Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
7
Caricato da
79lalalaCopyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
7
Caricato da
79lalalaCopyright:
Formati disponibili
Acta Dermatovenerol Croat 2016;24(2):130-136 CLINICAL ARTICLE
Clinical and Demographic Characteristics of Patients
with Molluscum Contagiosum Treated at the Univer-
sity Dermatology Clinic Maribor in a 5-year period
Katarina Trko, Mario Poljak, Miljenko Krimari, Jovan Miljkovi
Department of Dermatovenereology, University Clinical Centre Maribor, Maribor,
Slovenia; Institute of Microbiology and Immunology, Faculty of Medicine, University
of Ljubljana, Ljubljana, Slovenia; Faculty of Medicine, University of Maribor, Maribor,
Slovenia
Corresponding author: ABSTRACT Molluscum contagiosum virus (MCV) is a common skin pathogen in
Katarina Trko, MD both adults and children. In this prospective study, we clinically evaluated con-
secutive patients with molluscum contagiosum (MC) who had been examined
Department of Dermatovenereology
during a 5-year period at the second-largest dermatology clinic in Slovenia and
University Clinical Centre Maribor described their main demographic and clinical characteristics, concomitant dis-
Ljubljanska 5 eases, and treatment success. The study included 188 patients, of which 121
2000 Maribor (64%) were men and 67 (36%) were women. A total of 135 (72%) patients were
adults, with lesions that were most commonly located in the anogenital region
Slovenia (98%) and were probably sexually acquired. Two adult patients were diagnosed
katarina.trcko@gmail.com with concurrent human immunodeficiency virus (HIV) infection. Fifty-three
(28%) patients were children with a mean age of 5.7 years, most commonly pre-
senting with lesions on the torso and extremities (85%). In adults, the infection
Received: December 5, 2015
most commonly occurred in male patients, while in children it was slightly more
Accepted: May 10, 2016 common in female patients. At presentation, 58% of patients had more than
5 MC lesions. A total of 30% of the included children had concomitant atopic
dermatitis. We did not observe an increased occurrence of MCV infection in pa-
tients with atopic dermatitis. All patients were treated with curettage of the le-
sions. The cure rate at the first follow-up visit after 2 months was relatively high
(63%), and recurrences were not associated with the number or site of lesions
at presentation or with concomitant atopic dermatitis.
KEY WORDS: molluscum contagiosum, sexually transmitted infection, curet-
tage
INTRODUCTION
Molluscum contagiosum (MC) is a common der- infects humans (3). MCV is epidermotropic and rep-
matologic disease caused by the molluscum conta- licates in the cytoplasm of cells, producing cytoplas-
giosum virus (MCV) (1). MCV is a large, brick-shaped, mic inclusions and enlargement of infected cells (4).
double-stranded DNA poxvirus and is the sole mem- To date, four subtypes of MCV have been identified
ber of the genus Molluscipoxvirus (2). After eradication (5,6). The subtype 1 comprises approximately 76-97%
of smallpox, MCV is the only poxvirus that exclusively of the cases (7,8). The subtype 2 is more prevalent in
ACTA DERMATOVENEROLOGICA CROATICA
130
Trko et al. Acta Dermatovenerol Croat
Patients with molluscum contagiosum 2016;24(2):130-136
patients infected with human immunodeficiency vi- treatment of refractory MC in immunocompromised
rus (HIV) (9), while subtypes 3 and 4 are relatively rare patients with topical imiquimod (36-38), systemic or
(5,6,9). intralesional interferon alpha (39-41), parenteral or
topical cidofovir (42-44), pulsed dye laser, or photo-
Characteristics of patients with mollus- dynamic therapy (45,46).
cum contagiosum In this prospective study we clinically evaluated
MCV infection is characterized by a single or mul- in detail consecutive patients with MC who had been
tiple flesh-colored umbilicated papules (10). MCV is examined during a 5-year period at the second larg-
transmitted directly from person to person or by au- est dermatology clinic in Slovenia, and described
toinoculation (11). MC is relatively frequent in chil- their main demographic and clinical characteristics,
dren, and although lesions may appear anywhere, in concomitant diseases, and treatment success. To the
children they commonly occur on the face, torso, and best of our knowledge, this is the largest clinical study
extremities (12). In adults, MC is regarded as a sexu- of MC in Central Europe to date.
ally transmitted infection (STI), thus all adult patients
with MC should be carefully screened for other STI PATIENTS AND METHODS
and appropriately counselled. In adults, MC charac- The study included consecutive patients who
teristically involves the genital region, but extrageni- were clinically suspected of MC and were treated at
tal appearance can be more commonly observed in the Department of Dermatology and Venereal Dis-
persons with immunosuppressive conditions, espe- eases at the University Medical Centre (UMC) Maribor,
cially HIV/AIDS (13). The characteristic umbilicated Slovenia, from October 2009 to April 2015. The main
papules develop after an estimated incubation of inclusion criterion was lesions that could be clinically
2 weeks to 6 months or longer (11,14). Clinical find- defined as MC and were located either on the skin,
ings are the most important for establishing the final torso, or extremities or in the anogenital region. Di-
diagnosis. MC is generally self-limited and regresses agnosis was established based on the characteristic
spontaneously after several months or years (15,16). clinical picture commonly characterized by an umbil-
However, treatment is recommended and also fre- ical papule of less than 1 cm in diameter. We obtained
quently required by the patient, as it is beneficial in data on patient age, sex, concomitant diseases, site,
preventing further MCV transmission or autoinocu- and number of lesions. Patients clinically suspected
lation. Currently, there is no specific treatment for of acquiring MC by a sexual route underwent tests for
MC, and according to the literature, no treatment is HIV, syphilis, and hepatitis B and C. We photographed
definitively effective (17). Patients are usually treated the lesions using a digital camera before treatment.
by physical removal of the lesions by curettage or Patients were treated with curettage of all visible
cryotherapy (18,19). Both methods are painful and lesions. If required, lidocaine plus procaine (EMLA
have the potential for scarring and hypopigmenta- cream) was applied on the treated spot 1 hour before
tion. Other physical methods, such as mechanical ex-
the procedure. At a follow-up visit 2 months after the
pression of the lesion core, electrosurgery, and laser
end of treatment the success of treatment or even-
therapy, might also be beneficial (20,21).
tual recurrence of the disease were assessed.
There are many options for topical treatment
The study was approved by the Medical Eth-
that have been tried for treating MC. These include
ics Committee of the Republic of Slovenia (consent
cantharidin (22), 0.5% podophyllotoxin cream (23),
number: 101/12/09). Written consent was obtained
imiquimod 5% cream (24,25), potassium hydroxide
from all patients older than 18 years of age and from
(26,27), and salicylic acid (19). Topical retinoids such
parents of those aged 17 years or less. For categori-
as tretinoin (0.1 or 0.5 % cream, or 0.025% gel) and
cal variables, statistical analyses were performed with
adapalene have been described as treatment options
the exact test with a Monte Carlo approximation due
(28,29). Topical phenol (20), silver nitrate 40% paste
to unbalanced data. A probability of P<0.05 was con-
(30), intralesional injections with Candida antigen
sidered statistically significant. The data were ana-
(31), and trichloroacetic acid 70% (32) or iodine (33)
lyzed using the statistical software IBM SPSS, version
have also been used for the treatment of MC. System-
22 (SPSS Inc., Chicago, IL, USA).
ic therapy with oral cimetidine, an H2 antihistamine,
has been found successful in the treatment of MC in
one study (34). In HIV-infected patients with refrac- RESULTS
tory disease, antiretroviral treatment has been as- In the period from October 2009 to April 2015,
sociated with reports of significant improvement of we examined a total of 188 patients with MC lesions,
MC lesions (35). There are also reports of successful of which 121 were men (64%) and 67 were women
ACTA DERMATOVENEROLOGICA CROATICA
131
Trko et al. Acta Dermatovenerol Croat
Patients with molluscum contagiosum 2016;24(2):130-136
Table 1. Study group demographics
Variable Number of %
patients
All patients 188 100.0
Men 121 64.4
Women 67 35.6
Age >17 years 135 71.8
Age <17 years 53 28.2
Men aged >17 years 100 74.0
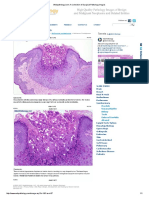
Women aged >17 years 35 26.0 Figure 1. Penile molluscum contagiosum in a 25-year-old
Men aged <17 years 21 40.0 man.
Women aged <17 years 32 60.0
a difference was not statistically significant (P=0.066).
Two adult patients were diagnosed with an HIV infec-
(36%). Patient age was 0-62 years. Of these, 135 were
tion, and four patients also had genital warts.
adult patients aged 17 years or older (range 17-62
years; mean age 27.5 years) who developed MC At presentation, all patients were treated with cu-
mainly as a result of a sexual/anogenital contact with rettage and had all visible lesions removed. At the 2-
an infected individual, and 53 were children aged 0- month follow-up visit, recurrence of MC lesions was
16 years (mean age 5.7 years) who had non-sexually recorded in 69/188 (37%) patients. The disease re-
transmitted MC lesions (Figure 1, Figure 2). In adults, curred in 51/135 (38%) of all included adult patients,
we noticed more male patients (74%), whereas in chil- while recurrence was observed in 18/53 (34%) of chil-
dren there were more female patients (60%) (Table 1). dren. There was no statistically significant difference
The difference in the distribution by sex was statisti- with regard to recurrence by age (P=0.737). All re-
cally significant (P<0.001). currences of MC in adults were observed in the ano-
In adults, lesions were distributed primarily in the genital region, whereas in children the disease most
anogenital region 133/135 (98%), while MC lesions in commonly recurred on the torso and the extremities.
children were most commonly observed on the torso In adults, MC recurrence was more often observed in
b the extremities 45/53 (85%) (Table 2). The differ-
and patients with more than 5 lesions, and the same was
ence in lesion distribution between adults and chil- found in children. However, the difference was not
dren was statistically significant (P<0.001). statistically significant compared to patients with less
lesions (P=0.376 and P=0.565, respectively) (Table 3).
At examination, 78/135 (58%) of adult patients
and 32/53 (60%) of children had more than 5 MC le- Disease recurrence was observed in only 5 out of
sions. The difference in frequency of those with 5 or 16 children with atopic dermatitis and in 13 out of
more MC lesions between adults and children was 37 children without atopic dermatitis. The difference
not statistically significant (P=0.869). was not statistically significant (P=1.00).
All adult patients had a larger number of lesions
(>5) in the anogenital region, whereas in children we
DISCUSSION
observed more lesions (>5) on the torso and the ex- Although MC is a relatively frequent disease, there
tremities. is a lack of large published cohort studies in the avail-
Out of the 53 children included, 16 (30%) pre- able literature, especially from Central and Eastern
sented with atopic dermatitis. We found more than Europe.
5 lesions in 81% of children with atopic dermatitis This prospective clinical study presents main de-
(n=16), while this was observed in only 52% of chil- mographics, lesion localization, concomitant diseas-
dren without atopic dermatitis. (n=37). However, the es, and results of the most common treatment of the
Table 2. Distribution of molluscum contagiosum (MC) lesions
Sites of lesions Torso and extremities Head and neck Anogenital region P
All patients (N=188) 47 4 137
Children (n=53) 45 4 4
<0.001
Adults (n=135) 2 0 133
ACTA DERMATOVENEROLOGICA CROATICA
132
Trko et al. Acta Dermatovenerol Croat
Patients with molluscum contagiosum 2016;24(2):130-136
tion (51). In our cohort, distribution of the disease in
children did not differ significantly by sex; however
there was a slight predominance of female patients. A
similar distribution by sex was also established in the
above mentioned study (48).
Children usually present with multiple MC lesions
on the face, torso, and extremities. 10-50% of children
have lesions in the genital region, which is usually a
result of autoinoculation (52). Very rare non-genital
lesions in adult patients point to the importance of
sexual transmission of MCV infections in adults (53),
whereas non-genital lesions are prevalent in children,
and lesions in the anogenital region are probably a
Figure 2. Molluscum contagiosum lesions on the inner result of autoinoculation (16). In our adult patients,
thigh of a 4-year-old boy
MC lesions were most commonly located in the ano-
genital region (98%), while in children they were most
commonly observed on the torso and extremities
cohort of patients with MC treated at the UMC Mari-
(58%). Only 7% of all evaluated children had lesions
bor in a 5-year period. According to literature data,
in the anogenital region; we strongly believe that this
the disease has a bimodal age of onset and most
is a result of autoinoculation.
commonly occurs in children and young adults (47).
Although MCV infection more commonly develops in At presentation, the majority of adults had more
children than adults (48,49), we treated more adult than 5 MC lesions. Children had a larger number of le-
patients than children at our hospital in the observed sions (>5) on the torso and extremities and less than
period. The most probable reason is that children 5 lesions on the head or neck and in the anogenital
with MC in our medical system are usually evaluated region. This leads to the conclusion that parents de-
and treated by school medicine practitioners and cide to visit the physician earlier if lesions are located
only fraction is referred to tertiary hospital. The mean on the visible parts (face) or in the anogenital region.
age of our adult patient was 27.5 years, which is in Adult patients often come to the dermatologist quite
accordance with the literature since MCV infection late, when the infection has already spread.
in adults most commonly occurs between 20 and 29 According to the literature data, MCV infection
years of age, similarly as with other sexually transmit- most commonly occurs in children with atopic der-
ted diseases (STD) (11,50). matitis (54,55), although this result was contradicted
The mean age of children included in our study by some studies (56). In our study, 30% of children
who had a non-sexually transmitted MC infection with MC also had concomitant atopic dermatitis. Chil-
was 5.7 years, which is comparable to the results of dren with atopic dermatitis more commonly present-
other studies, which found that MC is most com- ed with a larger number of lesions (>5) than children
mon in children aged 8 years or younger (48). In our without atopic dermatitis, but the difference was not
study, MC was recorded 3 times more commonly in statistically significant (P=0.066).
adult men than in women patients. This is similar to MC is a frequently found in HIV patients (57), with
published data, where the authors found that MC prevalence and incidence of the disease reflecting
occurred twice more commonly in the male popula- advanced immunodeficiency (58). Extragenital distri-
bution of lesions is characteristic in patients infected
Table 3. Comparison between adults and children with HIV, with lesions appearing somewhat more
with lesions recurrence at 2-month follow-up visit commonly on the face (59). Two of our patients pre-
sented with HIV infection, and none of them had been
Recurrence Adults Children
receiving antiretroviral therapy at the time of pre-
All patients (N=69) 51 18
sentation. The HIV-positive male patient had lesions
Anogenital region 51 2
located in the pubic region, and the HIV-positive fe-
Trunk and extremities 0 16 male patient had them on the abdomen. Incidentally
Number of lesions (>5) 32 12 identified HIV infection points to the importance of
Number of lesions (<5) 19 6 testing all adult patients for other STIs, as adult pa-
Concomitant atopic dermatitis 0 5 tients with sexually-transmitted MC lesions are at a
Without atopic dermatitis 51 13 higher risk of developing other STIs (60).
ACTA DERMATOVENEROLOGICA CROATICA
133
Trko et al. Acta Dermatovenerol Croat
Patients with molluscum contagiosum 2016;24(2):130-136
Curettage is the most frequent and effective 4. Buller RM, Burnett J, Chen W, Kreider J. Replica-
method for removing MC with the least recurrences tion of molluscum contagiosum virus. Virology
and complications (19). In a previous study, the le- 1995;213:655-9.
sions were fully cured in 80% of patients after only 5. Porter CD, Blake NW, Cream JJ, Archard LC. Mol-
one treatment (19). Other descriptive studies in the luscum contagiosum virus. Mol Cell Biol Hum Dis
literature recorded a lower cure rate, namely 34% (22 Ser 1992;1:233-57.
out of 64) and 39% (29 out of 75) (18,61). The num-
6. Nakamura J, Muraki Y, Yamada M, Hatano Y, Nii S.
ber of lesions, concomitant AD, and the number of
Analysis of molluscum contagiosum virus genom-
involved anatomical sites were described as the risk
es isolated in Japan. J Med Virol 1995;46:339-48.
factors for treatment failure (18). In our study, the
cure rate after a single treatment with curettage was 7. Porter CD, Blake NW, Archard LC, Muhlemann MF,
63% (119/188). At the 2-month follow-up visit, lesion Rosedale N, Cream JJ. Molluscum contagiosum
recurrence was identified in the rest of the patients virus types in genital and nongenital lesions. Br J
(69/188, 37%), both adults and children. Considering Dermatol 1989;120:37-41.
that most adult patients had lesions in the anogenital 8. Scholz J, Rsen-Wolff A, Bugert J, Reisner H, White
region, recurrences in this site were expected. Some- MI, Darai G, et al. Epidemiology of molluscum con-
what later, recurrences occurred in those patients tagiosum using genetic analysis of the viral DNA. J
who had a larger number of lesions (>5), but the dif- Med Virol 1989;27:87-90.
ference was not statistically significant. Recurrences 9. Yamashita H, Uemura T, Kawashima M. Mole-
in children were most commonly observed in those cular epidemiologic analysis of Japanese patients
who had lesions on the trunk and extremities, as well with molluscum contagiosum. Int J Dermatol
as in those who presented with more than 5 lesions 1996;35:99-105.
at examination. The differences were not statistically 10. Gottlieb SL, Myskowski PL. Molluscum contagio-
significant. We did not find a statistically significant sum. Int J Dermatol 1994;33:453-61.
difference in disease recurrence in children with
11. Brown ST, Nalley JF, Kraus SJ. Molluscum contagio-
atopic dermatitis as compared to children without
sum. Sex Transm Dis 1981;8:227-34.
atopic dermatitis.
12. Smith KJ, Yeager J, Skelton H. Molluscum con-
CONCLUSION tagiosum: its clinical, histopathologic, and im-
munohistochemical spectrum. Int J Dermatol
In the largest clinical study of MC in Central Eu- 1999;38:664-72.
rope to date, MC was more commonly observed in
13. Czelusta A, Yen-Moore A, Van der Straten M, Car-
adult men compared to adult women, whereas age
rasco D, Tyring SK. An overview of sexually trans-
distribution was the reverse in children. Adults in our
mitted diseases. Part III. Sexually transmitted
study most commonly presented with MC lesions in
diseases in HIV-infected patients. J Am Acad Der-
the anogenital region, whereas in children they were
matol 2000;43:409-32.
most commonly located on the torso and extremities.
We did not observe an increased occurrence of MCV 14. Birthistle K, Carrington D. Molluscum contagio-
infection in patients with atopic dermatitis. The cure sum virus. J Infect 1997;34:21-8.
rate following treatment with curettage was relative- 15. Brown J, Janniger CK, Schwartz RA, Silverberg NB.
ly high (63%), and recurrences were not associated Childhood molluscum contagiosum. Int J Der-
with the number of lesions or concomitant atopic matol 2006;45:93.
dermatitis. 16. Lee R, Schwartz RA. Pediatric molluscum contagi-
osum: reflections on the last challenging poxvirus
References: infection, Part 1. Cutis 2010;86:230.
1. Buller RM, Palumbo GJ. Poxvirus pathogenesis. 17. van der Wouden JC, van der Sande R, van Suijle-
Microbiol Rev 1991;55:80-122. kom-Smit LW, Berger M, Butler CC, Koning S. Inter-
2. Moss, B. Poxviridae. In: Fields BN, Knipe DM, Ho- ventions for cutaneous molluscum contagiosum.
wley PM, editors. Fields virology: 6th ed. Vol 2. Cochrane Database Syst Rev 2009;CD004767.
Philadelphia: Wolters Kluwer Health/ Lippincott 18. Simonart T, De Maertelaer V. Curettage treatment
Williams & Wilkins; 2013. pp. 2129 - 59. for molluscum contagiosum: a follow-up survey
3. Breman JG, Arita I. The confirmation and main- study. Br J Dermatol 2008;159:1144.
tenance of smallpox eradication. N Engl J Med 19. Hanna D, Hatami A, Powell J, Marcoux D, Maari
1980;303:1263-73. C, Savard P, et al. A prospective randomized trial
ACTA DERMATOVENEROLOGICA CROATICA
134
Trko et al. Acta Dermatovenerol Croat
Patients with molluscum contagiosum 2016;24(2):130-136
comparing the efficacy and adverse effects of four 33. Ohkuma M. Molluscum contagiosum treated with
recognized treatments of molluscum contagio- iodine solution and salicylic acid plaster. Int J Der-
sum in children. Pediatr Dermatol 2006;23:574-9. matol 1990;29:443-5.
20. Weller R, OCallaghan CJ, MacSween RM, White MI. 34. Dohil M, Prendiville JS. Treatment of mollus-
Scarring in Molluscum contagiosum: comparison cum contagiosum with oral cimetidine: clini-
of physical expression and phenol ablation. BMJ cal experience in 13 patients. Pediatr Dermatol
1999;319:1540. 1996;13:310-2.
21. Michel JL. Treatment of molluscum contagiosum 35. Horn CK, Scott GR, Benton EC. Resolution of severe
with 585 nm collagen remodeling pulsed dye la- molluscum contagiosum on effective antiretrovi-
ser. Eur J Dermatol 2004;14:103-6. ral therapy. Br J Dermatol 1998;138:715-7.
22. Coloe J, Morrell DS. Cantharidin use among pedia- 36. Strauss RM, Doyle EL, Mohsen AH, Green ST. Suc-
tric dermatologists in the treatment of molluscum cessful treatment of molluscum contagiosum
contagiosum. Pediatr Dermatol 2009;26:405-8. with topical imiquimod in a severely immuno-
23. Syed TA, Lundin S, Ahmad M. Topical 0.3% and compromised HIV-positive patient. Int J STD AIDS
0.5% podophyllotoxin cream for self-treatment 2001;12:264-6.
of molluscum contagiosum in males. A placebo- 37. Brown CW Jr, ODonoghue M, Moore J, Tharp M.
controlled, double-blind study. Dermatology Recalcitrant molluscum contagiosum in an HIV-
1994;189:65-8. afflicted male treated successfully with topical
24. Arican O. Topical treatment of molluscum con- imiquimod. Cutis 2000;65:363-6.
tagiosum with imiquimod 5% cream in Turkish 38. Gardner LS, Ormond PJ. Treatment of multiple gi-
children. Pediatr Int 2006;48:403-5. ant molluscum contagiosum in a renal transplant
25. Skinner RB Jr. Treatment of molluscum contagio- patient with imiquimod 5% cream. Clin Exp Der-
sum with imiquimod 5% cream. J Am Acad Der- matol 2006;31:452-3.
matol 2002;47:S221-4. 39. Hourihane J, Hodges E, Smith J, Keefe M, Jones A,
26. Short KA, Fuller LC, Higgins EM. Double-blind, Connett G. Interferon alpha treatment of mollus-
randomized, placebo-controlled trial of the use of cum contagiosum in immunodeficiency. Arch Dis
topical 10% potassium hydroxide solution in the Child 1999;80:77-9.
treatment of molluscum contagiosum. Pediatr 40. Bhm M, Luger TA, Bonsmann G. Disseminated
Dermatol 2006;23:279-81. giant molluscum contagiosum in a patient with
27. Romiti R, Ribeiro AP, Romiti N. Evaluation of the idiopathic CD4+ lymphocytopenia. Successful er-
effectiveness of 5% potassium hydroxide for the adication with systemic interferon. Dermatology
treatment of molluscum contagiosum. Pediatr 2008;217:196-8.
Dermatol 2000;17:495. 41. Nelson MR, Chard S, Barton SE. Intralesional inter-
28. Papa CM, Berger RS. Venereal herpes-like mollus- feron for the treatment of recalcitrant molluscum
cum contagiosum: treatment with tretinoin. Cutis contagiosum in HIV antibody positive individuals-
1976;18:537-40. a preliminary report. Int J STD AIDS 1995;6:351-2.
29. Scheinfeld N. Treatment of molluscum contagio- 42. Ibarra V, Blanco JR, Oteo JA, Rosel L. Efficacy of
sum: a brief review and discussion of a case suc- cidofovir in the treatment of recalcitrant mollus-
cessfully treated with adapelene. Dermatol Online cum contagiosum in an AIDS patient. Acta Derm
J 2007;13:15. Venereol 2000;80:315-6.
30. Niizeki K, Hashimoto K. Treatment of molluscum 43. Erickson C, Driscoll M, Gaspari A. Efficacy of intrave-
contagiosum with silver nitrate paste. Pediatr nous cidofovir in the treatment of giant molluscum
Dermatol 1999;16:395-7. contagiosum in a patient with human immunode-
31. Maronn M, Salm C, Lyon V, Galbraith S. One-year ficiency virus. Arch Dermatol 2011;147:652-4.
experience with candida antigen immunothe- 44. Zabawski EJ Jr, Cockerell CJ. Topical cidofovir for
rapy for warts and molluscum. Pediatr Dermatol molluscum contagiosum in children. Pediatr Der-
2008;25:189-92. matol 1999;16:414-5.
32. Garrett SJ, Robinson JK, Roenigk HH Jr. Trichlo- 45. Nehal KS, Sarnoff DS, Gotkin RH, Friedman-Kien A.
roacetic acid peel of molluscum contagiosum in Pulsed dye laser treatment of molluscum conta-
immunocompromised patients. J Dermatol Surg giosum in a patient with acquired immunodefi-
Oncol 1992;18:855-8. ciency syndrome. Dermatol Surg 1998;24:533-5.
ACTA DERMATOVENEROLOGICA CROATICA
135
Trko et al. Acta Dermatovenerol Croat
Patients with molluscum contagiosum 2016;24(2):130-136
46. Moiin A. Photodynamic therapy for molluscum 55. Osio A, Deslandes E, Saada V, Morel P, Guibal F.
contagiosum infection in HIV-coinfected patients: Clinical characteristics of molluscum contagiosum
review of 6 patients. J Drugs Dermatol 2003;2:637- in children in a private dermatology practice in
9. the greater Paris area, France: a prospective study
47. Hanson D, Diven DG. Molluscum contagiosum. in 661 patients. Dermatology 2011;222:314-20.
Dermatol Online J. 2003;9:2. 56. Seize MB, Ianhez M, Cestari Sda C. A study of the
48. Dohil MA, Lin P, Lee J, Lucky AW, aller AS, Eic- correlation between molluscum contagiosum
henfield LF. The epidemiology of molluscum and atopic dermatitis in children. An Bras Der-
contagiosum in children. J Am Acad Dermatol matol 2011;86:663-8.
2006;54:47-54. 57. Matis WL, Triana A, Shapiro R, Eldred L, Polk BF,
49. Koning S, Bruijnzeels MA, van Suijlekom-Smit Hood AF. Dermatologic findings associated with
human immunodeficiency virus infection. J Am
LW, van der Wouden JC. Molluscum contagio-
Acad Dermatol 1987;17:746-51.
sum in Dutch general practice. Br J Gen Pract
1994;44:417-9. 58. Koopman RJ, van Merrinboer FC, Vreden SG,
Dolmans WM. Molluscum contagiosum; a mar-
50. Becker TM, Blount JH, Douglas J, Judson FN. Trends
ker for advanced HIV infection. Br J Dermatol
in molluscum contagiosum in the United States,
1992;126:528-9.
1966-1983. Sex Transm Dis 1986;13:88-92.
59. Thompson CH, de Zwart-Steffe RT, Donovan B.
51. Lewis EJ, Lam M. An update on molluscum conta-
Clinical and molecular aspects of molluscum con-
giosum. Cutis 1997;60:29-34.
tagiosum infection in HIV-1 positive patients. Int J
52. Postlethwaite R, Watt JA, Hawley TG, Simpson I, STD AIDS 1992;3:101-6.
Adam H. Features of molluscum contagiosum in
60. Skerlev M, Husar K. Mollusca Contagiosa. In Gross
the north-east of Scotland and in Fijian village G, Tyring SK, editors. Sexually transmitted infec-
settlements. J Hyg 1967;65:281-91. tions and sexually transmitted diseases. 1st ed.
53. Oriel JD. The increase in molluscum contagiosum. Berlin/New York:Springer; 2011. pp. 547-52.
Br Med J (Clin Res Ed) 1987;294:74. 61. Monteagudo B, Cabanillas M, Acevedo A, de las-
54. Kakourou T, Zachariades A, Anastasiou T, Architec- Heras C, Surez-Amor O, Ramrez-Santos A, et al.
tonidou E, Georgala S, Theodoridou M. Molluscum Curettage for the treatment of molluscum conta-
contagiosum in Greek children: a case series. Int J giosum: a descriptive study. Actas Dermosifiliogr
Dermatol 2005;44:221-3. 2011;102:157-8.
ACTA DERMATOVENEROLOGICA CROATICA
136
Potrebbero piacerti anche
- 4 Male and Female Reproductive Organs PPTDocumento71 pagine4 Male and Female Reproductive Organs PPTDanica Marie Sibay100% (2)
- Delivery Room RequirementsDocumento12 pagineDelivery Room RequirementsHannah Ros Quitorio IINessuna valutazione finora
- Virtual Pig Dissection Worksheet 06-07 2Documento6 pagineVirtual Pig Dissection Worksheet 06-07 2api-255054890Nessuna valutazione finora
- Organizing Nursing Service and Patient CareDocumento32 pagineOrganizing Nursing Service and Patient CareGrashia100% (2)
- Reaction Paper The Physician OutlineDocumento3 pagineReaction Paper The Physician OutlinePhoebe SuboNessuna valutazione finora
- 3) A Comparative Study of Topical Cantharidin and Intralesional PPD To Treat Molluscum ContagiosumDocumento6 pagine3) A Comparative Study of Topical Cantharidin and Intralesional PPD To Treat Molluscum ContagiosumFathia KhattabNessuna valutazione finora
- Extrapulmonary Infections Associated With Nontuberculous Mycobacteria in Immunocompetent PersonsDocumento9 pagineExtrapulmonary Infections Associated With Nontuberculous Mycobacteria in Immunocompetent Personsrajesh Kumar dixitNessuna valutazione finora
- Cryptococcal Infection in Plhiv: Series of 12 Cases at The Laboratory of Mohammed Vi University Hospital of MarrakechDocumento6 pagineCryptococcal Infection in Plhiv: Series of 12 Cases at The Laboratory of Mohammed Vi University Hospital of MarrakechIJAR JOURNALNessuna valutazione finora
- Factors Associated with Invasive Fungal Infections in Multiple Myeloma PatientsDocumento4 pagineFactors Associated with Invasive Fungal Infections in Multiple Myeloma PatientsMario ChristopherNessuna valutazione finora
- Saini 2016 Calci NoseDocumento5 pagineSaini 2016 Calci NoseRym MesfarNessuna valutazione finora
- International Journal of Pediatric OtorhinolaryngologyDocumento7 pagineInternational Journal of Pediatric OtorhinolaryngologyAndi SuhriyanaNessuna valutazione finora
- Dok Til 1Documento15 pagineDok Til 1Nurul Ulya RahimNessuna valutazione finora
- Thrombosis Research: Full Length ArticleDocumento6 pagineThrombosis Research: Full Length ArticleCarol GomezNessuna valutazione finora
- BAPoma SkinDocumento13 pagineBAPoma SkinShriniwas Rushi100% (1)
- DKX 445Documento12 pagineDKX 445Aayush SinghNessuna valutazione finora
- Jurnal Ing MK 4Documento10 pagineJurnal Ing MK 4Iffah AlhikmahNessuna valutazione finora
- Treatment of Vitiligo With Narrow-Band UVB and Topical Gel Containing Catalase and Superoxide DismutaseDocumento5 pagineTreatment of Vitiligo With Narrow-Band UVB and Topical Gel Containing Catalase and Superoxide DismutaseBen IntosiusNessuna valutazione finora
- Jurnal KedokteranDocumento9 pagineJurnal KedokteranannisanoviaNessuna valutazione finora
- Nontüberküloz Lenf AdenitDocumento7 pagineNontüberküloz Lenf AdenitDogukan DemirNessuna valutazione finora
- Mucormycosis in Children: A Study of 22 Cases in A Mexican HospitalDocumento6 pagineMucormycosis in Children: A Study of 22 Cases in A Mexican Hospitalandrianiputri916Nessuna valutazione finora
- Covid 19 InfectionDocumento2 pagineCovid 19 InfectionGheavita Chandra DewiNessuna valutazione finora
- Venous and Arterial Thromboembolic Complications in COVID-19 PatientsDocumento6 pagineVenous and Arterial Thromboembolic Complications in COVID-19 PatientsWilliam MilesNessuna valutazione finora
- Risk Factor TaiwanDocumento5 pagineRisk Factor TaiwanMira ApriliaNessuna valutazione finora
- Lander HPV 2012Documento4 pagineLander HPV 2012Christian Yohan Sierra HurtadoNessuna valutazione finora
- A Study of Thrombocytopenia in Malaria and Its Prognostic SignificanceDocumento6 pagineA Study of Thrombocytopenia in Malaria and Its Prognostic SignificanceBobby Faisyal RakhmanNessuna valutazione finora
- Puig 2003Documento5 paginePuig 2003Sơn Chu HồngNessuna valutazione finora
- CDC Repeat Syphilis Infection and HIV Coinfection Among Men Who Have SexDocumento16 pagineCDC Repeat Syphilis Infection and HIV Coinfection Among Men Who Have SexkikiNessuna valutazione finora
- Profile of Tinea Corporis and Tinea Cruris in Dermatovenereology Clinic of Tertiery Hospital: A Retrospective StudyDocumento6 pagineProfile of Tinea Corporis and Tinea Cruris in Dermatovenereology Clinic of Tertiery Hospital: A Retrospective StudyRose ParkNessuna valutazione finora
- A Retrospective Study of Tuberculosis Prevalen - 2020 - Journal of Clinical TubeDocumento5 pagineA Retrospective Study of Tuberculosis Prevalen - 2020 - Journal of Clinical TubeKarina ChristantoNessuna valutazione finora
- s41416 023 02233 XDocumento7 pagines41416 023 02233 Xevita.irmayantiNessuna valutazione finora
- Non-Tuberculous Mycobacterial Lymphadenitis inDocumento4 pagineNon-Tuberculous Mycobacterial Lymphadenitis inAbdur Rachman Ba'abdullahNessuna valutazione finora
- Profile of tinea corporis and tinea cruris casesDocumento6 pagineProfile of tinea corporis and tinea cruris casesMarwiyahNessuna valutazione finora
- Journal TB Kulit PDFDocumento4 pagineJournal TB Kulit PDFdrmarhuNessuna valutazione finora
- Diţescu D Et Al 2021Documento15 pagineDiţescu D Et Al 2021ayasofiavrNessuna valutazione finora
- HPV E6E7 mRNA 1173 PatientDocumento9 pagineHPV E6E7 mRNA 1173 PatienthippopotamasNessuna valutazione finora
- Cost Utility of Vaccination Against COVID-19 in BrazilDocumento8 pagineCost Utility of Vaccination Against COVID-19 in BrazilTatiana DutraNessuna valutazione finora
- Int J Lab Hematology - 2017 - Galtseva - Minimal residual disease in multiple myeloma Benefits of flow cytometryDocumento9 pagineInt J Lab Hematology - 2017 - Galtseva - Minimal residual disease in multiple myeloma Benefits of flow cytometrymatanhibNessuna valutazione finora
- Duygu 2020Documento4 pagineDuygu 2020Aditya HendraNessuna valutazione finora
- Case Anemia Thalasemia FixDocumento4 pagineCase Anemia Thalasemia FixmasranraisaNessuna valutazione finora
- Conquering Your Pastendorsement Requirements Toward A TransparencyBased Mark of The MentalxseyjDocumento2 pagineConquering Your Pastendorsement Requirements Toward A TransparencyBased Mark of The Mentalxseyjraysea40Nessuna valutazione finora
- Bacterial and Fungal Coinfections in Covid 19 Patients Hospitalized During The New York City Pandemic SurgeDocumento5 pagineBacterial and Fungal Coinfections in Covid 19 Patients Hospitalized During The New York City Pandemic SurgeReynaldo CarvajalNessuna valutazione finora
- Article 1648703466Documento6 pagineArticle 1648703466Regla Mailyn Perez PerezNessuna valutazione finora
- Cutaneous TB in NigerDocumento4 pagineCutaneous TB in NigerRizky ErizkaNessuna valutazione finora
- Evaluation of Torch Infections and Specific Humoral Responses Against HSV in Patients With Nonsegmental VitiligoDocumento5 pagineEvaluation of Torch Infections and Specific Humoral Responses Against HSV in Patients With Nonsegmental VitiligoCentral Asian StudiesNessuna valutazione finora
- Jurnal 3 MoluskumDocumento2 pagineJurnal 3 MoluskumFausiah Ulva MNessuna valutazione finora
- Patterns of Adjuvant Treatment For Endometrial Cancer: The Experience of A Single Institution in MoroccoDocumento8 paginePatterns of Adjuvant Treatment For Endometrial Cancer: The Experience of A Single Institution in MoroccoIJAR JOURNALNessuna valutazione finora
- COVID-19 in Patients With LymphoproliferativeDocumento4 pagineCOVID-19 in Patients With LymphoproliferativeRaquel Sant'AnnaNessuna valutazione finora
- Clinical Features and CD4+ T Cells Count in AIDS Patients With CMV Retinitis - Correlation With MortalityDocumento6 pagineClinical Features and CD4+ T Cells Count in AIDS Patients With CMV Retinitis - Correlation With MortalitysyenadamaraNessuna valutazione finora
- Out 3Documento8 pagineOut 3FanialiahsaniNessuna valutazione finora
- Skin Disorder PBCDocumento6 pagineSkin Disorder PBCRatusweethella Intan Yudagrahania PuspitaNessuna valutazione finora
- Deep Neck Infections: Continuing Burden in Developing World: Original ArticleDocumento4 pagineDeep Neck Infections: Continuing Burden in Developing World: Original ArticlevaNessuna valutazione finora
- Le Cadet 2019Documento6 pagineLe Cadet 2019supaidi97Nessuna valutazione finora
- Epidemiology and Factors Related To The Survival of Metastatic Kidney Cancers: Retrospective Study at The Mohamed VI Center For The Cancer Treatment in Casablanca, MoroccoDocumento5 pagineEpidemiology and Factors Related To The Survival of Metastatic Kidney Cancers: Retrospective Study at The Mohamed VI Center For The Cancer Treatment in Casablanca, MoroccoInternational Journal of Innovative Science and Research TechnologyNessuna valutazione finora
- JDV 36 6Documento5 pagineJDV 36 6NandaNessuna valutazione finora
- Biomedicines 09 00171 v2Documento33 pagineBiomedicines 09 00171 v2farihNessuna valutazione finora
- Review Article: Profile of Corneal Ulcer in A Tertiary Eye Care CentreDocumento6 pagineReview Article: Profile of Corneal Ulcer in A Tertiary Eye Care CentreNadhila ByantNessuna valutazione finora
- Pi Is 1083879118313909Documento2 paginePi Is 1083879118313909Ljc JaslinNessuna valutazione finora
- International Journal of Infectious DiseasesDocumento8 pagineInternational Journal of Infectious DiseasesTiti IndasariNessuna valutazione finora
- Exploring vismodegig A non-surgical breakthrough in the management of advanced periocular basal cell carcinomaDocumento11 pagineExploring vismodegig A non-surgical breakthrough in the management of advanced periocular basal cell carcinomaGeorgios LavasidisNessuna valutazione finora
- Pathomorphological Peculiarities of Tuberculous Meningoencephalitis Associated With HIV InfectionDocumento6 paginePathomorphological Peculiarities of Tuberculous Meningoencephalitis Associated With HIV InfectionBesseMarwah AgusHusainNessuna valutazione finora
- CT Manifestations of Coronavirus Disease 2019 A RDocumento5 pagineCT Manifestations of Coronavirus Disease 2019 A ReugeniaNessuna valutazione finora
- De PrintatDocumento24 pagineDe PrintatDJ ATBNessuna valutazione finora
- Hodgkin Lymphoma: A Comprehensive OverviewDa EverandHodgkin Lymphoma: A Comprehensive OverviewAndreas EngertNessuna valutazione finora
- COVID-19 Mortality Review in Malaysia & Updates on Clinical Management of COVID-19Da EverandCOVID-19 Mortality Review in Malaysia & Updates on Clinical Management of COVID-19Nessuna valutazione finora
- Fast Facts: Blastic Plasmacytoid Dendritic Cell Neoplasm: Shedding light on a rare diseaseDa EverandFast Facts: Blastic Plasmacytoid Dendritic Cell Neoplasm: Shedding light on a rare diseaseNessuna valutazione finora
- Updated rhinitis diagnosis and management guidelinesDocumento84 pagineUpdated rhinitis diagnosis and management guidelinesdarkreign86Nessuna valutazione finora
- Diseases Pictures: SyringomaDocumento4 pagineDiseases Pictures: Syringoma79lalalaNessuna valutazione finora
- Diseases Pictures: SyringomaDocumento4 pagineDiseases Pictures: Syringoma79lalalaNessuna valutazione finora
- Molluscum Contagiosum: TweetsDocumento1 paginaMolluscum Contagiosum: Tweets79lalalaNessuna valutazione finora
- BR J Ophthalmol 2001 DUA 1379 83Documento5 pagineBR J Ophthalmol 2001 DUA 1379 8379lalalaNessuna valutazione finora
- Rhinitis Clasification PDFDocumento6 pagineRhinitis Clasification PDF79lalalaNessuna valutazione finora
- EPOS Guide to RhinosinusitisDocumento20 pagineEPOS Guide to RhinosinusitisMargaretha Syane LientunganNessuna valutazione finora
- PCH 32 s1 039 PDFDocumento4 paginePCH 32 s1 039 PDF79lalalaNessuna valutazione finora
- Chronic Rhinosinusitis 2012Documento305 pagineChronic Rhinosinusitis 2012Sorina StoianNessuna valutazione finora
- Molluscum Contagiosum:: What's New and True?Documento6 pagineMolluscum Contagiosum:: What's New and True?79lalalaNessuna valutazione finora
- Aria Rhinitis Allergic Pocket GuideDocumento8 pagineAria Rhinitis Allergic Pocket GuideAlfani FajarNessuna valutazione finora
- Clasification of Non Allergic Rhinitis Syndrome PDFDocumento4 pagineClasification of Non Allergic Rhinitis Syndrome PDF79lalalaNessuna valutazione finora
- Ophthalmic Surgery May 1988 19, 5 Medical DatabaseDocumento3 pagineOphthalmic Surgery May 1988 19, 5 Medical Database79lalalaNessuna valutazione finora
- Management of External Genital WartsDocumento7 pagineManagement of External Genital Warts79lalalaNessuna valutazione finora
- Dermatology Online Journal: 1/13/2017 Molluscum Contagiosum (Escholarship)Documento5 pagineDermatology Online Journal: 1/13/2017 Molluscum Contagiosum (Escholarship)79lalalaNessuna valutazione finora
- 18Documento4 pagine1879lalalaNessuna valutazione finora
- References: (Medline)Documento1 paginaReferences: (Medline)79lalalaNessuna valutazione finora
- 8-Hal 1-3Documento3 pagine8-Hal 1-379lalalaNessuna valutazione finora
- Molluscum Contagiosum: TweetsDocumento1 paginaMolluscum Contagiosum: Tweets79lalalaNessuna valutazione finora
- Molluscum Contagiosum Causes and SymptomsDocumento1 paginaMolluscum Contagiosum Causes and Symptoms79lalalaNessuna valutazione finora
- Molluscum Contagiosum Causes and SymptomsDocumento1 paginaMolluscum Contagiosum Causes and Symptoms79lalalaNessuna valutazione finora
- Treatment of Molluscum Contagiosum With Cantharidin: A Practical ApproachDocumento7 pagineTreatment of Molluscum Contagiosum With Cantharidin: A Practical Approach79lalalaNessuna valutazione finora
- Molluscum Contagiosum Causes and SymptomsDocumento1 paginaMolluscum Contagiosum Causes and Symptoms79lalalaNessuna valutazione finora
- Molluscum Contagiosum Causes and SymptomsDocumento1 paginaMolluscum Contagiosum Causes and Symptoms79lalalaNessuna valutazione finora
- C R o W E, M A R K A - Moluscum Contagiosum. Diakses Dari: - Pada Tanggal 27 Desember 2016Documento22 pagineC R o W E, M A R K A - Moluscum Contagiosum. Diakses Dari: - Pada Tanggal 27 Desember 201679lalalaNessuna valutazione finora
- Diseases Pictures: SyringomaDocumento3 pagineDiseases Pictures: Syringoma79lalalaNessuna valutazione finora
- Molluscum Contagiosum Causes and SymptomsDocumento1 paginaMolluscum Contagiosum Causes and Symptoms79lalalaNessuna valutazione finora
- References: Dermatol. 1989 Jun. 28 (5) :351-2Documento2 pagineReferences: Dermatol. 1989 Jun. 28 (5) :351-279lalalaNessuna valutazione finora
- References: Dermatol. 2008 Sep-Oct. 25 (5) :553-6Documento3 pagineReferences: Dermatol. 2008 Sep-Oct. 25 (5) :553-679lalalaNessuna valutazione finora
- Surekha. Molluscum Contagiosum. Diakses Dari: - Pada Tanggal 13 Januari 2017 Molluscum ContagiosumDocumento2 pagineSurekha. Molluscum Contagiosum. Diakses Dari: - Pada Tanggal 13 Januari 2017 Molluscum Contagiosum79lalalaNessuna valutazione finora
- Guidelines For Fcps-I (Medicine)Documento3 pagineGuidelines For Fcps-I (Medicine)Israr Ul HaqNessuna valutazione finora
- Married Women of Reproductive AgeDocumento26 pagineMarried Women of Reproductive Agelibagon rhuNessuna valutazione finora
- Maternal Child Health (MCH)Documento49 pagineMaternal Child Health (MCH)Dr. Rakshit SolankiNessuna valutazione finora
- Ventouse - FinalDocumento10 pagineVentouse - FinalJasmine KaurNessuna valutazione finora
- Medical School Notes: LabourDocumento5 pagineMedical School Notes: LabourKatherine NunnNessuna valutazione finora
- Pre Employment Occupational Health FormDocumento7 paginePre Employment Occupational Health Formlinks2309Nessuna valutazione finora
- Geraldine Gabriel: Work ExperienceDocumento2 pagineGeraldine Gabriel: Work ExperienceSiddharth NelsonNessuna valutazione finora
- Curriculum Vitae DR Andrea GilkisonDocumento5 pagineCurriculum Vitae DR Andrea GilkisonysNessuna valutazione finora
- Basic Splinting TechniquesDocumento5 pagineBasic Splinting TechniquesRicardo BragaNessuna valutazione finora
- Invaginasi Jurnal2 PDFDocumento4 pagineInvaginasi Jurnal2 PDFZieky YoansyahNessuna valutazione finora
- Neonatal JaundiceDocumento4 pagineNeonatal JaundiceChristian Eduard de DiosNessuna valutazione finora
- DR Asia ResumeDocumento2 pagineDR Asia ResumeAasiya TarannumNessuna valutazione finora
- Peptic Ulcer Disease (PUD) EditedDocumento9 paginePeptic Ulcer Disease (PUD) EditedRashed ShatnawiNessuna valutazione finora
- Operational Guidelines RBSKDocumento43 pagineOperational Guidelines RBSKanunad100% (1)
- SterilizationDocumento6 pagineSterilizationMaria Ella Joanne DapitonNessuna valutazione finora
- ControlDocumento12 pagineControlSirVietaNessuna valutazione finora
- Social Determinants of HealthDocumento2 pagineSocial Determinants of HealthBrían GohNessuna valutazione finora
- Shetty Darshana NileshDocumento1 paginaShetty Darshana NileshBipin JenaNessuna valutazione finora
- Asymptomatic Bacterial Vaginosis and Intermediate Flora As Risk Factors For Adverse Pregnancy OutcomeDocumento16 pagineAsymptomatic Bacterial Vaginosis and Intermediate Flora As Risk Factors For Adverse Pregnancy OutcomealvilNessuna valutazione finora
- Case Study A SungDocumento1 paginaCase Study A SungSharon Williams100% (1)
- Differentiate Between With AnswersDocumento14 pagineDifferentiate Between With AnswersStephy SojanNessuna valutazione finora
- Recent Advancement in Contraceptive TechnologyDocumento3 pagineRecent Advancement in Contraceptive Technologyjeelani saima83% (6)
- K-Klinis Dokter ObsgynDocumento6 pagineK-Klinis Dokter Obsgynadyat89Nessuna valutazione finora
- @MedicalBooksStore 2015 Office Based PDFDocumento237 pagine@MedicalBooksStore 2015 Office Based PDFGalih WicaksonoNessuna valutazione finora
- Aio Vol 1 Issue 1 Sep 2013Documento56 pagineAio Vol 1 Issue 1 Sep 2013aiojournalNessuna valutazione finora