Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Alchohol Retrosynthesis Page 3 (Organic Chemistry)
Caricato da
Ana-Marija Bartolincic0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
14 visualizzazioni1 paginaRETROSYNTHETIC APPROACH ON ALCHOHOL SNYTHESIS AND DEVELOPMENT
FOR MORE INFORMATION CHECK ON MY LINKED.IN PROFILE :
https://hr.linkedin.com/in/ana-marija-bartolincic-09883877
FOR MORE SUBSCRIOTIONS AND FREE DOWNLOADS IN CHEMISTRY, MATH AND PHYSICS CHECK MY FACEBOOK PAGE :
fb.me/chemistrymathphysicsprojects
Titolo originale
ALCHOHOL RETROSYNTHESIS PAGE 3 (ORGANIC CHEMISTRY)
Copyright
© © All Rights Reserved
Formati disponibili
PDF, TXT o leggi online da Scribd
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoRETROSYNTHETIC APPROACH ON ALCHOHOL SNYTHESIS AND DEVELOPMENT
FOR MORE INFORMATION CHECK ON MY LINKED.IN PROFILE :
https://hr.linkedin.com/in/ana-marija-bartolincic-09883877
FOR MORE SUBSCRIOTIONS AND FREE DOWNLOADS IN CHEMISTRY, MATH AND PHYSICS CHECK MY FACEBOOK PAGE :
fb.me/chemistrymathphysicsprojects
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PDF, TXT o leggi online su Scribd
0 valutazioniIl 0% ha trovato utile questo documento (0 voti)
14 visualizzazioni1 paginaAlchohol Retrosynthesis Page 3 (Organic Chemistry)
Caricato da
Ana-Marija BartolincicRETROSYNTHETIC APPROACH ON ALCHOHOL SNYTHESIS AND DEVELOPMENT
FOR MORE INFORMATION CHECK ON MY LINKED.IN PROFILE :
https://hr.linkedin.com/in/ana-marija-bartolincic-09883877
FOR MORE SUBSCRIOTIONS AND FREE DOWNLOADS IN CHEMISTRY, MATH AND PHYSICS CHECK MY FACEBOOK PAGE :
fb.me/chemistrymathphysicsprojects
Copyright:
© All Rights Reserved
Formati disponibili
Scarica in formato PDF, TXT o leggi online su Scribd
Sei sulla pagina 1di 1
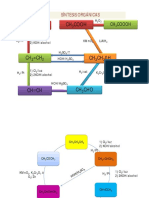
ALCHOHOL RESTROSYNTHESIS PAGE 3
LETS REMIND OURSELF OF THIRD WAY OF ACHOHOL SNYTHESIS
USING RETROGRIGNARD SNYTHESIS WITH PARTICIPATION OF
VICINAL GROUP:
OH
H3C
H3C
OH
CH2
H3C
H3C
electrons will go on both sides!!
TG2
one
molecules
has
become a diene and
another dienophile!
H2C
remind yourslef that when
you cut off the molecule its
synthons should at first be
the same size and shape so
you can find and recognize
them! and the charge add
later , dont be confused by
adding them at the same time
!
What does this reminds me
off?
You have to add a grignard
component in here so think
how and where!
H3C
CH3 MgBr
H3C
How to synthesise this?
H3C
H3C
Br
H3C
OH
1)Mg/H20
2)epoxide
H3C
TG2
Magnesium will make a
typical Grignard salt and
reminding that it will cut off
when added epoxide which
will convert into alchohol or
our targeted molecule TG2!
Potrebbero piacerti anche
- Handbook of Coordination Catalysis in Organic ChemistryDa EverandHandbook of Coordination Catalysis in Organic ChemistryNessuna valutazione finora
- 11 - Alcohol Ethers Thiols Wks KeyDocumento5 pagine11 - Alcohol Ethers Thiols Wks KeyMaria Aira Mendoza100% (1)
- Organic Reaction SummaryDocumento4 pagineOrganic Reaction SummaryRavindu HeshanNessuna valutazione finora
- Spectroscopy Nuclear Magnetic ResonanceDocumento54 pagineSpectroscopy Nuclear Magnetic ResonanceDamar Nurwahyu BimaNessuna valutazione finora
- Mechanism S.6 Chemistry-1Documento17 pagineMechanism S.6 Chemistry-1alexlweerebugembeNessuna valutazione finora
- Practice Makes Perfect in Chemistry: Oxidation-ReductionDa EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionValutazione: 5 su 5 stelle5/5 (1)
- Group 1 MOLE FRACTIONDocumento10 pagineGroup 1 MOLE FRACTIONjeromeespirituNessuna valutazione finora
- Universiti Kuala Lumpur: Malaysian Institute of Chemical & Bioengineering TechnologyDocumento4 pagineUniversiti Kuala Lumpur: Malaysian Institute of Chemical & Bioengineering TechnologyNufar MohmdNessuna valutazione finora
- Organic I Reactions (Complete) PDFDocumento10 pagineOrganic I Reactions (Complete) PDFStarrx714Nessuna valutazione finora
- Chemical Reactions: Soap Making: GSCI 1020 - Physical Science Laboratory Experiment #5Documento4 pagineChemical Reactions: Soap Making: GSCI 1020 - Physical Science Laboratory Experiment #5Rita L CaneloNessuna valutazione finora
- Organic Chemistry I: The Unofficial Reaction SheetDocumento11 pagineOrganic Chemistry I: The Unofficial Reaction SheetKarl WilsonNessuna valutazione finora
- Chemguide - Answers: Structural IsomerismDocumento2 pagineChemguide - Answers: Structural IsomerismEnny RachelNessuna valutazione finora
- January HW Sec 6Documento3 pagineJanuary HW Sec 6Freya SawNessuna valutazione finora
- CLS ENG 22 23 XII Che Target 5 Level 1 Chapter 13Documento62 pagineCLS ENG 22 23 XII Che Target 5 Level 1 Chapter 13Harsh JakharNessuna valutazione finora
- ALKANESDocumento73 pagineALKANESChona TuyNessuna valutazione finora
- LT - Batch A - Unit Test - 6 - CHE & BOT - 23.03.2023 - A Type PDFDocumento16 pagineLT - Batch A - Unit Test - 6 - CHE & BOT - 23.03.2023 - A Type PDFVENUGOPALARAONessuna valutazione finora
- Road Maps Organic Chemistry Set 8 Eklavya @JEEAdvanced - 2024Documento8 pagineRoad Maps Organic Chemistry Set 8 Eklavya @JEEAdvanced - 2024abhyudaipathwayNessuna valutazione finora
- Final Revision MCQ OrganicDocumento7 pagineFinal Revision MCQ Organiceeenus100% (1)
- Tutorial Letter 102/0/2021: General Chemistry 2Documento33 pagineTutorial Letter 102/0/2021: General Chemistry 2Tale BanksNessuna valutazione finora
- Mechanism S.6 ChemistryDocumento10 pagineMechanism S.6 Chemistryjordanmulumba34Nessuna valutazione finora
- NEPHAR 109 Practice Problems - 2 - G1&G2-1Documento3 pagineNEPHAR 109 Practice Problems - 2 - G1&G2-1Amirabbas SaffariNessuna valutazione finora
- Benzene Synthesis Page 51-53Documento3 pagineBenzene Synthesis Page 51-53Ung HhNessuna valutazione finora
- Organic Chemistry Unit - Test Review - Answer Key 1Documento6 pagineOrganic Chemistry Unit - Test Review - Answer Key 1Sumi SolangNessuna valutazione finora
- Preparación Alcanos-AcidosDocumento2 paginePreparación Alcanos-AcidosErickAntonyChavarríaGutiérrezNessuna valutazione finora
- Carbonyl Compounds 1Documento23 pagineCarbonyl Compounds 1Gowri ShankarNessuna valutazione finora
- SCHEME TRIAL CHEMISTRY SEM 3-Stpm 2013Documento11 pagineSCHEME TRIAL CHEMISTRY SEM 3-Stpm 2013Zuraini Arshad100% (1)
- 3 Functional Groups Alcohols:: Lecture Notes Chem 51B S. KingDocumento27 pagine3 Functional Groups Alcohols:: Lecture Notes Chem 51B S. KingHuấnĐìnhNguyễnNessuna valutazione finora
- Name of The Molecule Molecular Formula Hybridization Type Bond Angle GeometryDocumento7 pagineName of The Molecule Molecular Formula Hybridization Type Bond Angle GeometryKerala MekuriyaNessuna valutazione finora
- Alcohol Phenol and EtherDocumento78 pagineAlcohol Phenol and EtherBilal MalikNessuna valutazione finora
- Vollhardt Chapter 18 OChem PracticeDocumento23 pagineVollhardt Chapter 18 OChem PracticeDanNessuna valutazione finora
- Chapter 1 ALCOHOLDocumento65 pagineChapter 1 ALCOHOLNURUL AINUN MUHAMMAD NOR100% (1)
- Quiz Chapter 12 - Alcohols From CarbonylsDocumento16 pagineQuiz Chapter 12 - Alcohols From CarbonylsKaran RandhawaNessuna valutazione finora
- 20 Reaction of AlcoholsDocumento18 pagine20 Reaction of AlcoholsHamid Hussain HamidNessuna valutazione finora
- Chapter 16 SmithDocumento26 pagineChapter 16 SmithSandipan SahaNessuna valutazione finora
- Organic Chemistry I - Practice Exercise: Alkene Reactions and MechanismsDocumento9 pagineOrganic Chemistry I - Practice Exercise: Alkene Reactions and MechanismsElliot JamesNessuna valutazione finora
- Worksheet Class Xii - Chemistry Chapter - Haloalkanes and HaloarenesDocumento3 pagineWorksheet Class Xii - Chemistry Chapter - Haloalkanes and HaloarenesApratim NagNessuna valutazione finora
- Worksheet Class Xii - Chemistry Chapter - Haloalkanes and HaloarenesDocumento3 pagineWorksheet Class Xii - Chemistry Chapter - Haloalkanes and Haloarenesjiya jainNessuna valutazione finora
- Worksheet Class Xii - Chemistry Chapter - Haloalkanes and HaloarenesDocumento3 pagineWorksheet Class Xii - Chemistry Chapter - Haloalkanes and Haloarenesjiya jainNessuna valutazione finora
- 2 - JEE - Chemistry - Bio Molecules - MonosaccharidesDocumento19 pagine2 - JEE - Chemistry - Bio Molecules - MonosaccharidesMayank GoelNessuna valutazione finora
- Name: - DateDocumento2 pagineName: - Datejun joie jr. ruizNessuna valutazione finora
- Alkanes, Alkenes and AlkynesDocumento85 pagineAlkanes, Alkenes and AlkynesYoichi Kho100% (1)
- CH 44 Organic Reactions - Supp Ex 2Documento4 pagineCH 44 Organic Reactions - Supp Ex 2伊貝P-Nessuna valutazione finora
- CH 44 Organic Reactions - Supp Ex 2Documento4 pagineCH 44 Organic Reactions - Supp Ex 2伊貝P-Nessuna valutazione finora
- HKKX Fo"K Kred Iz'U: Part - I: Subjective QuestionsDocumento43 pagineHKKX Fo"K Kred Iz'U: Part - I: Subjective QuestionsKrishna Mohan ShuklaNessuna valutazione finora
- Practice Sn12Documento5 paginePractice Sn12Ram KrishnaNessuna valutazione finora
- Chapter 17: Alcohols and PhenolsDocumento29 pagineChapter 17: Alcohols and Phenols張湧浩Nessuna valutazione finora
- Combustion and Hydrate AnalysisDocumento2 pagineCombustion and Hydrate Analysisjonathen jaganNessuna valutazione finora
- Chapter3 Mole ConceptDocumento10 pagineChapter3 Mole Conceptmatyiman_123Nessuna valutazione finora
- NMR 27 APRIL To 27 May 20Documento34 pagineNMR 27 APRIL To 27 May 20Quratul AinNessuna valutazione finora
- Solutions Manual Chapter22Documento62 pagineSolutions Manual Chapter22더브레인코어과학관Nessuna valutazione finora
- CHM11-3 Balancing EquationsDocumento35 pagineCHM11-3 Balancing EquationsBenmar N. OcolNessuna valutazione finora
- Moles TestDocumento10 pagineMoles Testpirateduser666Nessuna valutazione finora
- Functional GroupCH5Documento36 pagineFunctional GroupCH5syedmcgarretNessuna valutazione finora
- Alkane-Full Notes FazliDocumento47 pagineAlkane-Full Notes Fazlijokowi123Nessuna valutazione finora
- Chemistry For Engineers Group 17 Assignment 3Documento5 pagineChemistry For Engineers Group 17 Assignment 3Vỹ KhangNessuna valutazione finora
- Retrosynthesis Examples Sec 2 With Wittig SynthesisDocumento1 paginaRetrosynthesis Examples Sec 2 With Wittig SynthesisAna-Marija BartolincicNessuna valutazione finora
- Achohol Retrosynthesis Page 5 With Examples of Phenol 1Documento1 paginaAchohol Retrosynthesis Page 5 With Examples of Phenol 1Ana-Marija BartolincicNessuna valutazione finora
- Ketone Retrosynthesis ExamplesDocumento1 paginaKetone Retrosynthesis ExamplesAna-Marija BartolincicNessuna valutazione finora
- Ketone Retrosynthesis SECTION 1Documento1 paginaKetone Retrosynthesis SECTION 1Ana-Marija BartolincicNessuna valutazione finora
- Definition of Limes and Continuity of FunctionDocumento4 pagineDefinition of Limes and Continuity of FunctionAna-Marija BartolincicNessuna valutazione finora
- Porous Inorganic Materials-Chemistry of Materials (Aluminosilicates, Aluminophosphates)Documento31 paginePorous Inorganic Materials-Chemistry of Materials (Aluminosilicates, Aluminophosphates)Ana-Marija BartolincicNessuna valutazione finora
- Kinetical and Mechanical Aspects of Metal HelicatesDocumento37 pagineKinetical and Mechanical Aspects of Metal HelicatesAna-Marija BartolincicNessuna valutazione finora
- Achohol Retrosynthesis (Organic Chemistry)Documento1 paginaAchohol Retrosynthesis (Organic Chemistry)Ana-Marija BartolincicNessuna valutazione finora
- Mathematical Absolute Value-Definition and RulesDocumento7 pagineMathematical Absolute Value-Definition and RulesAna-Marija BartolincicNessuna valutazione finora
- Hypocrisy, PoemDocumento1 paginaHypocrisy, PoemAna-Marija BartolincicNessuna valutazione finora
- Superconductivity 1Documento20 pagineSuperconductivity 1Madhur MayankNessuna valutazione finora
- Magnezij SuspstDocumento3 pagineMagnezij SuspstAna-Marija BartolincicNessuna valutazione finora
- Recycling LCD ScreenDocumento50 pagineRecycling LCD ScreenAna-Marija BartolincicNessuna valutazione finora
- CaseStudy2 WindmillDocumento8 pagineCaseStudy2 WindmillAnthony BergemannNessuna valutazione finora
- High Temperature Physicochemical Properties of High Alumina Blast Furnace SlagDocumento200 pagineHigh Temperature Physicochemical Properties of High Alumina Blast Furnace SlagBernardo Loureiro PattoNessuna valutazione finora
- Pec LTD.: Daily Welding Visual Inspection ReportDocumento5 paginePec LTD.: Daily Welding Visual Inspection ReportNatarajan MurugesanNessuna valutazione finora
- Ethanol EmittersDocumento2 pagineEthanol EmittersIosif CatalinNessuna valutazione finora
- OISD Std-108Documento15 pagineOISD Std-108Narayanan Menon100% (1)
- C4e 3 'Group 7 (The Halogens) ' HW SheetDocumento3 pagineC4e 3 'Group 7 (The Halogens) ' HW SheetNeen NaazNessuna valutazione finora
- Enhanced Degradation of Persistent Pharmaceuticals Found in Wastewater Treatment Ef Uents Using Tio2 Nanobelt PhotocatalystsDocumento14 pagineEnhanced Degradation of Persistent Pharmaceuticals Found in Wastewater Treatment Ef Uents Using Tio2 Nanobelt PhotocatalystsSourav SutradharNessuna valutazione finora
- Cooling A Pipe Filled With WaterDocumento78 pagineCooling A Pipe Filled With WatervyrgoNessuna valutazione finora
- Experiment 8 - The Preparation of AcetanlideDocumento12 pagineExperiment 8 - The Preparation of AcetanlideMark Ryan Tripole92% (13)
- Crystallography NotesDocumento96 pagineCrystallography NotesNafis AhmedNessuna valutazione finora
- M.SC - Physics - Syllabus 2015 - 2016Documento42 pagineM.SC - Physics - Syllabus 2015 - 2016r prathap100% (1)
- Bezaprint Black BDC - TdsDocumento2 pagineBezaprint Black BDC - TdsMahdiNessuna valutazione finora
- Delayed Coking Fractionators PTQ Q2 2009Documento5 pagineDelayed Coking Fractionators PTQ Q2 2009mahesh070Nessuna valutazione finora
- Isolation, Stabilization and Characterization of Xanthophyll From Marigold FlowerDocumento10 pagineIsolation, Stabilization and Characterization of Xanthophyll From Marigold FlowerpratheeshvbNessuna valutazione finora
- Theories of Aging PDFDocumento47 pagineTheories of Aging PDFBenedict LumabiNessuna valutazione finora
- Vitamin C ProjectDocumento40 pagineVitamin C ProjectJaid Mulla UTNessuna valutazione finora
- AT6002 - Unit 1 New FullDocumento36 pagineAT6002 - Unit 1 New FullƦoʛeʀ Ɩeo ƖɩbɩŋNessuna valutazione finora
- List of Important Metals and Their Ores With Chemical Formulas PDFDocumento2 pagineList of Important Metals and Their Ores With Chemical Formulas PDFAudibleNessuna valutazione finora
- Beggs - Brill Method PDFDocumento12 pagineBeggs - Brill Method PDFTiago Mendes TavaresNessuna valutazione finora
- Review Hydrogen Fuel CellsDocumento360 pagineReview Hydrogen Fuel CellsAdrian Delgado QuesadaNessuna valutazione finora
- AOC F013 SeriesDocumento2 pagineAOC F013 SeriesYap HSNessuna valutazione finora
- Fracture Resistance of Yttrium Oxide Partially-Stabilized Zirconia All-Ceramic Bridges After Veneering and Mechanical Fatigue TestingDocumento7 pagineFracture Resistance of Yttrium Oxide Partially-Stabilized Zirconia All-Ceramic Bridges After Veneering and Mechanical Fatigue TestingMostafa MedhatNessuna valutazione finora
- Programme of The M.Sc. (Other Than Mathematics, Statistics & Geography) (Part I) ExaminationDocumento4 pagineProgramme of The M.Sc. (Other Than Mathematics, Statistics & Geography) (Part I) ExaminationRajkumar PomajiNessuna valutazione finora
- ELEC-E8409 High Voltage Engineering Condition Monitoring of Electrical EquipmentDocumento46 pagineELEC-E8409 High Voltage Engineering Condition Monitoring of Electrical EquipmentRavinder SharmaNessuna valutazione finora
- Me8792 & Power Plant Engineering: Topic: Magneto-Hydro Dynamic (MHD) Generator-Power PlantDocumento25 pagineMe8792 & Power Plant Engineering: Topic: Magneto-Hydro Dynamic (MHD) Generator-Power PlantPalanivel Rajan A RNessuna valutazione finora
- APR GeneralDocumento53 pagineAPR GeneralAlex McMinnNessuna valutazione finora
- Transformation of SubstancesDocumento10 pagineTransformation of SubstancesRonnith NandyNessuna valutazione finora
- Corn RefineryDocumento31 pagineCorn RefineryAmit Arora100% (1)
- Hollo BlastDocumento16 pagineHollo BlastBraz Pataro NetoNessuna valutazione finora
- Casting PDFDocumento40 pagineCasting PDFphani301100% (1)
- Summary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisDa EverandSummary: Limitless: Upgrade Your Brain, Learn Anything Faster, and Unlock Your Exceptional Life By Jim Kwik: Key Takeaways, Summary and AnalysisValutazione: 5 su 5 stelle5/5 (8)
- The Obesity Code: Unlocking the Secrets of Weight LossDa EverandThe Obesity Code: Unlocking the Secrets of Weight LossValutazione: 4 su 5 stelle4/5 (6)
- The Marshmallow Test: Mastering Self-ControlDa EverandThe Marshmallow Test: Mastering Self-ControlValutazione: 4.5 su 5 stelle4.5/5 (60)
- Why We Die: The New Science of Aging and the Quest for ImmortalityDa EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityValutazione: 4.5 su 5 stelle4.5/5 (6)
- Summary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisDa EverandSummary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisValutazione: 4.5 su 5 stelle4.5/5 (44)
- Return of the God Hypothesis: Three Scientific Discoveries That Reveal the Mind Behind the UniverseDa EverandReturn of the God Hypothesis: Three Scientific Discoveries That Reveal the Mind Behind the UniverseValutazione: 4.5 su 5 stelle4.5/5 (52)
- Sully: The Untold Story Behind the Miracle on the HudsonDa EverandSully: The Untold Story Behind the Miracle on the HudsonValutazione: 4 su 5 stelle4/5 (103)
- To Explain the World: The Discovery of Modern ScienceDa EverandTo Explain the World: The Discovery of Modern ScienceValutazione: 3.5 su 5 stelle3.5/5 (51)
- When the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisDa EverandWhen the Body Says No by Gabor Maté: Key Takeaways, Summary & AnalysisValutazione: 3.5 su 5 stelle3.5/5 (2)
- Critical Care: A New Nurse Faces Death, Life, and Everything in BetweenDa EverandCritical Care: A New Nurse Faces Death, Life, and Everything in BetweenValutazione: 3.5 su 5 stelle3.5/5 (159)
- Sugar Crush: How to Reduce Inflammation, Reverse Nerve Damage, and Reclaim Good HealthDa EverandSugar Crush: How to Reduce Inflammation, Reverse Nerve Damage, and Reclaim Good HealthValutazione: 4 su 5 stelle4/5 (6)
- The Story of Philosophy: The Lives and Opinions of the Greater PhilosophersDa EverandThe Story of Philosophy: The Lives and Opinions of the Greater PhilosophersNessuna valutazione finora
- Alex & Me: How a Scientist and a Parrot Discovered a Hidden World of Animal Intelligence—and Formed a Deep Bond in the ProcessDa EverandAlex & Me: How a Scientist and a Parrot Discovered a Hidden World of Animal Intelligence—and Formed a Deep Bond in the ProcessNessuna valutazione finora
- Dark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseDa EverandDark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseValutazione: 3.5 su 5 stelle3.5/5 (69)
- 10% Human: How Your Body's Microbes Hold the Key to Health and HappinessDa Everand10% Human: How Your Body's Microbes Hold the Key to Health and HappinessValutazione: 4 su 5 stelle4/5 (33)
- Under Alien Skies: A Sightseer's Guide to the UniverseDa EverandUnder Alien Skies: A Sightseer's Guide to the UniverseValutazione: 4.5 su 5 stelle4.5/5 (17)
- Lessons for Survival: Mothering Against “the Apocalypse”Da EverandLessons for Survival: Mothering Against “the Apocalypse”Valutazione: 5 su 5 stelle5/5 (2)
- Masterminds: Genius, DNA, and the Quest to Rewrite LifeDa EverandMasterminds: Genius, DNA, and the Quest to Rewrite LifeNessuna valutazione finora
- Knocking on Heaven's Door: How Physics and Scientific Thinking Illuminate the Universe and the Modern WorldDa EverandKnocking on Heaven's Door: How Physics and Scientific Thinking Illuminate the Universe and the Modern WorldValutazione: 3.5 su 5 stelle3.5/5 (64)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincDa EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincValutazione: 3.5 su 5 stelle3.5/5 (137)