma cells in the lamina propria of the plasma cells43.
recipient mice40. Third, when a segment of Rag2 /
mouse intestine (with intact LTR signalling on lamina- IgA switching in the lamina propria
propria stromal cells) was transplanted next to the As mice that lack or have poorly developed Peyers
intestine of recipient LT-deficient mice (in which patches contain a large number of IgA+ plasma cells in
LTLTR interactions were completely absent), IgA+ their lamina propria, it seems that IgA+ B cells can be
plasma cells were detected only in the Rag2 / segment, generated in lymphoid tissues other than Peyers
but not in the host intestine40. Fourth, the administra- patches48,49. The notion that Peyers patches are not the
tion of LTR antagonists after birth led to a marked only location for the induction of mucosal IgA responses
decrease in the number of lamina-propria B cells and was strengthened by studies on the progeny of mice
plasma cells41. Therefore, signalling through LTR on treated during gestation with LTRimmunoglobulin
lamina-propria stromal cells is absolutely necessary for fusion protein, which is known to block the development
the presence of IgM+ B cells and IgA+ plasma cells in the of Peyers patches50,51. Because these mice, but not
small intestine. Although a decrease in the local concen- tumour-necrosis factor (TNF) and LT double-knockout
tration of adhesion molecules and chemokines might mice, which lack Peyers patches and MLNs, have antigen-
be one explanation for the absence of lamina-propria specific IgA in their lamina propria, it was proposed that
B cells as a result of impaired LTR signalling40,43,46, the MLNs have an important role in the induction of IgA
molecular mechanisms by which LTLTR interac- mucosal immune responses51.Although it is clear that this
tions selectively affect B-cell homing to the gut lamina pathway contributes to the generation of IgA+ plasma-cell
propria are unresolved. precursors derived from both B1 and B2 cells27, it is not
What factors are required for the migration of B1 cells absolutely required, as shown by LT-deficient mice,
OMENTUM to the gut lamina propria? Although less so than B2 cells, which lack both Peyers patches and MLNs but develop
A bilayered sheet of mesothelial peritoneal B1 cells do recirculate actively through the normal IgA+ plasma cells in the lamina propria after
cells connecting the spleen, bloodstream47. Homing of B1 cells to the peritoneal cavity injection with normal lymphocytes40.
pancreas, stomach and
transverse colon, terminating in
was found to depend mainly on CXCL13 (B-lymphocyte Direct evidence for class-switching to IgA-producing
an apron-like structure that chemoattractant, BLC), a chemokine that is produced by B cells outside Peyers patches and MLNs was obtained
contains adipocytes. cells in the OMENTUM and by peritoneal macrophages47. by studies of activation-induced cytidine deaminase
66 | JANUARY 2003 | VOLUME 3 www.nature.com/reviews/immunol
2002 Nature Publishing Group
REVIEWS
Box 1 | Events and markers in class-switch recombination
One genetic alteration that amplifies the diversity of the immune response is class-switch recombination (CSR). This
complex process takes place in activated B cells, and it changes the immunoglobulin heavy-chain constant-region
(CH) gene that will be expressed from the C region to one of the other CH genes. The result is a switch of the
immunoglobulin isotype from IgM/IgD to IgG, IgA or IgE, with similar antigenic specificity but with different biological
properties. CSR takes place between two regions comprising repetitive sequences of palindrome-rich motifs, known as
S regions, and it results in a looped-out deletion of the intervening DNA segments. CSR is preceded by the expression
of germline transcripts initiated from intronic promoters (I), which are located 5 to the S regions and are regulated
specifically by various cytokines. The close association between isotype specificity of germline transcription and the
recombination targeting of S regions by stimulation with a certain cytokine has led to the accessibility model that
germline transcription opens the chromatin structure of a specific S region and renders it accessible to the putative
recombinase. Activation-induced cytidine deaminase (AID), expression of which is induced specifically in activated
B cells, seems to be the only B-cell-specific marker that is essential for CSR100, and it is involved most probably in
recognition and cleavage of the target DNA by the switch recombinase. CSR is accompanied by looping-out deletion of
the DNA fragment containing C and other CH genes from the chromosome, followed by repair and ligation of the
broken DNA ends by the ubiquitously expressed non-homologous end-joining (NHEJ) repair system. The resultant
circular DNA contains an I promoter that is still responsive to cytokines and that directs the production of IC
transcripts known as circle transcripts. These circle transcripts are dependent on AID, and they disappear more quickly
than germline transcripts, AID or circular DNAs after removal of class-switch stimulation, making them the best
available marker for active CSR. D, diversity; J, joining; TGF-, transforming growth factor-; V, variable.
I C C I3 C3 I1 C1 I C
VDJ
5 S S S S 3
IgA-specific cytokine (TGF-)
Accessible chromatin
AID
-germline transcripts
Intermediate Looped-out circular DNA
Chromosomal product
VDJ I C
C I
Post-switched transcripts (IgA) -circle transcripts
(AID)-deficient mice52. AID, a potential RNA-editing in the lamina propria of AID-deficient mice, together
enzyme, is expressed specifically by germinal-centre with the presence of IgM+ B cells and IgA+ B cells in the
B cells53, and AID deficiency in humans and mice causes lamina propria of normal mice, indicates that IgA+
a complete block of CSR and SHM52,54. Interestingly, B cells might be generated in the ILFs, or the lamina
AID-deficient mice accumulate a large number of IgM+ propria outside the ILFs, from IgM+ B cells.
B cells and IgM+ plasma cells in their lamina propria55 If this is the case, then ongoing CSR should be
(FIG. 2). In addition, the lamina propria of normal mice detectable at these sites. However, until recently, the
contains IgM+ B cells and a small number of IgA+ detection of active, ongoing CSR was not easy, as no
B cells, as well as IgA+ plasma cells55,56 (FIG. 2). These two molecular marker was known that appears during
lamina-propria B-cell populations are found either in CSR and disappears rapidly after the class-switching
ILFs or scattered among IgA+ plasma cells in the villi. event. This problem has been overcome recently by the
The accumulation of IgM+ B cells and IgM+ plasma cells finding that the I promoter, located on the circular
NATURE REVIEWS | IMMUNOLOGY VOLUME 3 | JANUARY 2003 | 6 7
2002 Nature Publishing Group
REVIEWS
M CELLS DNA that is released during CSR, is still active and IgM+ B cells generate IgA+ B cells, which, under the
(Microfold cells). Specialized directs the production of IC transcripts, known as influence of factors that are produced by lamina-propria
epithelial cells that deliver circle transcripts57 (BOX 1). Kinetic analyses in stimulated stromal cells, differentiate to IgA+ plasma cells. More-
antigens by transepithelial
spleen B cells or in a B-lymphoma cell line58 showed that over, both in vitro culture experiments and in vivo
vesicular transport from the gut
lumen directly to intraepithelial circle transcripts appear shortly before or simultaneously transfer studies of IgA-depleted cells into Rag2 / mice
lymphocytes and to with surface immunoglobulin expression and disappear have shown that lamina-propria IgM+ B cells generate
subepithelial lymphoid tissues. more rapidly than other markers of CSR, such as the IgA+ B cells and plasma cells more quickly and more
expression of AID, germline transcripts59 or circular efficiently than do their Peyers patch counterparts55.
DNA6062, within one day of the class-switching event55,57.
Importantly, IgA+ B cells isolated from the lamina pro- Antigen recognition and activation in the gut
pria express not only -germline transcripts and AID, How are lamina-propria B cells activated for in situ IgA
but also -circle transcripts. This molecular profile pro- class-switching, and how do the immune cells at this site
vides direct evidence that lamina-propria IgA+ B cells sense the intestinal microflora?
are generated in situ from IgM+ B cells. Lamina-propria The model of Peyers patches as the main, or only,
IgM+ B cells seem to be committed to class-switching to site in the intestine that can, through M CELLS, bind,
IgA, because they express -germline transcripts and translocate and present bacterial antigens to B cells
AID. Indeed, when stimulated in vitro, lamina-propria (reviewed in REF. 63) has been challenged recently by two
a T-cell dependent
APC
TCR MHC Lamina propria
CD4+ AID
B7 T cell
CD28
CD40L CD40
IgA
IgM c
B Antigen
AID
TGF-
IgM
TACI,
BCMA or
SC SHM
BAFFR AID Selection
BAFF
From the bone marrow
or peritoneal cavity
APRIL
DC IL-10, IL-6
IgA+
PC d Differentiation
b T-cell independent
Bacteria
Gut lumen
Figure 3 | Possible pathways for the induction of IgA responses in the gut lamina propria. From the bone marrow or
peritoneal cavity, immunoglobulin M (IgM)+ B cells home to the gut lamina propria, where they are activated by antigens and CD40
ligand (CD40L) expressing T cells (a) or, alternatively, by antigens presented by lamina-propria dendritic cells (DCs) or by polyclonal
stimuli (b). In the presence of cytokines secreted by T cells (a), or soluble and membrane-bound BAFF and/or APRIL produced by
antigen-presenting cells (APCs) (b), activated B cells upregulate expression of activation-induced cytidine deaminase (AID), which is
an absolute requirement for class-switch recombination and somatic hypermutation (SHM). The factors that are secreted by lamina-
propria stromal cells (SCs), such as interleukin-6 (IL-6), IL-10 and transforming growth factor- (TGF-), favour not only preferential
class-switching to IgA (c), but also the differentiation of antigen-selected cells to IgA+ plasma cells (PCs) (d). APRIL, a proliferation-
inducing ligand; BAFF, B-cell-activating factor of the tumour-necrosis factor family; BCMA, B-cell maturation antigen; TACI,
transmembrane activator and CAML interactor; TCR, T-cell receptor.
68 | JANUARY 2003 | VOLUME 3 www.nature.com/reviews/immunol
2002 Nature Publishing Group
REVIEWS
COMMON VARIABLE findings. First, the presence of M cells is not restricted to might class-switch preferentially to IgA under the influ-
IMMUNODEFICIENCY the follicular-associated epithelium (FAE) of the Peyers ence of cytokines that are secreted by lamina-propria
SYNDROME patches, because the epithelium that covers the ILFs also stromal cells, then undergo terminal differentiation to
(CVID). The most common
contains M cells10,11,64. Second, DCs located in the gut IgA+ plasma cells (FIG. 3). Taken together, these observa-
symptomatic primary antibody
deficiency, characterized by epithelium65 or the lamina propria can sample intestinal tions indicate that the lamina propria (inside or outside
decreased levels of serum antigens directly66. ILFs) might be a site where T-cell-independent IgA
immunoglobulin. Most patients The role of DCs in surveillance of gut bacteria seems responses are generated77.
suffer from recurrent infections, to require that the integrity of the epithelium remains
predominantly of the respiratory
and gastrointestinal tracts. The
intact. To achieve this, lamina-propria DCs express pro- Biological relevance of IgA for gut homeostasis
incidence of malignancies, teins such as occludin, claudin 1 and zona occludens 1, What has led to the evolution of such a sophisticated
such as gastric carcinoma or which are required to open and close the tight junctions system to generate large amounts of IgA in the intes-
lymphoma, is increased in between epithelial cells67, and they project dendrites into tine? In other words, what is the physiological impor-
patients with CVID.
the lumen and sample the gut antigens66. It has been tance of IgA secretion in the gut?
INTUSSUSCEPTION proposed that lamina-propria DCs that interact with Selective IgA deficiency is the most common
The telescoping or prolapse of pathogenic bacteria might migrate out of the lamina humoral immunodeficiency in humans, occurring at a
one portion of the intestine into propria, whereas those that encounter commensals frequency of about 1 in 5002,000 (REF. 78). Furthermore,
an immediately adjacent might remain in situ, where they can probably present some patients with COMMON VARIABLE IMMUNODEFICIENCY
segment.
antigens and activate T cells and B cells located in the SYNDROME (CVID), who have low levels of IgA and IgG, as
lamina propria66. However, this has not been proven yet. well as a marked reduction in SHM, suffer from fre-
Lamina-propria T cells have an activated phenotype, quent gastrointestinal infections and develop a lympho-
as indicated by the high percentage of cells expressing the proliferative disorder of the small intestine known as
interleukin-2 receptor (IL-2R) and MHC class II mole-
cules, and by the increased production of cytokines
(such as IL-2, IL-4 and IL-5) that are involved in the gen- Box 2 | Nodular lymphoid hyperplasia
eration of IgA responses68,69. However, even in the
Nodular lymphoid hyperplasia is a rare
absence of T cells, it is possible that local B cells are acti-
lymphoproliferative disorder that is associated with
vated by antigen presentation by lamina-propria DCs or
common variable immunodeficiency syndrome (CVID),
by polyclonal stimulation by microbes captured by DCs
intestinal lymphoma and Gardner syndrome. But, it can
(FIG. 3). Activated B cells might then class-switch and dif-
be found also in the small intestine of adults without
ferentiate to IgA+ plasma cells under the influence of fac- immunodeficiency. The hyperplastic lymphoid follicles
tors secreted by lamina-propria stromal cells. In support are found most often in the small intestine as numerous
of this proposal, interactions between lipopolysaccharide small protrusions of 36 mm in diameter, and they are
(LPS)-stimulated B cells and lamina-propria stromal morphologically identical to the isolated lymphoid
cells greatly enhanced in vitro class-switching to IgA and follicles (ILFs) that are present normally in the
differentiation to IgA+ plasma cells, independent of T cells gastrointestinal tract. The symptoms are caused usually
or the CD40 signalling pathway55. IgA class-switching, by underlying conditions, such as gastrointestinal
which was induced to an equal extent in lamina-propria, infections or malabsorption, and only rarely by large
Peyers patch and spleen B cells, was induced mainly by follicles that can cause INTUSSUSCEPTION or bleeding.
transforming growth factor- (TGF-)7073 secreted by Although nodular follicular hyperplasia in itself does not
lamina-propria stromal cells, whereas stromal-cell- require therapy, it is important to differentiate it from
derived IL-6 (REFS 69,74) and IL-10 (REF. 75) might be other polyposis syndromes with which it is often
involved in the final differentiation to plasma cells. confused, to define the aetiology and eventually to treat
Moreover, activated lamina-propria DCs might be suf- the associated diseases (reviewed in REF. 81). The figure
ficient to induce the class-switching of B cells to IgA shows an endoscopic image of a duodenal segment
through a pathway that involves the engagement of with hyperplasia of ILFs in an adult patient (courtesy
BAFF (B-cell-activating factor of the tumour-necrosis of K. Suzuki, Department of Gastroenterology, Kyoto
University Hospital, Japan).
factor family) receptors76. After stimulation with LPS,
interferon- (IFN-) and IFN-, human DCs were
found to upregulate expression of the TNF-family
molecules BAFF and a proliferation-inducing ligand
(APRIL), which engage the receptors transmembrane
activator and CAML interactor (TACI), B-cell matura-
tion antigen (BCMA) and BAFF receptor (BAFFR) on
B cells76. In a TGF--sufficient environment, by as-yet-
unknown mechanisms, these interactions can induce
class-switching to IgA, by enhancing the accessibility
of the IgA locus and by the upregulation of expression
of AID76.
So, lamina-propria B cells that are activated either in
a BAFF-independent manner by polyclonal stimulation
or after antigen presentation by BAFF-expressing DCs
NATURE REVIEWS | IMMUNOLOGY VOLUME 3 | JANUARY 2003 | 6 9
2002 Nature Publishing Group
REVIEWS
a b bacteria abolished not only the ILF hyperplasia, but also
the induction of germinal-centre formation in all lym-
phoid tissues, continuous antigenic stimulation by an
excessive population of intestinal anaerobic bacteria is
probably responsible for both local and systemic B-cell
activation, as well as ILF hyperplasia and germinal-
centre formation, in AID-deficient mice12,52. So, it seems
that the IgA that is secreted into the gut lumen functions
not only to protect against viral and bacterial pathogens,
but also for homeostasis of the gut flora, which is essen-
tial to prevent over-stimulation of the non-mucosal
immune system.
Hypermutation and antigenic selection
Besides CSR, another AID-dependent process that
occurs mostly in germinal centres and induces further
diversification of the immunoglobulin repertoire of
activated B cells is SHM8690. Multiple rounds of muta-
tion of variable (V), diversity (D) and joining (J) exons,
followed by the selection of B cells with enhanced bind-
ing to antigens, result in so-called affinity maturation of
the humoral response. SHM is likely to be more frequent
Figure 4 | AID deficiency leads to hyperplasia of isolated lymphoid follicles in the gut
at mucosal sites87, where constant antigenic pressure
lamina propria. a | A duodenal segment of the small intestine from an AID-deficient mouse
showing many protruding follicles. b | These follicles consist of IgM+ B cells on a follicular dendritic
from a diverse bacterial flora91 requires a vast repertoire
cell (FDC) network (IgM, red; FDC, green). of antibody specificities.
Indeed, IgA+ and IgM+ plasma cells isolated from
human small intestine9294 or mouse small intestine95
nodular follicular hyperplasia7981 (BOX 2). The mecha- (S.F. and T.H., unpublished observations) are heavily
nisms for the development of follicular hyperplasia in mutated in their heavy-chain variable-region (VH)
humans remain unknown, although it has been pro- genes, and the frequency of mutation is more than two-
posed to occur as a result of local immune responses to fold greater than that of splenic IgA+ or IgM+ plasma
gut antigens7981. cells. SHM of intestinal B cells and plasma cells might
Although the importance of IgA-mediated protec- be important for homeostasis of the mucosal immune
tion at mucosal surfaces is not questioned82, the role of system, because IgA-deficient mice, which have
intestinal IgA in regulation of the gut microflora and mutated IgMs in their intestinal secretions, do not have
how dysregulation of the gut bacteria might affect the the ILF hyperplasia that is observed in AID-deficient
GALT system leading to pathological manifestations are mice (D. Metzger, personal communication).
controversial issues. This is due partly to the heterogen- That antigenic pressure leads to selection of gut
eity of the clinical manifestations in IgA-deficient B cells was indicated by repertoire studies of non-
patients and the existence of compensatory mechanisms mutated B cells isolated from the ILFs of AID-deficient
such as overproduction of IgM or IgG that make mice12. Not only was the repertoire of ILF B cells more
it difficult to assess the biological role of IgA. This is also diverse than that of B cells from Peyers patches or spleen,
a problem in animal models that have been generated to but also individual ILFs contained different dominant VH
investigate the physiological role of IgA such as IgA-, gene and VHDJH combinatorial diversity, indicating
J-chain- or pIgR-deficient mice. Under normal condi- that selection and clonal expansion of B cells probably
tions, these mice seem to be healthy, although they have take place in situ, depending on the prevailing antigenic
a greater number of activated B cells in Peyers patches diversity of local bacteria12.
(IgA-deficient mice)83, impaired intestinal anti-toxin
protection (J-chain-deficient mice)84 or elevated levels A model for the development of IgA responses
of serum IgG that reacts with intestinal bacteria (pIgR- Taken together, the available evidence indicates that the
deficient mice)85 compared with wild-type mice. simplest model of IgA+ plasma-cell generation in the gut
An animal model that seems to recapitulate the lamina propria would be: recruitment of IgM+ B cells;
pathology of CVID in humans is AID deficiency in mice. followed by activation and proliferation of IgM+ B cells,
AID-deficient mice develop many protruding follicular in either a T-cell-dependent or -independent manner,
structures along the small intestine, which indicate through interactions with lamina-propria DCs and
MT/ MICE hypertrophia of ILFs12 (FIG. 4). This resembles the nodu- lamina-propria stromal cells; followed by class-switching
These mice carry a stop codon in lar follicular hyperplasia that is seen in humans with to IgA, then SHM and selection of IgA+ B cells by anti-
the first membrane exon of the CVID (BOX 2). Furthermore, AID-deficient mice have a gens captured by DCs; and finally, differentiation to IgA+
-chain constant region. They
lack IgM+ B cells, and B-cell
large increase in the number of non-pathogenic, but plasma cells (FIG. 3).
development is arrested at the anaerobic, bacteria in all segments of the small intestine12. This model would help to explain an apparently
pre-B-cell stage. As appropriate antibiotic treatment of the anaerobic unexpected finding that MT MICE, which have
/
70 | JANUARY 2003 | VOLUME 3 www.nature.com/reviews/immunol
2002 Nature Publishing Group
REVIEWS
a developmental block at the pro-B-cell stage96, do have Concluding remarks
intestinal IgA95. This IgA seems to be induced in Although recent findings have indicated new complexi-
response to the intestinal flora, or after experimental ties of the regulation and biological importance of IgA+
intestinal bacterial infection, even in the absence of sur- B-cell differentiation in the gut, more studies are
face IgM and T cells. The IgA repertoire in these mice required to understand the role of non-follicularly
was found to be diverse, with point mutations in the organized lymphoid elements in the gut lamina propria
complementarity-determining regions, which strongly in the induction of mucosal immune responses.
indicates the involvement of antigenic selection95. One Further analyses of the interactions between B cells,
additional assumption required to explain the above T cells, DCs, macrophages and stromal cells after anti-
observation is that the BCR-null B cells of MT/ mice gen presentation in the gut lamina propria are required
might be recruited to the lamina propria and rescued to solve outstanding questions regarding the molecular
from apoptosis in a T-cell-independent manner76,97. If mechanisms that are responsible for the induction of
so, they might proliferate and class-switch to IgA in immunity or tolerance in the intestinal mucosa.
response to soluble factors and cellular interactions Furthermore, a more comprehensive understanding
in the lamina propria. Once surface IgA is expressed, of the relationships between commensal bacteria98,99 and
antigenic stimulation might induce SHM, followed by the innate and adaptive immune systems should offer
positive selection and further differentiation to IgA+ new approaches for the therapy of gut inflammatory
plasma cells. pathology and for the design of oral vaccinations.
1. Mestecky, J., Moro, I. & Underdown, B. J. in Mucosal 18. Butcher, E. C. et al. Surface phenotype of Peyers patch 32. Briskin, M. J., McEvoy, L. M. & Butcher, E. C. MAdCAM-1
Immunology (eds Ogra, P. et al.) 133152 (Academic Press, germinal center cells: implications for the role of germinal has homology to immunoglobulin and mucin-like adhesion
San Diego, 1999). centers in B-cell differentiation. J. Immunol. 129, 26982707 receptors and to IgA1. Nature 363, 461464 (1993).
2. Matsunaga, T. & Rahman, A. What brought the adaptive (1982). 33. Berlin, C. et al. 47 integrin mediates lymphocyte binding to
immune system to vertebrates? The jaw hypothesis and the 19. Weinstein, P. D. & Cebra, J. J. The preference for switching the mucosal vascular addressin MAdCAM-1. Cell 74, 185
seahorse. Immunol. Rev. 166, 177186 (1998). to IgA expression by Peyers patch germinal-center B cells is (1993).
3. Brandtzaeg, P. et al. Regional specialization in the mucosal likely due to the intrinsic influence of their microenvironment. 34. Wagner, N. et al. Critical role for 7 integrins in formation of
immune system: what happens in the microcompartments? J. Immunol. 147, 41264135 (1991). the gut-associated lymphoid tissue. Nature 382, 366370
Immunol. Today 20, 141151 (1999). 20. Cebra, J. J. Influences of microbiota on intestinal immune (1996).
4. van Egmond, M. et al. IgA and the IgA Fc receptor. Trends system development. Am. J. Clin. Nutr. 69, 1046S1051S 35. Cyster, J. G. Chemokines and cell migration in secondary
Immunol. 22, 205211 (2001). (1999). lymphoid organs. Science 286, 20982102 (1999).
5. Mostov, K. E. Transepithelial transport of immunoglobulins. 21. Lebman, D. A., Griffin, P. M. & Cebra, J. J. Relationship 36. Okada, T. et al. Chemokine requirements for B-cell entry to
Annu. Rev. Immunol. 12, 6384 (1994). between expression of IgA by Peyers patch cells and lymph nodes and Peyers patches. J. Exp. Med. 196, 6575
6. Hanson, L. A. Comparative immunological studies of the functional IgA memory cells. J. Exp. Med. 166, 14051418 (2002).
immunoglobulins of human milk and of blood serum. Int. (1987). 37. Bowman, E. P. et al. The intestinal chemokine thymus-
Arch. Allergy Appl. Immunol. 18, 241253 (1961). 22. Lycke, N. in Mucosal T Cells (ed. MacDonald, T.) 209234 expressed chemokine (CCL25) attracts IgA antibody-
7. Tomasi, T. B., Tan, E. M., Solomon, A. & Predergast, R. A. (Karger, Basel, 1998). secreting cells. J. Exp. Med. 195, 269275 (2002).
Characteristics of an immune system common to certain 23. Crabbe, P. A., Nash, D. R., Bazin, H., Eyssen, H. & Butcher and colleagues provide evidence that TECK
external secretions. J. Exp. Med. 121, 101142 (1965). Heremans, J. F. Immunohistochemical observations on (CCL25) is an IgA+ B-cell chemotactic factor, which
8. Cebra, J. J. & Shroff, K. E. in Handbook of Mucosal lymphoid tissues from conventional and germ-free mice. attracts IgA-committed B cells from the spleen,
Immunology (eds Ogra, P. et al.) 151157 (Academic Press, Lab. Invest. 22, 448457 (1970). Peyers patches and mesenteric lymph nodes to the
San Diego, 1994). 24. Boursier, L., Farstad, I. N., Mellembakken, J. R., lamina propria.
9. Kelsall, B. & Strober, W. in Mucosal Immunology (eds Ogra, P. Brandtzaeg, P. & Spencer, J. IgVH gene analysis suggests 38. Kunkel, E. J. et al. Lymphocyte CC-chemokine receptor 9
et al.) 293317 (Academic Press, San Diego, 1999). that peritoneal B cells do not contribute to the gut immune and epithelial thymus-expressed chemokine (TECK)
10. Moghaddami, M., Cummins, A. & Mayrhofer, G. system in man. Eur. J. Immunol. 32, 24272436 (2002). expression distinguish the small intestinal immune
Lymphocyte-filled villi: comparison with other lymphoid 25. Kroese, F. G. et al. Many of the IgA-producing plasma cells compartment: epithelial expression of tissue-specific
aggregations in the mucosa of the human small intestine. in murine gut are derived from self-replenishing precursors in chemokines as an organizing principle in regional immunity.
Gastroenterology 115, 14141425 (1998). the peritoneal cavity. Int. Immunol. 1, 7584 (1989). J. Exp. Med. 192, 761768 (2000).
11. Hamada, H. et al. Identification of multiple isolated lymphoid This study provides the first evidence that many IgA- 39. Papadakis, K. A. et al. The role of thymus-expressed
follicles on the antimesenteric wall of the mouse small producing plasma cells in the lamina propria of the chemokine and its receptor CCR9 on lymphocytes in the
intestine. J. Immunol. 168, 5764 (2002). mouse small intestine are derived from peritoneal B1 regional specialization of the mucosal immune system.
References 10 and 11 give detailed morphological cells. J. Immunol. 165, 50695076 (2000).
descriptions of isolated lymphoid follicles in human 26. Macpherson, A. J. et al. A primitive T-cell-independent 40. Kang, H. S. et al. Signaling via LTR on the lamina propria
and mouse small intestine, respectively. mechanism of intestinal mucosal IgA responses to stromal cells of the gut is required for IgA production. Nature
12. Fagarasan, S. et al. Critical roles of activation-induced commensal bacteria. Science 288, 22222226 (2000). Immunol. 3, 576582 (2002).
cytidine deaminase (AID) in the homeostasis of gut flora. The first demonstration that anti-commensal IgA 41. Newberry, R. D., McDonough, J. S., McDonald, K. G. &
Science 298, 14241427 (2002). antibodies derived from B1 cells are not natural Lorenz, R. G. Postgestational lymphotoxin/lymphotoxin-
This paper implicates class-switch recombination and antibodies, but are induced specifically in response to receptor interactions are essential for the presence of
somatic hypermutation of gut B cells as being antigenic stimulation; this study also indicates that intestinal B lymphocytes. J. Immunol. 168, 49884997
essential for the maintenance of bacterial this pathway is independent of T cells or follicular (2002).
homeostasis in the gut. lymphoid-tissue organization. References 40 and 41 were the first to show that
13. McIntyre, T. & Strobel, W. in Mucosal Immunology (eds 27. Bos, N. A. et al. Monoclonal immunoglobulin A derived from lymphotoxin- receptor signalling on lamina-propria
Ogra, P. et al.) 319356 (Academic Press, San Diego, 1999). peritoneal B cells is encoded by both germ line and stromal cells is required for the presence of B cells in
14. Craig, S. W. & Cebra, J. J. Peyers patches: an enriched somatically mutated VH genes and is reactive with the lamina propria and for IgA production; these
source of precursors for IgA-producing immunocytes in the commensal bacteria. Infect. Immun. 64, 616623 (1996). studies emphasize the sufficiency of the lamina-
rabbit. J. Exp. Med. 134, 188200 (1971). 28. Guy-Grand, D., Griscelli, C. & Vassalli, P. The gut-associated propria environment for the generation of IgA+ plasma
15. Craig, S. W. & Cebra, J. J. Rabbit Peyers patches, lymphoid system: nature and properties of the large dividing cells.
appendix, and popliteal lymph node B lymphocytes: a cells. Eur. J. Immunol. 4, 435443 (1974). 42. Chaplin, D. D. & Fu, Y. Cytokine regulation of secondary
comparative analysis of their membrane immunoglobulin 29. McWilliams, M., Phillips-Quagliata, J. M. & Lamm, M. E. lymphoid organ development. Curr. Opin. Immunol. 10,
components and plasma-cell precursor potential. Mesenteric lymph node B lymphoblasts which home to the 289297 (1998).
J. Immunol. 114, 492502 (1975). small intestine are precommitted to IgA synthesis. J. Exp. 43. Fagarasan, S. et al. Alymphoplasia (aly)-type nuclear factor-
16. Tseng, J. Transfer of lymphocytes of Peyers patches Med. 145, 866875 (1977). B-inducing kinase (NIK) causes defects in secondary
between immunoglobulin allotype congenic mice: 30. Springer, T. A. Traffic signals for lymphocyte recirculation and lymphoid tissue chemokine receptor signaling and homing
repopulation of the IgA plasma cells in the gut lamina leukocyte emigration: the multistep paradigm. Cell 76, of peritoneal cells to the gut-associated lymphatic tissue
propria. J. Immunol. 127, 20392043 (1981). 301314 (1994). system. J. Exp. Med. 191, 14771486 (2000).
17. Tseng, J. A population of resting IgMIgD double-bearing 31. Holzmann, B., McIntyre, B. W. & Weissman, I. L. 44. Miyawaki, S. et al. A new mutation, aly, that induces a
lymphocytes in Peyers patches: the major precursor cells Identification of a murine Peyers patch-specific lymphocyte generalized lack of lymph nodes accompanied by
for IgA plasma cells in the gut lamina propria. J. Immunol. homing receptor as an integrin molecule with an -chain immunodeficiency in mice. Eur. J. Immunol. 24, 429434
132, 27302735 (1984). homologous to human VLA-4. Cell 56, 3746 (1989). (1994).
NATURE REVIEWS | IMMUNOLOGY VOLUME 3 | JANUARY 2003 | 7 1
2002 Nature Publishing Group
REVIEWS
45. Shinkura, R. et al. Alymphoplasia is caused by a point 63. Kraehenbuhl, J. P. & Neutra, M. R. Epithelial M cells: 87. Gonzalez-Fernandez, A. & Milstein, C. Analysis of somatic
mutation in the mouse gene encoding NF-B-inducing differentiation and function. Annu. Rev. Cell. Dev. Biol. 16, hypermutation in mouse Peyers patches using
kinase. Nature Genet. 22, 7477 (1999). 301332 (2000). immunoglobulin light-chain transgenes. Proc. Natl Acad.
46. Koike, R. et al. Analysis of expression of lymphocyte 64. Rosner, A. J. & Keren, D. F. Demonstration of M cells in the Sci. USA 90, 98629866 (1993).
homing-related adhesion molecules in ALY mice deficient in specialized follicle-associated epithelium overlying isolated 88. Nagaoka, H., Muramatsu, M., Yamamura, N., Kinoshita, K.
lymph nodes and Peyers patches. Cell. Immunol. 180, lymphoid follicles in the gut. J. Leukocyte Biol. 35, 397404 & Honjo, T. Activation-induced deaminase (AID)-directed
6269 (1997). (1984). hypermutation in the immunoglobulin S region: implication
47. Ansel, K. M., Harris, R. B. & Cyster, J. G. CXCL13 is 65. Maric, I., Holt, P. G., Perdue, M. H. & Bienenstock, J. Class II of AID involvement in a common step of class-switch
required for B1-cell homing, natural antibody production and MHC antigen (Ia)-bearing dendritic cells in the epithelium of recombination and somatic hypermutation. J. Exp. Med.
body-cavity immunity. Immunity 16, 6776 (2002). the rat intestine. J. Immunol. 156, 14081414 (1996). 195, 529534 (2002).
48. Neumann, B., Luz, A., Pfeffer, K. & Holzmann, B. Defective 66. Rescigno, M. et al. Dendritic cells express tight junction 89. Kinoshita, K. & Honjo, T. Linking class-switch recombination
Peyers patch organogenesis in mice lacking the 55-kD proteins and penetrate gut epithelial monolayers to sample with somatic hypermutation. Nature Rev. Mol. Cell. Biol.
receptor for tumor-necrosis factor. J. Exp. Med. 184, bacteria. Nature Immunol. 2, 361367 (2001). 2, 493503 (2001).
259264 (1996). This paper describes a new mechanism by which 90. Honjo, T., Kinoshita, K. & Muramatsu, M. Molecular
49. Vajdy, M., Kosco-Vilbois, M. H., Kopf, M., Kohler, G. & lamina-propria dendritic cells sample bacteria directly mechanism of class-switch recombination: linkage with
Lycke, N. Impaired mucosal immune responses in from the gut lumen. somatic hypermutation. Annu. Rev. Immunol. 20, 165196
interleukin-4-targeted mice. J. Exp. Med. 181, 4153 67. Farquhar, M. G. & Palade, G. E. Junctional complexes in (2002).
(1995). various epithelia. J. Cell Biol. 17, 375412 (1963). 91. Umesaki, Y. & Setoyama, H. Structure of the intestinal flora
50. Rennert, P. D., James, D., Mackay, F., Browning, J. L. & 68. Harriman, G. R., Kunimoto, D. Y., Elliott, J. F., Paetkau, V. responsible for development of the gut immune system in a
Hochman, P. S. Lymph node genesis is induced by signaling & Strober, W. The role of IL-5 in IgA B-cell differentiation. rodent model. Microbes Infect. 2, 13431351 (2000).
through the lymphotoxin- receptor. Immunity 9, 7179 J. Immunol. 140, 30333039 (1988). 92. Fischer, M. & Kuppers, R. Human IgA- and IgM-secreting
(1998). 69. Beagley, K. W. et al. Interleukins and IgA synthesis. Human intestinal plasma cells carry heavily mutated VH region
51. Yamamoto, M. et al. Alternate mucosal immune system: and murine interleukin-6 induce high-rate IgA secretion in genes. Eur. J. Immunol. 28, 29712977 (1998).
organized Peyers patches are not required for IgA IgA-committed B cells. J. Exp. Med. 169, 21332148 (1989). 93. Boursier, L., Dunn-Walters, D. K. & Spencer, J. Characteristics
responses in the gastrointestinal tract. J. Immunol. 164, 70. Coffman, R. L., Lebman, D. A. & Shrader, B. Transforming of IgVH genes used by human intestinal plasma cells from
51845191 (2000). growth factor- specifically enhances IgA production by childhood. Immunology 97, 558564 (1999).
52. Muramatsu, M. et al. Class-switch recombination and lipopolysaccharide-stimulated murine B lymphocytes. 94. Boursier, L., Dunn-Walters, D. K. & Spencer, J. Sequence
hypermutation require activation-induced cytidine J. Exp. Med. 170, 10391044 (1989). analysis of light-chain genes from human intestinal plasma
deaminase (AID), a potential RNA-editing enzyme. Cell 71. Sonoda, E. et al. Transforming growth factor- induces IgA cells demonstrates that genes are almost all in-frame and
102, 553563 (2000). production and acts additively with interleukin-5 for IgA highly mutated, and most genes are highly mutated when
Together with reference 54, this paper shows that production. J. Exp. Med. 170, 14151420 (1989). in-frame and minimally mutated when out-of-frame. Eur. J.
class-switch recombination and somatic 72. van Ginkel, F. W. et al. Partial IgA deficiency with increased Immunol. 30, 29082917 (2000).
hypermutation depend on AID, a putative RNA-editing TH2-type cytokines in TGF-1 knockout mice. J. Immunol. 95. Macpherson, A. J. et al. IgA production without or chain
enzyme, which is expressed specifically by activated 163, 19511957 (1999). expression in developing B cells. Nature Immunol. 2,
B cells. 73. Cazac, B. B. & Roes, J. TGF- receptor controls B-cell 625631 (2001).
53. Muramatsu, M. et al. Specific expression of activation- responsiveness and induction of IgA in vivo. Immunity 13, 96. Kitamura, D., Roes, J., Kuhn, R. & Rajewsky, K. A B-cell-
induced cytidine deaminase (AID), a novel member of the 443451 (2000). deficient mouse by targeted disruption of the membrane
RNA-editing deaminase family in germinal-center B cells. 74. Ramsay, A. J. et al. The role of interleukin-6 in mucosal IgA exon of the immunoglobulin chain gene. Nature 350,
J. Biol. Chem. 274, 1847018476 (1999). antibody responses in vivo. Science 264, 561563 (1994). 423426 (1991).
54. Revy, P. et al. Activation-induced cytidine deaminase (AID) 75. Fayette, J. et al. Human dendritic cells skew isotype 97. Lam, K. P., Kuhn, R. & Rajewsky, K. In vivo ablation of
deficiency causes the autosomal recessive form of the switching of CD40-activated naive B cells towards IgA1 and surface immunoglobulin on mature B cells by inducible gene
hyper-IgM syndrome (HIGM2). Cell 102, 565575 (2000). IgA2. J. Exp. Med. 185, 19091918 (1997). targeting results in rapid cell death. Cell 90, 10731083
55. Fagarasan, S., Kinoshita, K., Muramatsu, M., Ikuta, K. & 76. Litinskiy, M. B. et al. DCs induce CD40-independent (1997).
Honjo, T. In situ class switching and differentiation to IgA- immunoglobulin class switching through BLyS and APRIL. 98. Hooper, L. V. & Gordon, J. I. Commensal hostbacterial
producing cells in the gut lamina propria. Nature 413, Nature Immunol. 3, 822829 (2002). relationships in the gut. Science 292, 11151118 (2001).
639643 (2001). The first demonstration that dendritic cells can trigger 99. Hooper, L. V. et al. Molecular analysis of commensal
The first demonstration at the molecular level class-switch recombination of B cells directly, in a hostmicrobial relationships in the intestine. Science 291,
(methodology described in reference 57) that class- CD40-independent manner, through BAFF and APRIL. 881884 (2001).
switching to IgA takes place in situ in the lamina 77. Fagarasan, S. & Honjo, T. T-independent immune response: 100. Okazaki, I. M., Kinoshita, K., Muramatsu, M., Yoshikawa, K.
propria ; the dominance of IgA in the gut is explained new aspects of B-cell biology. Science 290, 8992 (2000). & Honjo, T. The AID enzyme induces class-switch
by the capacity of lamina-propria stromal cells 78. Mestecky, J. et al. in Mucosal Immunology (eds Ogra, P. recombination in fibroblasts. Nature 416, 340345 (2002).
to enhance preferential class-switching and et al.) xxiiixliii (Academic Press, San Diego, 1999).
differentiation to IgA-producing plasma cells. 79. Levy, Y. et al. Defect in IgV gene somatic hypermutation in Acknowledgements
56. Kamata, T. et al. Increased frequency of surface IgA-positive common variable immuno-deficiency syndrome. Proc. Natl This study was supported in part by a Center of Excellence Grant
plasma cells in the intestinal lamina propria and decreased Acad. Sci. USA 95, 1313513140 (1998). from the Ministry of Education, Science, Sports and Culture of
IgA excretion in hyper IgA (HIGA) mice, a murine model of 80. Bastlein, C. et al. Common variable immunodeficiency Japan. We thank Y. Doi, K. Kinoshita, M. Muramatsu, H. Nagaoka
IgA nephropathy with hyperserum IgA. J. Immunol. 165, syndrome and nodular lymphoid hyperplasia in the small and K. Suzuki for their contributions to both the work cited and the
13871394 (2000). intestine. Endoscopy 20, 272275 (1988). writing of this manuscript. Because of the extent and complexity of
57. Kinoshita, K., Harigai, M., Fagarasan, S., Muramatsu, M. & 81. Burt, R. W. & Jacoby, R. F. in Textbook of Gastroenterology the mucosal immunology field, we could not discuss many interest-
Honjo, T. A hallmark of active class-switch recombination: (eds Yamada, T., Alpers, D., Laine, L., Owyang, C. & ing studies, and we apologize to those excellent scientists whose
transcripts directed by I promoters on looped-out circular Powell, D.) 19952022 (Lippincott William & Wilkins, work could not be cited.
DNAs. Proc. Natl Acad. Sci. USA 98, 1262012623 (2001). Philadelphia, 1999).
58. Nakamura, M. et al. High frequency class switching of an 82. Russell, M., Kilian, M. & Lamm, M. in Mucosal Immunology
IgM+ B-lymphoma clone CH12F3 to IgA+ cells. Int. Immunol. (eds Ogra, P. et al.) 225240 (Academic Press, San Diego, Online links
8, 193201 (1996). 1999).
59. Stavnezer-Nordgren, J. & Sirlin, S. Specificity of 83. Harriman, G. R. et al. Targeted deletion of the IgA constant DATABASES
immunoglobulin heavy-chain switch correlates with activity region in mice leads to IgA deficiency with alterations in The following terms in this article are linked online to:
of germline heavy chain genes prior to switching. EMBO J. expression of other Ig isotypes. J. Immunol. 162, LocusLink: http://www.ncbi.nlm.nih.gov/LocusLink/
5, 95102 (1986). 25212529 (1999). 47 | AID | APRIL | BAFF | BAFFR | BCMA | CCL25 | CCR9 |
60. Iwasato, T., Shimizu, A., Honjo, T. & Yamagishi, H. Circular 84. Lycke, N., Erlandsson, L., Ekman, L., Schon, K. & CD40 | claudin 1 | CXCL13 | IFN- | IFN- | IL-2 | IL-2R | IL-4 | IL-5 |
DNA is excised by immunoglobulin class-switch Leanderson, T. Lack of J chain inhibits the transport of gut IL-6 | IL-10 | LT | LTR | MADCAM1 | Nik | occludin | pIgR | Rag2 |
recombination. Cell 62, 143149 (1990). IgA and abrogates the development of intestinal antitoxic TACI | TGF- | TNF | zona occludens 1
61. von Schwedler, U., Jack, H. M. & Wabl, M. Circular DNA is a protection. J. Immunol. 163, 913919 (1999). OMIM: http://www.ncbi.nlm.nih.gov/Omim/
product of the immunoglobulin class-switch rearrangement. 85. Johansen, F. E. et al. Absence of epithelial immunoglobulin CVID | Gardner syndrome
Nature 345, 452456 (1990). A transport, with increased mucosal leakiness, in polymeric
62. Matsuoka, M., Yoshida, K., Maeda, T., Usuda, S. & Sakano, H. immunoglobulin receptor/secretory component-deficient FURTHER INFORMATION
Switch circular DNA formed in cytokine-treated mouse mice. J. Exp. Med. 190, 915922 (1999). Tasuku Honjos lab: http://www.kyoto-
splenocytes: evidence for intramolecular DNA deletion in 86. Berek, C., Berger, A. & Apel, M. Maturation of the immune u.ac.jp/kokuryu/kyotouniv/cur03.htm
immunoglobulin class switching. Cell 62, 135142 (1990). response in germinal centers. Cell 67, 11211129 (1991). Access to this interactive links box is free online.
72 | JANUARY 2003 | VOLUME 3 www.nature.com/reviews/immunol
2002 Nature Publishing Group
Potrebbero piacerti anche
- Immunoglobulin ADocumento11 pagineImmunoglobulin AFIAL FAIDA ASSYIFANessuna valutazione finora
- Understanding Antibodies and Their StructureDocumento48 pagineUnderstanding Antibodies and Their StructureLA TSHTTNessuna valutazione finora
- Giovanna 2012Documento5 pagineGiovanna 2012Jessica OktavianusNessuna valutazione finora
- Jenis dan bagian antibodi serta peran dalam sistem kekebalan tubuhDocumento26 pagineJenis dan bagian antibodi serta peran dalam sistem kekebalan tubuhJundy RabbaniNessuna valutazione finora
- Siga UsusDocumento9 pagineSiga UsusMira MaindraNessuna valutazione finora
- 1 s2.0 S1074761319303176 MainDocumento22 pagine1 s2.0 S1074761319303176 MainFaiz SheeNessuna valutazione finora
- The Biological Functions of The Complement SystemDocumento9 pagineThe Biological Functions of The Complement SystemAishah NajihahNessuna valutazione finora
- 1 s2.0 S1931312807001953 MainDocumento2 pagine1 s2.0 S1931312807001953 MainKavyarani RathodNessuna valutazione finora
- Terminology: Nomenclature of Mucosa-Associated Lymphoid TissueDocumento8 pagineTerminology: Nomenclature of Mucosa-Associated Lymphoid TissueJessica AlegríaNessuna valutazione finora
- Extrafollicular Responses in Humans and SLEDocumento13 pagineExtrafollicular Responses in Humans and SLERaul ReyesNessuna valutazione finora
- Immunoglobulins/Antibody: Presented By: Shashi Regd. No. 11006142 Roll No. B15 Section RP8003Documento17 pagineImmunoglobulins/Antibody: Presented By: Shashi Regd. No. 11006142 Roll No. B15 Section RP8003Shashi SharmaNessuna valutazione finora
- Wheeler 2007 Milk Immmune FactorsDocumento12 pagineWheeler 2007 Milk Immmune FactorsKumiho LimNessuna valutazione finora
- Fimmu 03 00130Documento18 pagineFimmu 03 00130Isabella1237Nessuna valutazione finora
- 2014 Artículo RegionalizaciónDocumento19 pagine2014 Artículo RegionalizaciónVerónica RangelNessuna valutazione finora
- Antibodies For MBBS PDFDocumento63 pagineAntibodies For MBBS PDFTANDINessuna valutazione finora
- Peterson2014 PDFDocumento13 paginePeterson2014 PDFLeonardoNessuna valutazione finora
- 64a957e1f81b70001842ef6d - ## - Human Health and Diseases Handwritten Notes (Of Lecture 03)Documento6 pagine64a957e1f81b70001842ef6d - ## - Human Health and Diseases Handwritten Notes (Of Lecture 03)sourajeetsahoo2610Nessuna valutazione finora
- T Display of Active Enzymes On The Cell Surface of Escherichia Coli Using PgsA Anchor Protein and Their Application To BioconversionDocumento9 pagineT Display of Active Enzymes On The Cell Surface of Escherichia Coli Using PgsA Anchor Protein and Their Application To BioconversionCamilo ChávezNessuna valutazione finora
- Chitosan-Alginate Microcapsules For Oral Delivery of Egg YolkDocumento8 pagineChitosan-Alginate Microcapsules For Oral Delivery of Egg YolkRo BellingeriNessuna valutazione finora
- The Role of Dimeric IgA in Mucosal ImmunityDocumento7 pagineThe Role of Dimeric IgA in Mucosal ImmunitysuperlalacuteNessuna valutazione finora
- Pathogenic Escherichia ColiDocumento18 paginePathogenic Escherichia ColizorbaxNessuna valutazione finora
- Kolitis UlseratifDocumento6 pagineKolitis UlseratifnurNessuna valutazione finora
- 14activation of B Lymphocytes-IDocumento11 pagine14activation of B Lymphocytes-IAleena KhanNessuna valutazione finora
- GLAROS 2021 - Limited Access To Antigen Drives Generation of Early B Cell Memory While Restraining The Plasmablast ResponseDocumento30 pagineGLAROS 2021 - Limited Access To Antigen Drives Generation of Early B Cell Memory While Restraining The Plasmablast ResponseJoão Pedro NunesNessuna valutazione finora
- Immunologyserology Module 1 and 2Documento4 pagineImmunologyserology Module 1 and 2Rhenjay Ferrer LagoyNessuna valutazione finora
- Devina Farahamida - 021911133033 - Tugas Mandiri Mikrobiologi - Dasar ImunologiDocumento9 pagineDevina Farahamida - 021911133033 - Tugas Mandiri Mikrobiologi - Dasar ImunologiDevina FarahamidaNessuna valutazione finora
- Kuby5 13 ComplementDocumento20 pagineKuby5 13 ComplementASDASDDD2Nessuna valutazione finora
- Adaptive Immunity - 1Documento15 pagineAdaptive Immunity - 1FN FajrinNessuna valutazione finora
- Immunoglobulins, Mucosal Immunity and Vaccination in Teleost FishDocumento14 pagineImmunoglobulins, Mucosal Immunity and Vaccination in Teleost FishBayuNessuna valutazione finora
- Fimmu 04 00189Documento6 pagineFimmu 04 00189jeffersonbborges99Nessuna valutazione finora
- Bacille Calmette-Gue Rin Vaccine Reprograms Human Neonatal Lipid Metabolism in Vivo and in VitroDocumento24 pagineBacille Calmette-Gue Rin Vaccine Reprograms Human Neonatal Lipid Metabolism in Vivo and in VitroMaria DruszczyńskaNessuna valutazione finora
- Autophagy in PreeclampsiaDocumento16 pagineAutophagy in PreeclampsiaeginabilaNessuna valutazione finora
- Células Plasmáticas Ingles A ProgramaçãoDocumento8 pagineCélulas Plasmáticas Ingles A ProgramaçãoAnimed Animed Clínica Veterinária DracenaNessuna valutazione finora
- Immunological Aspects of GastrointestinalDocumento12 pagineImmunological Aspects of GastrointestinalFebniNessuna valutazione finora
- 1b PDFDocumento7 pagine1b PDFAndrea Il MiodoNessuna valutazione finora
- Activacion de B Cells y Producción de Abs (2) CLASE RICHARD GARCIADocumento49 pagineActivacion de B Cells y Producción de Abs (2) CLASE RICHARD GARCIAZuleynis MendozaNessuna valutazione finora
- Non-IgE Mediated Food AllergyDocumento8 pagineNon-IgE Mediated Food AllergyDede NovHyNessuna valutazione finora
- Different Human Gut Models Reveal The Distinct Fermentation Patterns of Arabinoxylan Versus Inulin Pieter Van Den Abbeele 2013Documento9 pagineDifferent Human Gut Models Reveal The Distinct Fermentation Patterns of Arabinoxylan Versus Inulin Pieter Van Den Abbeele 2013Mamen Casado MuñozNessuna valutazione finora
- Tugas 9-DikonversiDocumento25 pagineTugas 9-DikonversiDevina FarahamidaNessuna valutazione finora
- CholesterolDocumento12 pagineCholesterolankit sharmaNessuna valutazione finora
- Biochemistry of Adaptive ImmunityDocumento22 pagineBiochemistry of Adaptive Immunityshreyansh singhNessuna valutazione finora
- 3.4 Microbiology PDFDocumento31 pagine3.4 Microbiology PDFQueena Yan Yie LeeNessuna valutazione finora
- BMC ImmunologyDocumento9 pagineBMC ImmunologyYuniarNessuna valutazione finora
- Instant Download Cell Biology 3rd Edition Ebook PDF PDF ScribdDocumento36 pagineInstant Download Cell Biology 3rd Edition Ebook PDF PDF Scribdconstance.whitesell736100% (40)
- Papel Da IgA e Interações Com MicrobiotaDocumento12 paginePapel Da IgA e Interações Com MicrobiotaFernando Cezar dos SantosNessuna valutazione finora
- The Establishment of Resident Memory B Cells in The Lung Requires Local Antigen EncounterDocumento19 pagineThe Establishment of Resident Memory B Cells in The Lung Requires Local Antigen EncounterjiangjieNessuna valutazione finora
- Antimicrobial Agents that Inhibit Cell Wall SynthesisDocumento13 pagineAntimicrobial Agents that Inhibit Cell Wall SynthesisMansi singhNessuna valutazione finora
- Review on Mucosal Immune ResponseDocumento17 pagineReview on Mucosal Immune Responsehngdf bfbnNessuna valutazione finora
- L1 - StaphylococciDocumento35 pagineL1 - StaphylococcitheresmachNessuna valutazione finora
- Overview of Complement Activation and RegulationDocumento14 pagineOverview of Complement Activation and RegulationAnna SkrobińskaNessuna valutazione finora
- Perspectives: Do Antibodies Select A Healthy Microbiota?Documento8 paginePerspectives: Do Antibodies Select A Healthy Microbiota?fernando chiaraNessuna valutazione finora
- International Journal of Medical MicrobiologyDocumento10 pagineInternational Journal of Medical MicrobiologyArthur Zanetti Nunes FernandesNessuna valutazione finora
- Subversion of Trafficking, Apoptosis, and Innate Immunity by Type III Secretion System EffectorsDocumento12 pagineSubversion of Trafficking, Apoptosis, and Innate Immunity by Type III Secretion System EffectorsDiego TulcanNessuna valutazione finora
- Immunoglobulin Structure and FunctionsDocumento26 pagineImmunoglobulin Structure and Functionssazia gaurNessuna valutazione finora
- Microbiology and Molecular Biology 2022Documento10 pagineMicrobiology and Molecular Biology 2022Shahida MMGNessuna valutazione finora
- Kuliah OB II - Mucosal Immune System-2017Documento22 pagineKuliah OB II - Mucosal Immune System-2017wayanNessuna valutazione finora
- Micro F2.chapter 6 - 2022Documento3 pagineMicro F2.chapter 6 - 2022Gamal Eldien ElsaiedNessuna valutazione finora
- Cellular and Molecular Mechanisms of Inflammation: Receptors of Inflammatory Cells: Structure—Function RelationshipsDa EverandCellular and Molecular Mechanisms of Inflammation: Receptors of Inflammatory Cells: Structure—Function RelationshipsCharles G. CochraneNessuna valutazione finora
- The Human IgG Subclasses: Molecular Analysis of Structure, Function and RegulationDa EverandThe Human IgG Subclasses: Molecular Analysis of Structure, Function and RegulationFarouk ShakibNessuna valutazione finora
- Presentation Illustrating The 2017 Classification of Seizure TypesDocumento54 paginePresentation Illustrating The 2017 Classification of Seizure TypesMohamad Syaikhul IslamNessuna valutazione finora
- Bordetella PertusisDocumento16 pagineBordetella PertusisAnailil MoralesNessuna valutazione finora
- DR Sunil Sharma Senior Resident Dept. of Pulmonary Medicine Dept. of Pulmonary MedicineDocumento45 pagineDR Sunil Sharma Senior Resident Dept. of Pulmonary Medicine Dept. of Pulmonary MedicineAnailil MoralesNessuna valutazione finora
- Inmunidad Del Tracto Respiratorio Superior PDFDocumento11 pagineInmunidad Del Tracto Respiratorio Superior PDFAnailil MoralesNessuna valutazione finora
- Cells: Airway Epithelial Cell Cilia and Obstructive Lung DiseaseDocumento19 pagineCells: Airway Epithelial Cell Cilia and Obstructive Lung DiseaseAnailil MoralesNessuna valutazione finora
- Ultrasound of Fetal Cardiac AnomaliesDocumento14 pagineUltrasound of Fetal Cardiac AnomaliesAnailil MoralesNessuna valutazione finora
- Inmunologia Asma y EpocDocumento10 pagineInmunologia Asma y EpocAnailil MoralesNessuna valutazione finora
- Amígdalas y Barreras Mucosas.Documento14 pagineAmígdalas y Barreras Mucosas.Anailil MoralesNessuna valutazione finora
- 08 0119 PDFDocumento8 pagine08 0119 PDFAnailil MoralesNessuna valutazione finora
- Basic Concepts in Lung Immunology: System ThatDocumento6 pagineBasic Concepts in Lung Immunology: System ThatAnailil MoralesNessuna valutazione finora
- AntivirL B y T en PulmonDocumento9 pagineAntivirL B y T en PulmonAnailil MoralesNessuna valutazione finora
- Ultrasound of Fetal Cardiac AnomaliesDocumento14 pagineUltrasound of Fetal Cardiac AnomaliesAnailil MoralesNessuna valutazione finora
- Pathogenesis of acute pancreatitisDocumento7 paginePathogenesis of acute pancreatitisAnailil MoralesNessuna valutazione finora
- English ProjectDocumento10 pagineEnglish ProjectHarshman Singh HarshmanNessuna valutazione finora
- ccpc15 Supportive and Preventive WorkbookDocumento30 pagineccpc15 Supportive and Preventive WorkbookJeremy HamptonNessuna valutazione finora
- Pump IntakeDocumento6 paginePump IntakeAnonymous CMS3dL1T100% (1)
- Ragavendhar Seeks Entry Software JobDocumento2 pagineRagavendhar Seeks Entry Software JobfferferfNessuna valutazione finora
- Optimal Inspection and Preventive Maintenance Scheduling of Mining EquipmentDocumento9 pagineOptimal Inspection and Preventive Maintenance Scheduling of Mining EquipmentLucia QuispeNessuna valutazione finora
- RFID Receiver Antenna Project For 13.56 MHZ BandDocumento5 pagineRFID Receiver Antenna Project For 13.56 MHZ BandJay KhandharNessuna valutazione finora
- Manufacturing Tech-1Documento6 pagineManufacturing Tech-1Vikram Rao0% (1)
- Soil Testing Lab Results SummaryDocumento2 pagineSoil Testing Lab Results SummaryMd SohagNessuna valutazione finora
- PoiconverterDocumento2 paginePoiconvertertaco6541Nessuna valutazione finora
- Resp Part 4Documento95 pagineResp Part 4Kristian CadaNessuna valutazione finora
- 1 Starter WBDocumento88 pagine1 Starter WBHYONessuna valutazione finora
- Catalogue MinicenterDocumento36 pagineCatalogue Minicentermohamed mahdiNessuna valutazione finora
- Title Page Title: Carbamazepine Versus Levetiracetam in Epilepsy Due To Neurocysticercosis Authors: Akhil P SanthoshDocumento16 pagineTitle Page Title: Carbamazepine Versus Levetiracetam in Epilepsy Due To Neurocysticercosis Authors: Akhil P SanthoshPrateek Kumar PandaNessuna valutazione finora
- Tipolo WH Gantt ChartDocumento15 pagineTipolo WH Gantt ChartMayeterisk RNessuna valutazione finora
- Educational Leadership Platform PaperDocumento4 pagineEducational Leadership Platform Paperapi-273087939Nessuna valutazione finora
- Differentiation SS2Documento88 pagineDifferentiation SS2merezemenike272Nessuna valutazione finora
- EDUC 5 - QuestionairesDocumento7 pagineEDUC 5 - QuestionairesWilliam RanaraNessuna valutazione finora
- Lake Isle of Innisfree Lesson Plan BV ZGDocumento4 pagineLake Isle of Innisfree Lesson Plan BV ZGapi-266111651100% (1)
- I Am Sharing 'Pregnancy Shady' With YouDocumento48 pagineI Am Sharing 'Pregnancy Shady' With YouNouran AlaaNessuna valutazione finora
- Thank you for purchasing your remap from HDI Tuning LtdDocumento2 pagineThank you for purchasing your remap from HDI Tuning LtdMaks LebanNessuna valutazione finora
- Classification of MatterDocumento2 pagineClassification of Matterapi-280247238Nessuna valutazione finora
- Enterprise Architecture A General OverviewDocumento45 pagineEnterprise Architecture A General OverviewraducooNessuna valutazione finora
- VANSINA, Jan. Art History in AfricaDocumento250 pagineVANSINA, Jan. Art History in AfricaRaphaelTim100% (1)
- Pankaj Screener 10 Oct 2014Documento127 paginePankaj Screener 10 Oct 2014Sadul Singh Naruka100% (1)
- Literature Revisited: Understanding Key Concepts and Applying Analytical SkillsDocumento31 pagineLiterature Revisited: Understanding Key Concepts and Applying Analytical SkillsMuhammad Furqan Aslam Awan100% (2)
- Rha GGBS 27 4Documento12 pagineRha GGBS 27 4KhaDeja MawraNessuna valutazione finora
- Financial Reporting Statement Analysis Project Report: Name of The Company: Tata SteelDocumento35 pagineFinancial Reporting Statement Analysis Project Report: Name of The Company: Tata SteelRagava KarthiNessuna valutazione finora
- (Nima Naghibi) Rethinking Global Sisterhood Weste PDFDocumento220 pagine(Nima Naghibi) Rethinking Global Sisterhood Weste PDFEdson Neves Jr.100% (1)
- OJT Form 03 Performance EvaluationDocumento2 pagineOJT Form 03 Performance EvaluationResshille Ann T. SalleyNessuna valutazione finora
- Color TheoryDocumento28 pagineColor TheoryEka HaryantoNessuna valutazione finora