Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Effects of Dietary Addition of Cellulase and A Saccharomyces Cerevisiae Fermentation Product On Nutrient Digestibility, Rumen Fermentation and Enteric Methane Emissions in Growing Goats
Caricato da
MaulitaTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Effects of Dietary Addition of Cellulase and A Saccharomyces Cerevisiae Fermentation Product On Nutrient Digestibility, Rumen Fermentation and Enteric Methane Emissions in Growing Goats
Caricato da
MaulitaCopyright:
Formati disponibili
Archives of Animal Nutrition
ISSN: 1745-039X (Print) 1477-2817 (Online) Journal homepage: http://www.tandfonline.com/loi/gaan20
Effects of dietary addition of cellulase and a
Saccharomyces cerevisiae fermentation product
on nutrient digestibility, rumen fermentation and
enteric methane emissions in growing goats
Qi Lu, Jian Wu, Min Wang, Chuanshe Zhou, Xuefeng Han, Edwin Nicholas
Odongo, Zhiliang Tan & Shaoxun Tang
To cite this article: Qi Lu, Jian Wu, Min Wang, Chuanshe Zhou, Xuefeng Han, Edwin Nicholas
Odongo, Zhiliang Tan & Shaoxun Tang (2016) Effects of dietary addition of cellulase and a
Saccharomyces cerevisiae fermentation product on nutrient digestibility, rumen fermentation
and enteric methane emissions in growing goats, Archives of Animal Nutrition, 70:3, 224-238,
DOI: 10.1080/1745039X.2016.1163002
To link to this article: http://dx.doi.org/10.1080/1745039X.2016.1163002
Published online: 31 Mar 2016.
Submit your article to this journal
Article views: 7
View related articles
View Crossmark data
Full Terms & Conditions of access and use can be found at
http://www.tandfonline.com/action/journalInformation?journalCode=gaan20
Download by: [University of California, San Diego]
Date: 04 April 2016, At: 14:57
ARCHIVES OF ANIMAL NUTRITION, 2016
VOL. 70, NO. 3, 224238
http://dx.doi.org/10.1080/1745039X.2016.1163002
Eects of dietary addition of cellulase and a Saccharomyces
cerevisiae fermentation product on nutrient digestibility,
rumen fermentation and enteric methane emissions in
growing goats
Downloaded by [University of California, San Diego] at 14:57 04 April 2016
Qi Lua,b, Jian Wua,b, Min Wanga, Chuanshe Zhoua, Xuefeng Hana,
Edwin Nicholas Odongoc, Zhiliang Tana and Shaoxun Tanga
a
Key Laboratory for Agro-Ecological Processes in Subtropical Region, and South-Central Experimental
Station of Animal Nutrition and Feed Science in Ministry of Agriculture, Institute of Subtropical Agriculture,
The Chinese Academy of Sciences, Changsha, Hunan, P.R. China; bCollege of Resources and Environment,
University of Chinese Academy of Sciences, Beijing, P. R. China; cAnimal Production and Health Section,
Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture, International Atomic Energy
Agency, Vienna, Austria
ABSTRACT
ARTICLE HISTORY
This study was designed to assess the eectiveness of dietary
cellulase (243 U/g, derived from Neocallimastix patriciarum) and a
Saccharomyces cerevisiae fermentation product (yeast product) on
ruminal fermentation characteristics, enteric methane (CH4) emissions and methanogenic community in growing goats. The experiment was conducted in a 5 5 Latin square design using ve
Xiangdong black wether goats. The treatments included a Control
and two levels of cellulase (0.8 g and 1.6 g/kg dry matter intake
(DMI), i.e. 194 U/kg and 389 U/kg DMI, respectively) crossed over
with two levels (6 g or 12 g/kg DMI) of the yeast product. There
were no signicant dierences regarding feed intake, apparent
digestibility of organic matter, neutral detergent bre and acid
detergent bre among all the treatments. In comparison with the
Control, the ruminal ammonia N concentration was decreased
(p = 0.001) by cellulase and yeast product addition. The activities
of carboxymethylcellulase and xylanase were decreased after cellulase addition. Moreover, dietary cellulase and yeast product
addition led to a signicant reduction (p < 0.05) of enteric CH4
emissions although the diversity and copy numbers of methanogens among treatments were not dissimilar. The present results
indicate that the combination of cellulase and yeast fermentation
product can reduce the production of CH4 energy and mitigate
the enteric CH4 emissions to a certain degree.
Received 23 September 2015
Accepted 2 March 2016
KEYWORDS
cellulase; digestibility; feed
supplements; methane
production; ruminants; yeast
1. Introduction
Methanogenesis is physiologically essential in the rumen to avoid hydrogen accumulation; otherwise excess hydrogen pressure leads to an inhibition of dehydrogenase
activity, further resulting in NADH accumulation, which is negative for rumen fermentation (Wang et al. 2014). The enteric methane (CH4) emissions from livestock
CONTACT Shaoxun Tang
shaoxuntang@163.com
2016 Informa UK Limited, trading as Taylor & Francis Group
Downloaded by [University of California, San Diego] at 14:57 04 April 2016
ARCHIVES OF ANIMAL NUTRITION
225
agriculture contribute to about 9% of global anthropogenic greenhouse gas emissions
(Gill et al. 2010) and the energy loss associated with enteric CH4 emissions represents
about 59% of GE (Martin et al. 2010; Buddle et al. 2011). Thus, the strategies on
enteric CH4 mitigation have already been a hot area of research.
As reported by Arriola et al. (2011), brolytic enzyme application decreases the
estimated CH4 production in dairy cows, but increases apparent total tract digestibility
(ATTD) of acid detergent bre (ADF) and neutral detergent bre (NDF). Moreover,
our group has evaluated the eects of cellulase (derived from Neocallimastix patriciarum) on ruminal fermentation and CH4 production in vitro (Tang et al. 2013) and in
vivo (Lu et al. 2015). The results revealed that cellulase inclusion decreases CH4
production of roughages and improves dry matter (DM) digestibility in vitro, whereas
the enteric CH4 emissions corrected by ADF intake tended to decline after addition of
cellulase to diets of growing goats.
On the other hand, Hristov et al. (2010) have pointed out that the addition of a yeast
fermentation product can decrease the CH4 emissions from manure to a certain extent,
although the enteric CH4 production was not aected. Nevertheless, Newbold and Rode
(2006) have proposed that the ruminal methanogenesis can be diminished by addition
of a yeast product. In a study of Mwenya et al. (2004), the application of a yeast product
derived from T. sericeum to diets of sheep resulted in a decline of enteric CH4
emissions.
Simultaneously, Tang et al. (2008) have demonstrated that the combination of a yeast
product and brolytic enzymes can improve the in vitro fermentation of low-quality
cereal straws. Furthermore, a signicant interaction on in vitro DM and organic matter
(OM) disappearances occurs when a yeast fermentation product (Diamond V Mills,
Inc., Cedar Rapids, IA) and a concentrated mixture of brolytic enzymes (Yingheng
Biotech Ltd, Guangdong Province, China; containing 13,000 units of hydroxyethyl
cellulase and 2100 units xylanase per gram) were applicated at 5.0 g and 7.5 g per kg
of fermented straws, respectively. On the other hand, Lu et al. (2015) have also been
cognizant of the signicant variations between in vitro and in vivo ndings. On the
basis of these ndings, this study was designed to assess the combinative eectiveness of
cellulase (derived from Neocallimastix patriciarum) and a yeast fermentation product
(derived from Saccharomyces cerevisiae) on ruminal fermentation characteristics,
enteric CH4 emissions and methanogenic community diversity in vivo in growing goats.
2. Materials and methods
2.1. Animals, diets and treatments
All procedures involving animals were approved by the Animal Care and Use
Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences,
Changsha, China.
Five Xiangdong black growing wether goats (a local breed in the south of China,
19.7 1.3 kg body weight (BW)) were assigned to a 5 5 Latin square design. All goats
were individually housed in metabolic cages and had free access to fresh water. The
experimental diets were formulated to meet 1.3 times the maintenance requirement of
metabolisable energy (ME) as suggested by Lu and Zhang (1996). The dietary
226
Q. LU ET AL.
Table 1. Ingredients and composition of experimental diet.
Downloaded by [University of California, San Diego] at 14:57 04 April 2016
Contents
Ingredients [g/kg DM]
Rice straw
Corn meal
Fat powder
Soybean meal
NaCl
Premix*
Composition (analysed)
Organic matter [g/kg DM]
Crude protein [g/kg DM]
Calcium [g/kg DM]
Phosphorus [g/kg DM]
Neutral detergent bre [g/kg DM]
Acid detergent bre [g/kg DM]
Metabolisable energy [MJ/kg]
55.0
31.1
2.14
9.90
0.86
1.00
922
85
2.4
1.7
502
279
8.86
Notes: DM, dry matter; *premix composition per kg diet: 68 mg
FeSO4 H2O, 44 mg CuSO4 5H2O, 411 g CoCl2 6H2O,
1.70 mg KIO3, 211 mg MnSO4 H2O, 126 mg ZnSO4 H2O,
56 g NaSeO3, 462 mg MgSO4 7H2O, 737 IU vitamin A,
8.29 mg vitamin E, 4.0 g NaHCO3, 5.1 g carrier; Calculated
according to Lu and Zhang (1996).
ingredients and nutrient composition are given in Table 1. The diets (rice straw, 55%;
concentrate, 45%) were equally oered twice at 08:00 h and 18:00 h at a daily rate of
600 g DM per goat. Before producing the nal diets, the cellulase and the yeast
fermentation product from S. cerevisiae were evenly mixed with the concentrate.
Even though feed refusals were minimal during the sampling phase, orts and feed
intakes were recorded daily.
Treatments included a Control and two supplementation levels of cellulase crossed
over with two levels of a yeast fermentation product from S. cerevisiae. The cellulase
derived from N. patriciarum (puried powder with an activity of 243 U/g; Hunan
Youtell Biochemical., Ltd. Yueyang, China) and was applicated at 0.8 and 1.6 g/kg DM
intake (DMI), i.e., 194 U/kg and 389 U/kg DMI (treatments CEL0.8 and CEL1.6,
respectively). The yeast fermentation product (following named as yeast product)
was derived from S. cerevisiae (solid powder, Diamond V Mills, Inc. Cedar Rapids, IA),
and a dehydrated and inactivated S. cerevisiae fermentation batch containing all soluble
metabolites of yeast fermentation which was produced under a controlled production
process. The yeast product was fed at 6.0 and 12 g/kg DMI (treatments YP6 and YP12,
respectively). The kind of cellulase and yeast product including their dose rates was
selected according to our previous experimental results (Tang et al. 2008, 2013; Lu et al.
2015). The cellulase activity was assayed according to the procedures as described by
Colombatto and Beauchemin (2003).
2.2. Experimental design and sampling
The experiment included ve periods each lasting 16 d. In every period, days 18 were used
for dietary adaptation and days 916 were used for sample collection. Samples of diets, orts,
faeces and urine were collected from days 912. The goats were kept in metabolic cages
Downloaded by [University of California, San Diego] at 14:57 04 April 2016
ARCHIVES OF ANIMAL NUTRITION
227
which enabled the separation of urine from faeces. Faeces and urine were collected from
goats twice daily before feeding (08:00 h and 18:00 h). Samples of the diets were collected at
feeding and were frozen (20C) until analysis. Orts were weighed, recorded and sampled
daily for each goat. Faeces were weighed daily and subsamples (10% of total weight) were
frozen at -20C for subsequent analyses of DM, OM, NDF, ADF and energy. Further
subsamples (2% of total weight) were acidied with 10% sulphuric acid (H2SO4) and stored
at 20C for nitrogen (N) determination. For each goat, the total volume of urine was
recorded daily. A 2% urine subsample was acidied with 10% H2SO4 and stored at 20C
for N determination. Meanwhile, a 10% subsample was taken, in which 10% H2SO4 was
added to keep pH less than 3.0 for the determination of N and energy. Within each period,
the subsamples of faeces and urine were pooled by goat.
CH4 emissions were sampled from days 13 to 15, one day for adaptation and two
consecutive days for sampling. For measurement, all goats were moved into ve
respiratory metabolism chambers as described by Verstegen et al. (1987).
Temperature was maintained at 15C with 70% relative humidity. Simultaneously,
ventilation rate was set at 45 m3/h. CH4 emissions were measured per chamber in
15-min intervals as described in detail by Verstegen et al. (1987).
After determination of gaseous exchange in the respiratory metabolism chambers, all
goats were moved back to the metabolic cages. On day 16, samples of rumen uid
(50 ml) were collected at 0, 2, 4, 6 and 8 h after the morning feeding using a stomach
tube. For each goat, a representative sample of each collection time was stored immediately at 80C for DNA extraction. Then, all the ruminal uid samples from all time
points of one goat were combined. A portion of 20 ml ruminal uid was then ltrated
through four layers of cheesecloth, whilst pH value was immediately determined with a
pH meter (Model PHS-3 C, Shanghai Precision Science Instrument Co., LTD, China).
A portion of this strained ruminal uid (2 ml) was stored at 20C for ammonia-N
(NH3-N) analysis. Thereafter, a second aliquot of ltrated ruminal uid (2 ml) was
centrifuged (15,000g for 10 min). The supernatant uid was acidied with 25% (w/v)
metaphosphoric acid at a ratio of 10:1, homogenised and stored at 20C for analysis of
volatile fatty acids (VFA). Another portion of 25 ml ruminal uid was mixed with 0.1 M
citrate phosphate buer (pH = 6.6) at a ratio of 1:1. Then, the mixture was rubbed
evenly at 39C and squeezed through four layers of cheesecloth. This straining uid was
frozen (20C) for analysis of ruminal enzymatic activity.
2.3. Chemical analysis
The pooled fresh samples of faeces, diets and orts were dried in a forced-air oven at 65
C for 72 h for analyses of DM, OM, NDF, ADF and N. DM for all samples was
determined by oven-drying at 105C for 24 h. OM content was determined by combustion of samples at 550C for 8 h in a mue furnace according to the methods of AOAC
(2002). NDF and ADF were measured according to the procedures of Van Soest et al.
(1991). N was determined by using a ow injection analyser (FIAstar 5000, FOSS,
Sweden). The amounts of ingested N (IN) [g/d], faecal N (FN) [g/d] and urinary N
(UN) [g/d] were used to calculate N balance as following:
Digested N (DN) [g/d] = IN FN,
228
Q. LU ET AL.
Downloaded by [University of California, San Diego] at 14:57 04 April 2016
Retained N (RN) [g/d] = IN FN UN,
Availability of retained N [%] = {(IN FN UN)/IN} 100%,
Availability of digested N [%] = {(IN FN UN)/(IN-FN)} 100%,
Apparent N digestibility [%] = {(IN FN)/IN} 100%.
Energy, including dietary gross energy (GE), faecal energy (FE) and urinary energy
(UE), was determined by an isothermal automatic calorimeter (5E-AC8018, Changsha
Kaiyuan Instruments Co., Ltd, China). Before UE analysis, a pretreatment was needed.
Briey, a quantitative portion of urine (10 ml) was dropped onto the lter paper and
dried at 65C. Thereafter, the dried lter paper was pressed into cylindrical pieces for
the measurement of urinary caloric value. Also, a lter paper without urine was
burned to correct the UE value. The CH4 energy, digested energy (DE) and metabolisable energy (ME) were calculated as following:
CH4 energy [MJ/d] = CH4 volume [l/d] 0.0395 [MJ/l], (Islam et al. 2000);
DE [MJ/kg DM] = {GE [MJ/d] FE [MJ/d]}/DMI [kg/d],
ME [MJ/kg DM] = {GE [MJ/d] FE [MJ/d] UE [MJ/d] CH4E [MJ/d]}/DMI
[kg/d].
The ruminal enzymatic activities, including extracellular carboxymethylcellulase
(CMCase) and xylanase and intracellular CMCase and xylanase, were determined by
the modied procedures described by Sun et al. (2007) and Jiao et al. (2014). Before the
analyses of enzymatic activities, the frozen ruminal samples needed a pretreatment.
Briey, the samples were thawed and centrifuged at 22,000g for 10 min at 4C. The
supernatants were kept for the measurements of extracellular CMCase and extracellular
xylanase. Meanwhile, the buer (0.1 M citrate phosphate, pH = 6.6) was transferred
into the sediment tubes at an amount equal to the supernatant taken out, and then
homogenised and centrifuged at 3750g for 10 min at 4C. The supernatant uid was
stored for the measurements of intracellular CMCase and xylanase. At the same time,
the total activity of CMCase or xylanase was expressed as the sum of extracellular
CMCase or xylanase and intracellular CMCase or xylanase, respectively. The enzymatic
activities were measured based on the absorbance of enzymatic reaction at 540 nm
using a spectrophotometer (UV-2450, Shimadzu, Japan). Glucose and xylose were the
standards for CMCase and xylanase, respectively. One IU was equal to the enzyme
activity that released 1 mol glucose or xylose per minute per ml of enzyme solution at
pH 6.6 and 39C.
The frozen samples for VFA analysis were thawed and centrifuged at 10,000g and 4C
for 15 min. The supernatants were analysed for VFA by gas chromatography (HP5890,
Agilent Technologies Co. Ltd, USA). NH3-N was determined by the procedures described
by Tang et al. (2013).
All ruminal uid samples (stored at 80C for DNA extraction) at ve sampling time
points per goat per period in each treatment were pooled into one representative
sample by taking 1 ml of each subsample. Twenty-ve representative samples (2 ml
per sample) were prepared, and then DNA was extracted using the QIAamp DNA Stool
Mini kit (QiagenGmbH, Hilden, Germany) according to the manufacturers instructions. The DNA was eluted with 50 l DNAase-free 1 diethylpyrocarbonate (DEPC)
Downloaded by [University of California, San Diego] at 14:57 04 April 2016
ARCHIVES OF ANIMAL NUTRITION
229
water and stored at 20C for further analysis. The DNA concentration was determined
by spectrophotometry (NanoDrop Technologies Inc., DE, USA).
The 16S rRNA gene amplication of methanogens was carried out using the universal
primer pair Ar109f (5-ACG/TGCTCAGTAACACGT-3) and Ar912r (5CTCCCCCGCCAATTCCTTTA-3) (Lueders and Friedrich 2000). The 25 l PCR reaction system consisted of 12.5 l premix, 1 l forward and 1 l reverse primers (10 M),
9.5 l 1 DEPC and 1 l DNA template (50 ng/l). Amplication parameters were as
follows: 5 min at 94C, 28 cycles for 60 s at 94C, 60 s at 52C and 10 min at 72C.
Additionally, in the current study, the community diversity of methanogens was proled
by the method described as terminal restriction fragment length polymorphism (TRFLP). Thus, the 5 end of Ar109r should be labelled with carboxyuorescein-5-succimidyl ester (FAM) dye for the further analysis of T-RFLP and it was not necessary for qPCR
determination.
For T-RFLP analysis, the amplied PCR products were puried and enzymatically
digested in a 30 l reaction system including 17 l 1 DEPC, 2 l buer, 10 l PCR
product (~ 0.2 g) and 1 l Hha I restriction enzyme (Thermo Fisher Scientic Inc.).
Then the samples were incubated at 37C for 3 h and the digestion was stopped by
heating at 80C for 20 min. Samples were sent to Shanghai Sunny Biotechnology Co.,
Ltd for analysis.
The quantitative analyses of methanogens were measured by the procedures described
in our earlier work (Jiao et al. 2014), in which the primers for qPCR were Met630 F (5GGATTAGATACCCSGGTAGT-3) and Met803 R (5-GTTGARTCCAATTAAAC
CGCA-3) previously validated by Hook et al. (2009). Briey, the qPCR assays were
run in an ABI 7900HT sequence detection system (Applied Biosystems, Foster City, CA,
USA) with a total volume of 10 l using SYBR Premix Ex Taq (Takara, Japan) following
the proposed protocol. The standard curve was generated for methanogens using serial
dilution of plasmid DNA copies that contained the exact 16S rRNA gene inserts. As
described by Chen et al. (2011), the nal copy numbers of targeted methanogens per
gram of ruminal contents were calculated by relating the Ct value to the standard curves.
In addition, the numbers would be converted into log10 for further statistical analysis.
CH4 concentration was analysed by gas chromatography (GC7890A, Agilent,
USA) equipped with a Flame Ionisation Detector (FID) and a Hayesep Q packing
column (Tang et al. 2013). The temperatures of column and injector were set for
3 min at 60C and 100C, respectively. The N2 was used as carrier gas at a ow rate
of 21 ml/min.
2.4. Statistical analysis
Firstly, data were analysed using MIXED procedures of SAS (SAS Inst. Inc., Cary, NC)
according to Latin square design; the model consisted of animal, period and treatment
as xed eects. Subsequently, the PROC MIXED procedures of SAS were used for the
data analysis according to a 2 2 factorial arrangement of treatments, the xed eects
in the model including cellulase, yeast product and their interaction. Statistical dierences were declared at p < 0.05. Dierences at 0.05 p 0.10 were considered as a
tendency towards signicance.
230
Q. LU ET AL.
For the T-RFLP analysis, the terminal restriction fragments (T-RF) were calculated
including peaks ranging from 50 to 500 bp and analysed using Peak Scanner 1.0
software (Applied Biosystems). The relative abundance of the individual T-RF was
calculated by dividing the peak area by the total area of all T-RF. The data were
standardised by exclusive of the peaks accounting for less than 0.5% of the total peak
area. Principle component analysis (PCA) of T-RF was conducted in R-Studio:
integrated development environment for R Version 0.96.122 (http://www.rstu
dio.org).
Downloaded by [University of California, San Diego] at 14:57 04 April 2016
3. Results
In Table 2, nutrient intakes, faecal output and the ATTD of OM, NDF and ADF are
listed for the experimental animals. Throughout the study, the intakes of OM, NDF and
ADF were similar between treatments. Likewise, no dierences in the respective faecal
outputs were observed. Similarly, due to similar nutrient intake and faecal output,
ATTD of nutrients did not dier between treatments.
Table 2. Eects of dietary cellulase (CEL) and yeast product (YP) addition on nutrient intake, faecal
output and apparent total tract digestibility in goats.
p-Values
Treatment (T)
CEL0.8#
Control
Intake [g DM/d]
OM*
NDF
ADF
Faecal output [g DM/d]
OM
NDF
ADF
Digestibility [%]
OM
NDF
ADF
YP6
YP12
480
232
143
489
237
148
483
234
145
141
83
56
140
81
53
152
89
59
70.6
64.3
60.8
71.4
65.8
64.4
68.6
61.7
59.1
CEL1.6
YP12
SEM
CEL
YP
CEL YP
483
234
145
441
227
144
21.1
4.88
1.92
0.525
0.655
0.620
0.344
0.358
0.452
0.341
0.350
0.412
0.458
0.798
0.624
134
80
52
140
85
56
8.67
5.65
3.61
0.718
0.791
0.642
0.451
0.713
0.650
0.409
0.336
0.273
0.788
0.828
0.728
3.64
2.63
2.60
0.666
0.689
0.556
0.775
0.980
0.762
0.282
0.247
0.219
0.639
0.971
0.730
YP6
72.2
65.8
64.3
65.2
61.9
61.2
Notes: Control, without addition of cellulase and yeast product #CEL0.8, cellulase added at 0.8 g/kg DM; CEL1.6,
cellulase added at 1.6 g/kg DM; YP6, yeast product added at 6 g/kg DM; $YP12, yeast product added at 12 g/kg DM;
SEM, standard error of means; *OM, organic matter; NDF, neutral detergent bre; ADF, acid detergent bre.
Table 3. Eects of dietary cellulase (CEL) and yeast product (YP) addition on energy balance in goats.
p-Values
Treatment (T)
CEL0.8#
CEL1.6
Control YP6 YP12$
YP6
YP12
Ingested gross energy [MJ/d]
9.25
9.41
9.30
9.30
8.47
Faecal energy [MJ/d]
3.01
2.97
3.25
2.88
2.99
Urinary energy [MJ/d]
0.65
0.79
0.68
0.67
0.61
0.70a
0.60b 0.62ab 0.66ab 0.59b
CH4 energy [MJ/d]
12.21
12.4
11.77
12.51
12.33
DE [MJ/kg DM]
9.43
9.59
9.24
9.80
9.90
ME [MJ/kg DM]
SEM
0.416
0.181
0.075
0.020
0.360
0.429
T
0.523
0.677
0.510
0.015
0.645
0.833
CEL
0.345
0.445
0.220
0.763
0.460
0.454
YP CEL YP
0.340
0.455
0.391
0.695
0.265
0.761
0.358
0.079
0.374
0.624
0.832
0.702
Notes: Control, without addition of cellulase and yeast product #CEL0.8, cellulase added at 0.8 g/kg DM; CEL1.6,
cellulase added at 1.6 g/kg DM; YP6, yeast product added at 6 g/kg DM; $YP12, yeast product added at 12 g/kg DM;
SEM, standard error of means; DE, digestible energy; ME, metabolisable energy.
Downloaded by [University of California, San Diego] at 14:57 04 April 2016
ARCHIVES OF ANIMAL NUTRITION
231
There were no eects of feeding dietary cellulase and yeast product on the daily
ingested GE and the FE and UE among all treatments (Table 3). For CH4 energy, a
tendency (p = 0.079) was observed for an interaction eect of cellulase and yeast
product. However, dietary addition of cellulase and yeast product had no eect on
DE and ME in goats.
Table 4 presents the eects of dietary addition of cellulase and yeast product on N
balance in goats. Throughout the study, the N intakes and outputs (of faeces and urine)
were similar between treatments. No dierences in N balance were observed between
cellulase or yeast product treatments and the Control.
As shown in Table 5, the ruminal pH values were not aected by dietary addition of
the yeast product, whereas the ruminal pH values were increased (p = 0.019) with the
increment of cellulase addition in the diets. The ruminal concentrations of NH3-N and
butyrate were signicant dierent among treatments (p < 0.01), while the dietary
addition of cellulase and yeast product did not aect the ruminal concentrations of
total VFA, acetate, propionate and the ratio of acetate to propionate. Nevertheless, with
the increment of cellulase addition in the diets, the ruminal butyrate concentration was
Table 4. Eects of dietary cellulase (CEL) and yeast product (YP) addition on nitrogen (N) balance in
goats.
p-Values
Treatment (T)
CEL0.8#
Ingested N [g/d]
Faecal N [g/d]
Urinary N [g/d]
Digested N [g/d]
Retained N [g/d]
Availability
[% of digested N]
[% of retained N]
Digestibility [%]
CEL1.6
Control
7.13
2.41
2.77
4.72
1.95
YP6
7.19
2.50
3.51
4.68
1.31
YP12$
7.14
2.70
3.14
4.44
1.45
YP6
7.13
2.36
3.10
4.78
1.68
YP12
7.16
2.39
3.06
4.77
1.71
SEM
0.021
0.146
0.242
0.144
0.298
T
0.349
0.487
0.362
0.490
0.615
CEL
0.491
0.224
0.308
0.250
0.383
YP
0.787
0.520
0.390
0.488
0.721
CEL YP
0.203
0.653
0.472
0.518
0.785
38.38
26.40
66.61
28.92
19.70
65.61
32.19
20.42
62.47
33.91
23.75
67.18
34.37
24.01
66.98
5.076
3.651
2.087
0.768
0.694
0.513
0.546
0.400
0.252
0.632
0.842
0.519
0.697
0.894
0.567
Notes: Control, without addition of cellulase and yeast product #CEL0.8, cellulase added at 0.8 g/kg DM; CEL1.6,
cellulase added at 1.6 g/kg DM; YP6, yeast product added at 6 g/kg DM; $YP12, yeast product added at 12 g/kg DM;
SEM, standard error of means.
Table 5. Eects of dietary cellulase (CEL) and yeast product (YP) addition on the ruminal pH,
ammonia-N and molar proportions of volatile fatty acids (VFA) in goats.
p-Values
Treatment (T)
CEL0.8#
Control
YP6
pH
6.35
6.37
NH3-N [mg/dl]
12.23a
9.72b
Total VFA [mM]
82.76
89.41
VFA concentration [mol/100 mol]
Acetate (A)
78.35
78.95
Propionate (P)
14.60
14.23
6.82ab
Butyrate
7.05ab
Ratio A:P
5.38
5.48
CEL1.6
YP12$
6.33
10.66ab
87.30
YP6
6.40
9.90b
83.08
YP12
6.40
10.23b
86.30
SEM
0.022
0.444
2.040
T
0.146
0.001
0.109
CEL
0.019
0.763
0.078
YP
0.354
0.133
0.787
CEL YP
0.497
0.468
0.198
79.60
14.17
6.23b
5.81
78.53
14.30
7.17a
5.45
78.66
13.99
7.35a
5.66
0.413
0.317
0.211
0.131
0.237
0.739
0.003
0.134
0.074
0.863
0.001
0.415
0.304
0.522
0.325
0.014
0.487
0.664
0.063
0.565
Notes: Control, without addition of cellulase and yeast product #CEL0.8, cellulase added at 0.8 g/kg DM; CEL1.6,
cellulase added at 1.6 g/kg DM; YP6, yeast product added at 6 g/kg DM; $YP12, yeast product added at 12 g/kg DM;
SEM, standard error of means.
232
Q. LU ET AL.
Table 6. Eect of dietary cellulase (CEL) and yeast product (YP) addition on the ruminal enzymatic
activity in goats.
p-Values
Treatment (T)
CEL0.8#
Downloaded by [University of California, San Diego] at 14:57 04 April 2016
Control
YP6
Carboxymethylcellulase activity [IU/l]
Intracellular
24.0a
15.2ab
38.9b
Extracellular
54.6a
54.1b
Total
78.6a
Xylanase activity [IU/l]
54.5a
Intracellular
72.9a
Extracellular
232.3a 171.1b
Total
305.2a 225.6b
CEL1.6
SEM
CEL
YP
CEL YP
21.2ab
48.7ab
69.9ab
2.48
3.33
5.50
0.015
0.003
0.003
0.004
0.001
0.001
0.635
0.985
0.826
0.955
0.970
0.961
70.4a
221.2ab
291.6ab
6.03
14.6
20.0
0.155
0.038
0.055
0.033
0.025
0.023
0.528
0.137
0.204
0.874
0.151
0.276
YP12$
YP6
YP12
13.9b
38.8b
52.7b
22.2ab
48.5ab
70.8ab
59.0a
210.2ab
269.2ab
67.7a
220.5a
288.2a
Notes: Control, without addition of cellulase and yeast product #CEL0.8, cellulase added at 0.8 g/kg DM; CEL1.6,
cellulase added at 1.6 g/kg DM; YP6, yeast product added at 6 g/kg DM; $YP12, yeast product added at 12 g/kg DM;
SEM, standard error of means; DE, digestible energy; ME, metabolisable energy.
increased (p = 0.001) and both the concentrations of total VFA (p = 0.078) and acetate
(p = 0.074) tended to be decreased. The ratio of acetate to propionate was increased
(p = 0.014) with the increment of yeast product addition. Furthermore, there was a
tendency for an interaction (p = 0.063) between addition of cellulase and yeast product
on the ruminal butyrate concentration.
Table 6 shows the activities of CMCase and xylanase in the rumen for all treatments.
CMCase (including the extracellular, intracellular and total CMCase) activities were
signicantly decreased (p < 0.05) in treatment CEL0.8 as compared with the Control
(except for the intracellular CMCase activity of treatment CEL0.8-YP6). While the
activities of intracellular xylanase were not signicantly dierent between treatments,
the activities of extracellular and total xylanase was decreased after feeding low concentrations of cellulose (treatments CEL0.8-YP6 and CEL0.8-YP12). Additionally, all
the determined enzymatic activities were greater (p < 0.05) for treatment CEL1.6 than
for those of treatment CEL0.8.
Data in Table 7 revealed the eects of dietary cellulase and yeast product addition on
the enteric CH4 emissions in goats. The enteric CH4 emissions corrected for OM and
NDF intake were declined (p < 0.05) in all supplemented groups when compared with
Table 7. Eects of dietary cellulase (CEL) and yeast product (YP) addition on the enteric methane
emissions in goats.
p-Values
Treatment (T)
CEL0.8
Control
CH4 emission
[l/kg DM intake]
[l/kg OM* intake]
[l/kg NDF intake]
CH4 energy of ingested GE$ [%]
Methanogen copy number
[log10/ml ruminal uid]
38.0a
41.2a
77.1a
7.5a
6.35a
YP6
YP12$
CEL1.6
YP6
YP12
32.5b 33.1ab 35.2ab 32.3b
35.3b 35.9b 38.2ab 35.0b
65.6b 66.8b 71.0ab 66.0b
6.4b
6.7ab 7.0ab 6.3b
6.40a 6.37a 6.45a 6.56a
SEM
CEL
YP
CEL YP
1.17
1.26
2.5
0.22
0.111
0.021
0.020
0.031
0.011
0.665
0.552
0.558
0.470
0.742
0.199
0.445
0.439
0.557
0.398
0.609
0.106
0.105
0.131
0.045
0.443
Notes: Control, without addition of cellulase and yeast product #CEL0.8, cellulase added at 0.8 g/kg DM; CEL1.6,
cellulase added at 1.6 g/kg DM; YP6, yeast product added at 6 g/kg DM; $YP12, yeast product added at 12 g/kg DM;
SEM, standard error of means.
Downloaded by [University of California, San Diego] at 14:57 04 April 2016
ARCHIVES OF ANIMAL NUTRITION
233
Figure 1. Eects of dietary cellulase and yeast product addition on methanogen diversity in goats.
Notes: PC1, rst principal component explaining 60.05% of variance to separate samples; PC2,
second principal component explaining 22.69% of variance to separate samples; CEL0.8 and
CEL1.6, cellulase added at 0.8 and 1.6 g/kg DM, respectively; YP6 and YP12, yeast product added
at 6 and 12 g/kg DM, respectively.
the Control, except for treatment CEL1.6-YP6. Compared with the Control, the enteric
CH4 emissions (corrected for DM intake) and CH4 energy of ingested GE [%] were
signicantly decreased (p < 0.05) in treatment CEL0.8-YP6 and CEL1.6-YP12 as. The
total copy numbers of methanogens (converted into log10) were not dierent between
treatments.
To evaluate the dierences in methanogens population diversity among dierent
treatments, PCA of T-RFs was conducted and the results revealed that the diversity of
methanogens were similar among dierent treatments (Figure 1). In Figure 1, PC1
means the rst principal component explaining 60.05% of variance for separate samples
and PC2 meant the second principal component explaining 22.69% of variance for
separate samples. The relative abundance of methanogenic T-RF was without obvious
clustering rule, which means that no signicant dierences in the relative abundance of
T-RF among the ve dierent treatments exist.
4. Discussion
The ruminal pH, NH3-N and VFA are vital parameters reecting the ruminal environment. In this study, the ruminal pH values were not altered after supplementation of
cellulase and yeast product when compared with the Control, while the pH values was
increased with the increasing levels of cellulase addition. Interestingly, in a previous
study (Lu et al. 2015), dietary cellulase and xylanase addition in growing goats
Downloaded by [University of California, San Diego] at 14:57 04 April 2016
234
Q. LU ET AL.
decreased the ruminal pH value when compared with the Control. The explanation
could be attributed to the function of yeast supplement (S. cerevisiae CNCM I-1077,
1010 cfu/d) as a booster, being helpful to maintain a higher rumen pH for rumen
fermentation (Bach et al. 2007). NH3-N is essential for microbial protein synthesis as
the preferred and major N source of rumen microbes (Bach et al. 2005). When
compared with the Control, a signicant reduction of NH3-N concentration in this
study was observed, except for treatment CEL0.8-YP12. These results corroborated with
ndings of earlier studies, where a yeast fermentation product from S. cerevisiae added
to diets of dairy cows at 56 g per animal and day (Hristov et al. 2010) decreased the
ruminal NH3-N concentration. The same eect was described by Gaafar et al. (2010),
who added exogenous brolytic enzymes to diets of lactating bualoes. In contradiction, increments of ruminal NH3-N concentration were claimed by Rajamma et al.
(2014) after inclusion of exogenous brolytic in bualo bulls, and by Mohamed et al.
(2009) after supplementation of a yeast product (containing S. cerevisiae at about 5 108
cfu/g plus growth media) in camels, while no dierences in ruminal NH3-N concentration were also reported with the addition of exogenous brolytic enzymes in lactating
dairy cattle (Arriola et al. 2011) and a yeast fermentation product in steers (56 g per
steer and day) (Lehloenya et al. 2008). These discrepancies could be due to the
dierences in animals (goats, dairy cows and camels), environmental factors, in particular feed type, and feed allocation method (Sutton et al. 2003; Beauchemin et al. 2004)
and the dierent nature of the yeast products. The combination of cellulase and yeast
product resulted in an increment of the ruminal butyrate concentration or acetate to
propionate ratio at the higher dose of cellulase or yeast product, respectively. This was
in agreement with the in vitro ndings of Tang et al. (2008), who reported that the
growth of dierent species of rumen microbes is responsible for the alteration of VFA
production and pattern after an addition of cellulase or yeast product.
The nutrient digestibility and N balance were not signicantly aected by the
addition of cellulase or yeast product in this trial, although nutrient digestibility have
been conrmed to be increased by an addition of exogenous brolytic enzyme (Arriola
et al. 2011) or a live yeast product derived from S. cerevisiae in diets of lactating dairy
cattle (Lascano et al. 2012). Tripathi and Karim (2010) reported that feed intake and
digestibility of OM, NDF, ADF and hemicelluloses are not changed by the addition of
the tested individual live yeast products (i.e., Kluyveromyces marximanus NRRL3234, S.
cerevisiae NCDC42 and S. uvarum ATCC9080) in lambs. In this study, rumen fermentation, total tract digestibility and N balance were not aected although enteric CH4
emissions were reduced. Besides the eect of cellulase and yeast product addition,
several other factors have been suggested to be responsible for these responses, including feed composition and feeding strategy (Desnoyers et al. 2009), yeast product source
(e.g., Kluyveromyces marximanus or S. cerevisiae), yeast product type (live or inactive
cultures) and animal species.
In the current study, the enteric CH4 emissions expressed as total daily emissions
corrected to the intake of DM, OM and NDF, respectively (Table 7), were decreased by the
addition of cellulase and the yeast product, although the evaluation of methanogens
revealed that the diversity of among treatments was similar. The reduction of enteric
CH4 emissions was in accordance with the data of CH4 energy accounted for ingested GE,
which was also decreased compared with the Control. As reported by Tang et al. (2013)
Downloaded by [University of California, San Diego] at 14:57 04 April 2016
ARCHIVES OF ANIMAL NUTRITION
235
and Lu et al. (2015), the enteric CH4 emissions were reduced (corrected to ADF intake)
with cellulase supplementation under in vitro conditions and in an in vivo trial in growing
goats. In addition, Mwenya et al. (2004) investigated an enteric CH4 reduction when a
yeast product (derived from T. sericeum, 1.22.3 107 cfu/g) was added to diets of sheep.
However, Hristov et al. (2010) have claimed that the enteric CH4 production is perhaps
not aected as much by methanogen abundance or diversity of the ruminal methanogen
populations when compared with methanogen-specic DGGE outcomes related to CH4
production in the rumen. Moreover, in the current experiment the sampling technique of
ruminal uid by an oesophageal tube may be a limiting factor for the analyses of
methanogens and enzyme activities as described by Sandri et al. (2014), who suggest
that the ruminal samples collected might not the representative samples of the solid phase
in the rumen. Because it contains small amounts of non-degraded feed particles, free
microbes, microbial remnants and metabolites in the uid fraction of rumen content, the
richness of bacterial populations is considered to be much lower than that of bacteria
adherent to large particles of feeds (Kong et al. 2010).
In comparison with the Control, a decline of ruminal CMCase and xylanase activities
(extracellular and total) were observed treatment CEL0.8-YP6, but higher inclusion
rates of cellulase enhanced their activities in the rumen to value not signicantly
dierent with the Control. This is in contrast to Lu et al. (2015), who have claimed
that the ruminal CMCase and xylanase activities were not inuenced by the addition of
cellulase and xylanase in goats. Furthermore, the CMCase and xylanase activities of
ruminal contents were not aected by a S. cerevisiae yeast product in some previous
studies (Hristov et al. 2010; Tripathi and Karim 2011). In addition, Giraldo et al. (2008)
have reported that an increment in ruminal enzymatic activity is not due to a direct
eect of the supplementation of the exogenous brolytic enzymes into the rumen.
Unfortunately, the eects of dietary exogenous cellulase and yeast product inclusion
on ruminal enzymatic activities were often neglected in other previous references, this
resulted in the diculty of comparing with the current ndings and it would be
necessary to be validated in further studies.
5. Conclusion
In the current study, the rumen fermentation, total tract digestibility, N balance and
energy balance of goats were not aected by dietary addition of exogenous cellulase and
a yeast fermentation product derived from S. cerevisiae, although the enteric CH4
emissions were reduced with a shift in CMCase and xylanase activities. However, in
spite of reduced enteric CH4 emission, the diversity and copy numbers of methanogens
were not changed. Because, the present ndings validated that the responses to cellulase
and yeast product supplementation in vitro did not completely match in vivo results, it
can be emphasised that in ruminants an ecient evaluation of exogenous enzymes or
yeast products should be proved under in vivo conditions.
Disclosure statement
No potential conict of interest was reported by the authors.
236
Q. LU ET AL.
Funding
The authors wish to acknowledge the nancial support received from the National Natural
Science Foundation of China [grant numbers 31561143009, 31320103917, 31460613, and
31472133].
Downloaded by [University of California, San Diego] at 14:57 04 April 2016
References
AOAC. 2002. Ocial methods of analysis. 16th ed. Washington, DC: Association of Ocial
Analytical Chemists.
Arriola KG, Kim SC, Staples CR, Adesogan AT. 2011. Eect of brolytic enzyme application to
low- and high-concentrate diets on the performance of lactating dairy cattle. J Dairy Sci.
94:832841.
Bach A, Calsamiglia S, Stern MD. 2005. Nitrogen metabolism in the rumen. J Dairy Sci. 88:921.
Bach A, Iglesias C, Devant M. 2007. Daily rumen pH pattern of loose-housed dairy cattle as
aected by feeding pattern and live yeast supplementation. Anim Feed Sci Technol. 136:146
153.
Beauchemin KA, Colombatto D, Morgavi DP. 2004. A rationale for the development of feed
enzyme products for ruminants. Can J Anim Sci. 84:2336.
Buddle BM, Denis M, Attwood GT, Altermann E, Janssen PH, Ronimus RS, Pinares-Patino CS,
Muetzel S, Neil Wedlock D. 2011. Strategies to reduce methane emissions from farmed
ruminants grazing on pasture. Vet J. 188:1117.
Chen YH, Penner GB, Li MJ, Oba M, Guan LL. 2011. Changes in bacterial diversity associated
with epithelial tissue in the beef cow rumen during the transition to a high-grain diet. Appl
Environ Microbiol. 77:57705781.
Colombatto D, Beauchemin KA. 2003. A proposed methodology to standardize the determination of enzymic activities present in enzyme additives used in ruminant diets. Can J Anim Sci.
83:559568.
Desnoyers M, Giger-Reverdin S, Bertin G, Duvaux-Ponter C, Sauvant D. 2009. Meta-analysis of
the inuence of Saccharomyces cerevisiae supplementation on ruminal parameters and milk
production of ruminants. J Dairy Sci. 92:16201632.
Gaafar HMA, Abdel-Raouf EM, EL-Reidy KFA. 2010. Eect of brolytic enzyme supplementation and ber content of total mixed ration on productive performance of lactating bualoes.
Slovak J Anim Sci. 43:147153.
Gill M, Smith P, Wilkinson JM. 2010. Mitigating climate change: the role of domestic livestock.
Animal. 4:323333.
Giraldo LA, Tejido M, Ranilla MJ, Ramos S, Carro MD. 2008. Inuence of direct-fed brolytic
enzymes on diet digestibility and ruminal activity in sheep fed a grass hay-based diet. J Anim
Sci. 86:16171623.
Hook SE, Northwood KS, Wright A-DG, McBride BW. 2009. Long-term monensin supplementation does not signicantly aect the quantity or diversity of methanogens in the rumen of
the lactating dairy cow. Appl Environ Microbiol. 75:374382.
Hristov AN, Varga G, Cassidy T, Long M, Heyler K, Karnati SK, Corl B, Hovde CJ, Yoon I. 2010.
Eect of Saccharomyces cerevisiae fermentation product on ruminal fermentation and nutrient utilization in dairy cows. J Dairy Sci. 93:682692.
Islam M, Abe H, Terada F, Iwasaki K, Tano R. 2000. Eects of levels of feed intake and
inclusion of corn on rumen environment, nutrient digestibility, methane emission and
energy and protein utilization by goats fed alfalfa pellets. Asian-Aus J Anim Sci. 13:1346
1346.
Jiao JZ, Wang PP, He ZX, Tang SX, Zhou CS, Han XF, Wang M, Wu DQ, Kang JH, Tan ZL.
2014. In vitro evaluation on neutral detergent ber and cellulose digestion by post-ruminal
microorganisms in goats. J Sci Food Agric. 94:17451752.
Downloaded by [University of California, San Diego] at 14:57 04 April 2016
ARCHIVES OF ANIMAL NUTRITION
237
Kong Y, Teather R, Forster R. 2010. Composition, spatial distribution, and diversity of the
bacterial communities in the rumen of cows fed dierent forages. FEMS Microbiol Ecol.
74:612622.
Lascano GJ, Heinrichs AJ, Tricarico JM. 2012. Substitution of starch by soluble ber and
Saccharomyces cerevisiae dose response on nutrient digestion and blood metabolites for
precision-fed dairy heifers. J Dairy Sci. 95:32983309.
Lehloenya KV, Krehbiel CR, Mertz KJ, Rehberger TG, Spicer LJ. 2008. Eects of propionibacteria
and yeast culture fed to steers on nutrient intake and site and extent of digestion. J Dairy Sci.
91:653662.
Lu DX, Zhang PY. 1996. Scientic technology of feeding goat. Beijing, China: China Agriculture
Press; p. 71126. (in Chinese).
Lu Q, Jiao JZ, Tang SX, He ZX, Zhou CS, Han XF, Wang M, Kang JH, Odongo NE, Tan ZL.
2015. Eects of dietary cellulase and xylanase addition on digestion, rumen fermentation and
methane emission in growing goats. Arch Anim Nutr. 69:251266.
Lueders T, Friedrich M. 2000. Archaeal population dynamics during sequential reduction
processes in rice eld soil. Appl Environ Microbiol. 66:27322742.
Martin C, Morgavi DP, Doreau M. 2010. Methane mitigation in ruminants: from microbe to the
farm scale. Animal. 4:351365.
Mohamed MI, Maareck YA, Abdel-Magid SS, Awadalla IM. 2009. Feed intake, digestibility,
rumen fermentation and growth performance of camels fed diets supplemented with a yeast
culture or zinc bacitracin. Anim Feed Sci Technol. 149:341345.
Mwenya B, Santoso B, Sar C, Gamo Y, Kobayashi T, Arai I, Takahashi J. 2004. Eects of including
1-4 galacto-oligosaccharides, lactic acid bacteria or yeast culture on methanogenesis as well as
energy and nitrogen metabolism in sheep. Anim Feed Sci Technol. 115:313326.
Newbold CJ, Rode LM. 2006. Dietary additives to control methanogenesis in the rumen. Int
Congress Ser. 1293:138147.
Rajamma K, Kumar SD, Rao RE, Nath ND. 2014. Eect of brolytic enzymes supplementation
on rumen fermentation of bualo bulls fed total mixed rations. Int J Agric Sci Vet Med.
2:106113.
Sandri M, Manfrin C, Pallavicini A, Stefanon B. 2014. Microbial biodiversity of the liquid
fraction of rumen content from lactating cows. Animal. 8:572579.
Sun ZH, Tan ZL, Liu SM, Tayo GO, Lin B, Teng B, Tang SX, Wang WJ, Liao YP, Pan YF,
et al. 2007. Eects of dietary methionine and lysine sources on nutrient digestion,
nitrogen utilization, and duodenal amino acid ow in growing goats. J Anim Sci.
85:33403347.
Sutton JD, Phipps RH, Beever DE, Humphries DJ, Hartnell GF, Vicini JL, Hard DL. 2003. Eect
of method of application of a brolytic enzyme product on digestive processes and milk
production in Holstein-Friesian cows. J Dairy Sci. 86:546556.
Tang SX, Tayo GO, Tan ZL, Sun ZH, Shen LX, Zhou CS, Xiao WJ, Ren GP, Han XF, Shen SB.
2008. Eects of yeast culture and brolytic enzyme supplementation on in vitro fermentation
characteristics of low-quality cereal straws. J Anim Sci. 86:11641172.
Tang SX, Zou Y, Wang M, Salem AZM, Odongo NE, Zhou CS, Han XF, Tan ZL, Zhang M, Fu YF,
et al. 2013. Eects of exogenous cellulase source on in vitro fermentation characteristics and
methane production of crop straws and grasses. Anim Nutr Feed Technol. 13:489505.
Tripathi MK, Karim SA. 2010. Eect of individual and mixed live yeast culture feeding on
growth performance, nutrient utilization and microbial crude protein synthesis in lambs.
Anim Nutr Feed Technol. 155:163171.
Tripathi MK, Karim SA. 2011. Eect of yeast cultures supplementation on live weight change,
rumen fermentation, ciliate protozoa population, microbial hydrolytic enzymes status and
slaughtering performance of growing lamb. Livest Sci. 135:1725.
Van Soest PJ, Robertson JB, Lewis BA. 1991. Methods for dietary ber, neutral detergent
ber and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 74:3583
3597.
238
Q. LU ET AL.
Downloaded by [University of California, San Diego] at 14:57 04 April 2016
Verstegen MWA, Van der Hel W, Brandsma HA, Henken AM, Bransen AM. 1987. The
Wageningen respiration unit for animal production research: A description of the equipment
and its possibilities. Energy metabolism in farm animals. eects of housing, stress and disease.
Dordrecht: Martinus Nijho; p. 2148.
Wang M, Sun XZ, Janssen PH, Tang SX, Tan ZL. 2014. Responses of methane production and
fermentation pathways to the increased dissolved hydrogen concentration generated by eight
substrates in in vitro ruminal cultures. Anim Feed Sci Technol. 194:111.
Potrebbero piacerti anche
- KP-210217-Recruitment Consultant - FINAL RevDocumento1 paginaKP-210217-Recruitment Consultant - FINAL RevMaulitaNessuna valutazione finora
- Spatial Uncertainty of A Geoid Undulation Model in Guayaquil, EcuadorDocumento11 pagineSpatial Uncertainty of A Geoid Undulation Model in Guayaquil, EcuadorMaulitaNessuna valutazione finora
- 1 GOCE IntroductionDocumento62 pagine1 GOCE IntroductionMaulitaNessuna valutazione finora
- Alternating Current Circuits and Electromagnetic WavesDocumento65 pagineAlternating Current Circuits and Electromagnetic WavesMaulitaNessuna valutazione finora
- Spatial Uncertainty of A Geoid Undulation Model in Guayaquil, EcuadorDocumento11 pagineSpatial Uncertainty of A Geoid Undulation Model in Guayaquil, EcuadorMaulitaNessuna valutazione finora
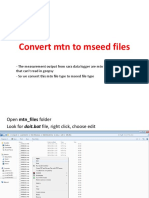
- Convert mtn files to mseed for dispersion analysisDocumento46 pagineConvert mtn files to mseed for dispersion analysisMaulitaNessuna valutazione finora
- Claprood Etal BSSA 2011Documento22 pagineClaprood Etal BSSA 2011Jennifer WatsonNessuna valutazione finora
- MMK Bab 5Documento96 pagineMMK Bab 5MaulitaNessuna valutazione finora
- Naskah Soal UN Fisika SMA 2012 Paket A81Documento13 pagineNaskah Soal UN Fisika SMA 2012 Paket A81agungfiristNessuna valutazione finora
- Long-Term Influence of Feeding Barley Treated With Lactic Acid and Heat On Performance and Energy Balance in Dairy CowsDocumento14 pagineLong-Term Influence of Feeding Barley Treated With Lactic Acid and Heat On Performance and Energy Balance in Dairy CowsMaulitaNessuna valutazione finora
- Effects of Dietary Addition of Cellulase and A Saccharomyces Cerevisiae Fermentation Product On Nutrient Digestibility, Rumen Fermentation and Enteric Methane Emissions in Growing GoatsDocumento16 pagineEffects of Dietary Addition of Cellulase and A Saccharomyces Cerevisiae Fermentation Product On Nutrient Digestibility, Rumen Fermentation and Enteric Methane Emissions in Growing GoatsMaulitaNessuna valutazione finora
- Effects of The Replacement of Concentrate and Fibre-Rich Hay by High-Quality Hay On Chewing, Rumination and Nutrient Digestibility in Non-Lactating Holstein CowsDocumento17 pagineEffects of The Replacement of Concentrate and Fibre-Rich Hay by High-Quality Hay On Chewing, Rumination and Nutrient Digestibility in Non-Lactating Holstein CowsMaulitaNessuna valutazione finora
- Chtest8 SolutionDocumento9 pagineChtest8 SolutionMaulitaNessuna valutazione finora
- Tabel ParabolaDocumento2 pagineTabel ParabolaMaulitaNessuna valutazione finora
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeDa EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeValutazione: 4 su 5 stelle4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingDa EverandThe Little Book of Hygge: Danish Secrets to Happy LivingValutazione: 3.5 su 5 stelle3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryDa EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryValutazione: 3.5 su 5 stelle3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceDa EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceValutazione: 4 su 5 stelle4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)Da EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Valutazione: 4 su 5 stelle4/5 (98)
- Shoe Dog: A Memoir by the Creator of NikeDa EverandShoe Dog: A Memoir by the Creator of NikeValutazione: 4.5 su 5 stelle4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureDa EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureValutazione: 4.5 su 5 stelle4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItDa EverandNever Split the Difference: Negotiating As If Your Life Depended On ItValutazione: 4.5 su 5 stelle4.5/5 (838)
- Grit: The Power of Passion and PerseveranceDa EverandGrit: The Power of Passion and PerseveranceValutazione: 4 su 5 stelle4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaDa EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaValutazione: 4.5 su 5 stelle4.5/5 (265)
- The Emperor of All Maladies: A Biography of CancerDa EverandThe Emperor of All Maladies: A Biography of CancerValutazione: 4.5 su 5 stelle4.5/5 (271)
- On Fire: The (Burning) Case for a Green New DealDa EverandOn Fire: The (Burning) Case for a Green New DealValutazione: 4 su 5 stelle4/5 (73)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersDa EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersValutazione: 4.5 su 5 stelle4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnDa EverandTeam of Rivals: The Political Genius of Abraham LincolnValutazione: 4.5 su 5 stelle4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaDa EverandThe Unwinding: An Inner History of the New AmericaValutazione: 4 su 5 stelle4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyDa EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyValutazione: 3.5 su 5 stelle3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreDa EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreValutazione: 4 su 5 stelle4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)Da EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Valutazione: 4.5 su 5 stelle4.5/5 (119)
- Her Body and Other Parties: StoriesDa EverandHer Body and Other Parties: StoriesValutazione: 4 su 5 stelle4/5 (821)
- Alternative Feeds GuideDocumento128 pagineAlternative Feeds GuideKadiwala Dairy Farm100% (1)
- AnnotatedDocumento9 pagineAnnotatedapi-483714607Nessuna valutazione finora
- Spelling List 4Documento70 pagineSpelling List 4BobbingtonionNessuna valutazione finora
- Alternative Plant Protein Sources For Pigs and ChickensDocumento23 pagineAlternative Plant Protein Sources For Pigs and ChickensalvinsernaNessuna valutazione finora
- SARA em RuminantesDocumento12 pagineSARA em RuminantesGustavo SchneidersNessuna valutazione finora
- Practical guide to dairy cattle nutrition and feeding managementDocumento74 paginePractical guide to dairy cattle nutrition and feeding managementUmesh AdigaNessuna valutazione finora
- Ps The Complete Book of BariaurDocumento133 paginePs The Complete Book of Bariaurpoisonink100% (2)
- M. M. Rahman - A Review of Oxalate Poisoning in Domestic Animals Tolerance and Performance AspectsDocumento10 pagineM. M. Rahman - A Review of Oxalate Poisoning in Domestic Animals Tolerance and Performance AspectsNayara YoNessuna valutazione finora
- 3rd Term s1 Agricultural Science 1Documento41 pagine3rd Term s1 Agricultural Science 1Adelowo DanielNessuna valutazione finora
- Biology BrochureDocumento2 pagineBiology Brochureapi-317164998Nessuna valutazione finora
- Biodegradation of Agricultural Wastes (Rice Straw and Sorghum Stalk) Into Substrates of Utilizable Products Using White Rot FungusDocumento7 pagineBiodegradation of Agricultural Wastes (Rice Straw and Sorghum Stalk) Into Substrates of Utilizable Products Using White Rot FungusInes HrastinskiNessuna valutazione finora
- Online English, Maths and Science Revision TestsDocumento8 pagineOnline English, Maths and Science Revision Testsshailesh mahtoNessuna valutazione finora
- ThesisDocumento64 pagineThesisHamdi ShaarNessuna valutazione finora
- Nutrient Requirements For CattleDocumento50 pagineNutrient Requirements For CattleMohaajanan AliNessuna valutazione finora
- Important animal feedstuffs and their classificationDocumento20 pagineImportant animal feedstuffs and their classificationFAZALNessuna valutazione finora
- Rumen MicrofloraDocumento14 pagineRumen MicrofloraDrRameem BlochNessuna valutazione finora
- Teeth 131109091818 Phpapp01Documento42 pagineTeeth 131109091818 Phpapp01ariyatiNessuna valutazione finora
- Voluntary Food Intake and Diet Selection in Farm Animals - J.M. Forbes - 2nd Ed - 2007 - (CABI)Documento462 pagineVoluntary Food Intake and Diet Selection in Farm Animals - J.M. Forbes - 2nd Ed - 2007 - (CABI)José Pedro Casagrande TrentínNessuna valutazione finora
- Anatomy and Physiology of The Goat PDFDocumento7 pagineAnatomy and Physiology of The Goat PDFHannan MirzaNessuna valutazione finora
- Methane Mitigation Strategies From Livestock by SK Asraf HossainDocumento78 pagineMethane Mitigation Strategies From Livestock by SK Asraf HossainKandice Burgess100% (1)
- Effect of Dietary Buffers on Milk Production and Physiology in Early LactationDocumento8 pagineEffect of Dietary Buffers on Milk Production and Physiology in Early LactationNICOL GIORJINA LOPEZ HUILLCANessuna valutazione finora
- Ch 2 MCQ Questions Answers Digestive System Human Body Organs Functions ProcessesDocumento24 pagineCh 2 MCQ Questions Answers Digestive System Human Body Organs Functions ProcessesRamesh KulkarniNessuna valutazione finora
- SSS 1 ANIMAL HUSBANDRY EXAM REVIEWDocumento2 pagineSSS 1 ANIMAL HUSBANDRY EXAM REVIEWejogheneta100% (3)
- Digestive PowerpointDocumento44 pagineDigestive PowerpointMauricio BeltranNessuna valutazione finora
- Feed Additives in Ruminant Nutrition FINALDocumento39 pagineFeed Additives in Ruminant Nutrition FINALjss_bustamanteNessuna valutazione finora
- How Sustainable Is Organic Agriculture in The Phils Rodel G. Maghirang Et AlDocumento180 pagineHow Sustainable Is Organic Agriculture in The Phils Rodel G. Maghirang Et AlAbner Obniala Venus Jr.Nessuna valutazione finora
- Fsa 3038 PDFDocumento6 pagineFsa 3038 PDFGenna Prama NugrohoNessuna valutazione finora
- Probiotic Use in Sheep FeedDocumento5 pagineProbiotic Use in Sheep FeedRiska AulyaNessuna valutazione finora
- Leafy Corn Silage Info GuideDocumento24 pagineLeafy Corn Silage Info GuideMargo Lee100% (1)
- Grade 11 TestDocumento5 pagineGrade 11 TestClinton O'NeilNessuna valutazione finora