Documenti di Didattica
Documenti di Professioni
Documenti di Cultura
Fate of Entamoeba Histolytica During Establishment of Amoebic Liver Abscess Analyzed by Quantitative Radioimaging and Histology
Caricato da
MaithreyanNandhakumarTitolo originale
Copyright
Formati disponibili
Condividi questo documento
Condividi o incorpora il documento
Hai trovato utile questo documento?
Questo contenuto è inappropriato?
Segnala questo documentoCopyright:
Formati disponibili
Fate of Entamoeba Histolytica During Establishment of Amoebic Liver Abscess Analyzed by Quantitative Radioimaging and Histology
Caricato da
MaithreyanNandhakumarCopyright:
Formati disponibili
INFECTION AND IMMUNITY, June 2002, p.
32083215
0019-9567/02/$04.000 DOI: 10.1128/IAI.70.6.32083215.2002
Copyright 2002, American Society for Microbiology. All Rights Reserved.
Vol. 70, No. 6
Fate of Entamoeba histolytica during Establishment of Amoebic Liver
Abscess Analyzed by Quantitative Radioimaging and Histology
Marie-Christine Rigothier,1 Hout Khun,2 Paulo Tavares,3,4 Ana Cardona,2
Michel Huerre,2 and Nancy Guilln3,4*
Laboratoire de Biologie et Contrle des Organismes Parasites, UPRES 398-IFR 75, Facult de Pharmacie, Universit Paris-Sud,
Chatnay-Malabry,1 and Unit dHistotechnologie et Pathologie,2 Unit de Biologie Cellulaire du Parasitisme,3 and
Unit de Pathognie Microbienne Molculaire,4 Institut Pasteur, Paris 75724, Cedex 15, France
Received 8 November 2001/Returned for modification 21 December 2001/Accepted 2 March 2002
The protozoan parasite Entamoeba histolytica is the causative agent of amoebiasis, a human disease characterized by dysentery and liver abscess. The physiopathology of hepatic lesions can be satisfactorily reproduced in the hamster animal model by the administration of trophozoites through the portal vein route.
Hamsters were infected with radioactively labeled amoebas for analysis of liver abscess establishment and
progression. The radioimaging of material from parasite origin and quantification of the number inflammation
foci, with or without amoebas, described here provides the first detailed assessment of trophozoite survival and
death during liver infection by E. histolytica. The massive death of trophozoites observed in the first hours
postinfection correlates with the presence of a majority of inflammatory foci without parasites. A critical point
for success of infection is reached after 12 h when the lowest number of trophozoites is observed. The process
then enters a commitment phase during which parasites multiply and the size of the infection foci increases
fast. The liver shows extensive areas of dead hepatocytes that are surrounded by a peripheral layer of parasites
facing inflammatory cells leading to acute inflammation. Our results show that the host response promotes
massive parasite death but also suggest also that this is a major contributor to the establishment of inflammation during development of liver abscess.
between the amoeba and the host cells during invasion of a
specific human organ.
Our current knowledge of ALA development is essentially
derived from studies with rodent models. Pioneer studies have
shown that ALA formation after inoculation of E. histolytica
into the portal vein of hamsters evolves through different
phases (12). During the early stages, trophozoites brought by
the bloodstream penetrate the liver sinusoids, where they are
rapidly surrounded by host defense neutrophils, forming inflammatory foci. Trophozoites are separated from the hepatocytes by a ring of inflammatory cells. Hepatocytes show degenerative changes and lyse. During later stages, the infection foci
acquire a characteristic organization with a central necrotic
region composed of cellular debris surrounded by two peripheric layers, the first of trophozoites and the second of immune
cells that separate the infection region from the hepatic tissue.
Interestingly, direct contact of hepatocytes with trophozoites is
observed only occasionally during infection, indicating that the
hepatocytes massive death may be partially caused by diffusible products. It was hypothesized that such products originate
from lysis of the neutrophils (12). The second rodent model
that has been used for ALA development studies is severe
combined immunodeficient mice (2), as well as normal mice or
neutrophil-depleted mice (10, 13). The histological features of
infected livers are similar to those found in the hamster. The
death of hepatocytes and of cells of the immune system during
E. histolytica invasion in these mice results not only from the
cytolytic activity of the trophozoites but also from an apoptotic
process (11). The not-yet-identified signaling pathway triggering apoptosis is independent of Fas-Fas ligand interactions and
of the tumor necrosis factor alpha pathway (9).
Amoebiasis is a widespread human parasitic disease caused
by the protozoan Entamoeba histolytica. The two major clinical
manifestations of E. histolytica infection are amoebic colitis
and liver amoebic abscess. Yearly, 50 million people develop
intestinal amoebiasis and it is estimated that 10% of individuals with amebic colitis will develop an amebic liver abscess.
Amoeba infection causes 100,000 deaths per year, ranking,
second after malaria, among the most deadly human parasitic
diseases caused by a protozoan (6).
During invasive amoebiasis, highly motile trophozoites invade the intestinal epithelium, causing extensive tissue damage
characterized by acute inflammation and ulceration with necrosis and hemorrhage. In contrast to intestinal amoebiasis,
invasion of the liver is characterized by the presence of nonmotile E. histolytica trophozoites that cause an acute inflammatory reaction. Well-individualized infection foci contain
mostly dead hepatocytes, polymorphonuclear leukocytes (PMN),
macrophages, and parasites. Differences in pathology between
amoebic colitis and amoebic liver abscess (ALA) likely result
from a variation of the E. histolytica virulence repertoire in the
two organs and from different responses of these organs to
amoeba infection (11). Understanding the physiopathology of
amoebiasis thus requires the development of new experimental
strategies that take into account the multifactorial cross talk
* Corresponding author. Mailing address: Unit de Biologie Cellulaire du Parasitisme and Unit de Pathognie Microbienne Molculaire, INSERM U389, Institut Pasteur, 28 Rue du Dr. Roux, Paris
75724, Cedex 15, France. Phone: 331-45-68-86-75. Fax: 331-45-68-8674. E-mail: nguillen@pasteur.fr.
Present address: Laboratoire de Gntique des Virus, CNRS,
91198 Gif-sur-Yvette Cedex, France.
3208
FATE OF E. HISTOLYTICA IN AMOEBIC LIVER ABSCESS
VOL. 70, 2002
We present here the first semiquantitative study of ALA
development in hamsters. The fate of radioactively labeled
trophozoites inoculated in the portal vein was monitored and
quantified by histological analysis and radioimaging with a
Beta-Imager. These sensitive procedures allowed us to detect
quantitatively the amount of amoebic material in the liver and
to determine its distribution in tissue sections. We estimated
the survival and lysis rate of trophozoites reaching the liver
after intraportal inoculation. The results show that a large
number of trophozoites arriving in the liver die and that there
is a critical early time point at which the success of infection is
decided. The trophozoites that survive this early stage begin to
multiply and cause significant tissue damage.
MATERIALS AND METHODS
E. histolytica cultivation and radioactive labeling. Pathogenic E. histolytica
strain HM1:IMSS was cultivated axenically in TYI-S-33 medium (4). Highly
virulent trophozoites of strain HM1:IMSS (a gift of R. Perez Tamayo via Esther
Orozco, CINVESTAV, Mexico City, Mexico) were passed through hamster
livers every 2 months to maintain virulence.
Trophozoite cultures for intraportal inoculation were grown at 37C for 48 h
in closed bottles filled with medium. At 12 h before inoculation the trophozoites
were diluted if required to ca. 50 to 60% saturation and 35S-protein labeling mix
(NEN, Boston, Mass.) was added to 12 Ci/ml of medium. Radioactive labeling
was carried out in complete TYI-S-33 medium because we observed that trophozoites were less fit and grew slower in medium lacking cysteine normally used
to increase radioactivity incorporation. Before inoculation, the radioactive medium was decanted, and the adherent amoebas were washed twice with prewarmed medium before being detached by tapping the bottle and counted.
Hepatic inoculation procedure and preparation of infected liver sections.
Male Syrian golden hamsters (Mesocricetus auratus), aged 6 weeks, with an
average weight of 100 g, were inoculated intraportally with 4 105 HM1:IMSS
trophozoites. Treatment of animals and surgical procedures were done according
to the method of Tsutsumi et al. (12). Groups of three animals were sacrificed at
1, 3, 6, and 12 h and at 1, 3, 5, and 7 days after inoculation. Control noninfested
animals were sacrificed after 7 days. The livers were removed, inspected for the
presence of amebic abscesses, and photographed. The infected livers were then
treated for histological analysis and radioimaging analysis.
Quantitative determination of the radioactivity in liver sections. The quantitative determination of the radioactivity in liver sections was carried out by using
both a Micro-Imager and a Beta-Imager (Biospace, Paris, France) (3, 5). The
radioimaging procedure converts the detected energy produced by ionized particles into light and intensifies the corresponding localized light spot observed by
using a charge-coupled device camera (1). Data from digitized autoradiograms
were collected for 8 to 14 h from five liver sections and three animals at each time
point. The radioactivity was determined as the total counts per section.
Histology and immunohistochemistry. Dissected livers were fixed in 10%
buffered neutral formalin and embedded in paraffin. Serial 5-m sections were
stained with Harris hematoxylin and periodic acid-Schiff reagent. Immunohistochemistry was performed on sections from different livers with a human polyclonal antibody to E. histolytica. The sections were deparaffinized and immersed
in 200 l of citrate buffer. They were then incubated with the human polyclonal
antibody, washed three times in PBS, incubated with an anti-human immunoglobulin G, and visualized by the streptavidin-peroxidase method with aminoethyl carbazole as the chromogen.
Tissue preparation for sectioning and light microautoradiographic visualization. The localization of radioactive trophozoites was investigated by using the
paraffin section autoradiography procedure (8). Serial 5-m sections obtained
from infected livers were used. The number of sections depended on the average
size of inflammatory foci for each time point (see Results). Paraffin from these
sections was removed with xylene, and slides were incubated through a descending and then an ascending ethanol series (from 100 to 50% [vol/vol] and then the
reverse). Dried sections were processed for light microscope autoradiography by
overloading a coverslip coated with a nuclear emulsion (Kodak NTB2, diluted
1:1 with distilled water). After the slides were air dried for 8 h at room temperature, they were exposed for 2 to 4 weeks in light-proof boxes at 4C. Radiosensitive coverslips were developed for 3 min in Kodak D19 developer and fixed for
5 min in Kodak fixer. Sections were counterstained with Harris hematoxylin,
dehydrated, and mounted in Eukitt medium. The tissue and overlying silver
3209
grains were viewed and photographed with a Leica photomicroscope equipped
with a bright-field optics (DMRD Leitz; Leica) camera.
RESULTS
Radioimaging examination of ALA development. Intraportal
infestation of young adult hamsters with trophozoites is a wellestablished method for reproducing the different stages of
ALA formation. Parasites with 35S-labeled proteins were injected into the hamster portal vein. Sections of the infected
liver were prepared from animals sacrificed at different times
postinfestation ranging from 3 h to 7 days. The total radioactivity and its distribution in the complete tissue section were
examined by using a Micro-Imager (Fig. 1). Fine detail was
obtained by histological and immunological staining combined,
when required, with detection of radioactivity emission by silver precipitation (Fig. 1A, panel 3). Radioactive material is
strictly found in infected livers colocalizing with parasites or
with ghosts of dead amoebas in a basically background-free
environment. This method allows tracing the fate of the original trophozoites inoculated in the animal.
Visualization of amoeba radioactive material was also done
by using the Beta-Imager. Analysis of complete tissue sections
of infected liver showed strong individualized signals at 3 h
postinfestation, indicating that parasites had reached the liver
parenchyma at this early stage of the infection (Fig. 1B). As
infection proceeds the pattern of focal radioactivity distribution is maintained for 24 h, but then it gets diffused throughout
the complete section. The total amount of radioactivity in the
tissue sections, however, decreases slowly until 3 days postinfestation (Fig. 1C). The data suggest that most of the original
injected amoebas die or undergo cell division until 24 h postinfestation but that their radioactive proteins or breakdown
products are mostly retained in the organ tissue until day 3.
Radioactive products are then progressively eliminated from
the liver by metabolism.
Kinetics of liver infiltrates in the presence of E. histolytica.
The indication that a significant number of original trophozoites reaching the liver do not survive, a feature of ALA not yet
investigated, led us to analyze quantitatively their fate. The
sequence of events occurring at the tissue level during ALA
formation was monitored by histology (Fig. 2). The descriptive
histology of disease development reproduces particularly well
the seminal work of Tsutsumi et al. (12). Early stages of infection are characterized by acute cellular infiltration leading to
irregular liver lesions ranging from 70 to 150 m in diameter.
Infectious foci present a large number of inflammatory cells
surrounding normally a single parasite. In a significant number
of cases no trophozoite is found in the inflammatory focus. At
these early stages, parasites can be also seen in blood vessels
surrounded by PMN, monocytes, and a few lymphocytes. The
volume of infiltrates increases steadily as infection proceeds.
Necrosis appears at 24 h and becomes obvious at 48 h. The
hepatic lesions at this stage exhibit the characteristic features
of ALA: the centers of the abscesses are filled with amorphous
necrotic debris and edema, while viable amoebas form a peripheral layer that is surrounded by inflammatory cells delimiting the border between the area of necrosis and hepatic
parenchyma (Fig. 2, days 3 to 7).
The highest number of inflammatory foci is found in the
3210
RIGOTHIER ET AL.
INFECT. IMMUN.
FIG. 1. Radioimaging analysis of liver abscess formation. (A) Micrographs of dissected liver after 24 h of infection examined by Micro-Imager
and histology: 1, optical acquisition (10 magnification); 2, radiological acquisition of the same section, allowing localization of radioactive foci;
and 3, radioimmunohistochemical photomicrograph, allowing the identification of E. histolytica (immunodetection), radioactive amoeba proteins
(silver precipitation), and host cells (histological staining) (1,000 magnification). (B) Distribution of 35S radioactivity in liver slices of animals
infected with E. histolytica for the periods indicated in the micrographs. The radioactive material concentrates in discrete areas of the liver until
24 h, and then it exhibits a diffuse distribution before the signal becomes very weak after 72 h (results not shown). The bar under the panel indicates
the intensity of the radioactivity by a colored scale from dark (no signal) to red (intense signal). (C) Radioactivity in total liver sections at several
time points postinfection. Quantification made in the Beta-Imager is an average of the signal obtained during 1 h from five tissue sections from
three different animals and for each time point. The surface of liver sections was of 250 mm2 50 mm2. The bars represent the means plus the
standard deviations of 15 determinations for each time point.
VOL. 70, 2002
FATE OF E. HISTOLYTICA IN AMOEBIC LIVER ABSCESS
3211
FIG. 2. Histology of ALA progression. Sections of tissue liver from hamsters infected with E. histolytica for the periods indicated in the panels
were stained with hematoxylin to visualize the hamster cells (in blue) and immunolabeled to visualize the trophozoites (in red). Note the presence
of infiltrates containing PMN present already during the early stages of the infection. Several of these inflammatory foci appear without parasites.
Infiltration is massive after 1 day postinfection, and necrosis is very well established at 2 days. Coalescent foci generate abscesses that show a central
region of necrosis delimited by a ring of trophozoites surrounded by cells from the immune response, mainly PMN and macrophages. Bar, 20 m.
hamster liver during the early stages after intraportal inoculation of trophozoites (3 to 6 h), and this number decreases as
ALA develops. A majority of the foci do not contain visible
parasites (Fig. 3A). However, detection of radioactive material
by silver precipitation in the histology sections demonstrated
the presence of significant levels of radioactive protein of parasite origin in these foci (Fig. 4 and data not shown), originating most likely from dead trophozoites. We attribute the mas-
3212
RIGOTHIER ET AL.
INFECT. IMMUN.
FIG. 3. Quantitative analysis of inflammatory foci during development of ALA. (A) The total number of inflammatory foci (open bars) and foci
with trophozoites (patterned bars) were counted in total liver sections processed as as described in Fig. 2. Values correspond to the average from
counts of five tissue sections from three different animals for each time point indicated. The standard deviations are presented as a thin line at the
top of each bar. No inflammatory foci were found in the liver sections of control animals that were not infected. (B) Serial sectioning experiment
to quantify the total number of trophozoites per inflammatory foci. The 5-m tissue sections stained for histology observation are presented as
shaded bars, and the section number is on the right side. The average dimensions of inflammatory foci at 3, 6, and 12 h are drawn to scale (see
text) as dashed lines whose shape provides an idea of the focus form. One trophozoite is schematized in the interior of each focus to illustrate its
dimensions relative to the foci and to the tissue sectioning strategy.
sive amoeba killing to the acute inflammatory response
mediated by host immune system cells.
Histological observations suggest that an important number
of inflammatory foci do not contain parasites. A quantitative
assessment of the number of inflammatory foci with or without
intact trophozoites requires a statistically representative counting and serial sectioning of the foci to check at different planes
whether amoebas are present. A preliminary single-section
analysis was used to determine the average diameter of inflammatory foci for each time point of the infestation kinetics.
Visual inspection confirmed that inflammatory foci without
trophozoites are normally observed at 3, 6, and 12 h postinfection (Fig. 2). We then made serial sections of 5 m that
span 130% of the average height of the foci for these three
time points and also for 24 h. Sections spaced by 20 m (the
average diameter of a trophozoite is 25 m) were prepared for
histology staining (Fig. 3B). Observation of serial histology
sections provided a three-dimensional view of the inflamma-
tory foci at a resolution at which the presence of trophozoites
can be detected accurately. The study was only continued for
the time points 3, 6, and 12 h because the vast majority of foci
contained at least one trophozoite at 24 h postinfection, as was
already suspected from single-section observation (Fig. 2; data
not shown). The foci had a regular spherical or oval threedimensional shape at 3 and 6 h postinfection, whereas they
exhibited a more irregular form with lobular protuberances
after 12 h. The total number of foci without trophozoites (referred to as negative here) and with one or more trophozoites (positive) that were present in each serial section was
scored first. Second, foci that were negative in one section were
examined by inspection of serial sections along their complete
height. In this way, the percentage of negative foci in one
section that contained a parasite at a different plane of the
inflammatory focus was estimated. Starting from a central section in the series (e.g., section 9 for 3 and 6 h; Fig. 3B) and
from negative foci in that section, we analyzed 30 complete foci
TABLE 1. Quantitative determination of the number of inflammatory foci without trophozoites, with one trophozoite,
or with more than one trophozoite at 3, 6, and 12 h postinfectiona
Foci per tissue section
Time point
(h)
3
6
12
24
Avg focus diam in
m SD (n)
70.8 18.3 (98)
78.8 18.6 (85)
122.9 21.3 (35)
459.8 132.1 (45)
Ht (m) covered by
serial cutting
105
105
225
600
Foci with:
No. of
foci
Foci without
amoebas (%)
1 amoeba (%)*
2 amoebas (%)
Total (%)*
101
79
73
ND
44
51
86
ND
46
39
6
ND
10
10
8
ND
56
49
14
ND
a
The details of this experiment are described in the text. The average diameter of the inflammatory foci measured at different times postinfection in tissue sections
is shown in column 2, and the number of serial sections made to scan the complete height of foci at each time point is shown in column 3. n number of scored foci.
The total number of foci analyzed in serial sections (column 4) was then divided according to the finding of no trophozoites, one trophozoite, or more than one
trophozoite. The results in the right columns are presented as the percentage of the total number of foci. To calculate the values presented, we applied a correction
factor (*) to the number of foci without amoebas that takes into consideration the percentage of cases in which a negative focus becomes positive when its complete
height is analyzed in the serial sections [i.e., the percent foci without amoebas 100 (the total counts of foci without amoebas) (the total counts of foci without
amoebas) (the correction factor)/the total number of foci]. A correction factor was calculated for each experimental time point (see the text). The percentage of foci
with amoebas was then corrected based on the calculated percentage of foci without amoebas.
VOL. 70, 2002
FATE OF E. HISTOLYTICA IN AMOEBIC LIVER ABSCESS
3213
FIG. 4. Distribution of 35S-labeled material of trophozoite origin during ALA formation. E. histolytica was identified by immunolabeling,
inflammatory cells were identified by staining, and radioactivity was visualized by using a radiosensitive emulsion. Strong precipitation of silver
colocalizes with trophozoites, showing that radioactive material is brought in by amoebas. At early stages, silver grains are deposited on the surface
of endothelial cells (magnification view at 1 h). While infiltration develops, radioactive debris can be found in numerous inflammatory foci
associated in a number of cases with discernible ghosts of dead amoebas. The ghosts are characterized by a diffuse antiamoeba antibody staining
that overlaps with the silver grain concentration; this can be seen on the micrograph illustrating the 24-h infection silver grain concentration. Bar,
20 m.
3214
RIGOTHIER ET AL.
for 3 h postinfection: 31 for 6 h and 26 for 12 h. Among these
20, 29, and 4% were found to contain one or more trophozoites
for the time points 3, 6, and 12 h, respectively. These percentages were used as correction factors for the average counts of
individual serial sections (Table 1). The results with these
corrected data agree well with those from Fig. 3A and demonstrate the validity of quantifications based on tissue section
inspection.
In the early stages after intraportal inoculation of trophozoites (3 to 6 h), 40 to 60% of the inflammatory foci in the
hamster liver do not contain visible parasites (Fig. 3A and
Table 1). The success of liver abscess establishment has a
commitment step at 12 h postinfestation. A minimum number
of foci are reached at this time point, and only 10% of these
contained live trophozoites that certainly account for the success of ALA development (Fig. 3A and Table 1). Later, the
number of infection foci increases, and the majority of them
contain more than one live amoeba, indicating that trophozoite
cell division has resumed, and therefore the size of the lesion
increases (Fig. 2, 3, and 4 and data not shown). Well-organized
infectious foci can be counted up to 3 days, after which they
start to coalesce until the formation of uniform necrotic areas.
Quantification of infectious foci with 35S-labeled trophozoites. The fate of trophozoites and of their 35S-labeled proteins
was examined by immunolabeling and silver precipitation. Parasites revealed by an antiamoebic antibody were labeled specifically with radioactivity (Fig. 4). At the early stages, during
which the amoeba invades the liver, radioactivity is often encountered on the surface of endothelial cells (Fig. 4, 1 h, left
and right panels). The radioactive ghosts of dead amoebas
and/or debris can then be found in numerous inflammatory
foci. Ghosts are characterized by diffuse antibody staining that
overlaps with silver grains (Fig. 4, e.g., 3 and 12 h). Detection
of radioactive material by silver precipitation in the histology
sections demonstrated the presence of significant levels of radioactive protein of parasite origin in these foci (Fig. 4 and
data not shown), originating most likely from dead trophozoites. We attribute the massive amoeba killing to the acute
inflammatory response mediated by host immune system cells.
This observation reconciles the constant level of radioactivity
detected in complete liver sections (Fig. 1C) with the very
significant variations observed in inflammatory foci and
amoeba counts during the first days postinfestation (Fig. 3A
and Table 1). At advanced stages of ALA, labeling is found in
the central necrotic area of the lesion (Fig. 4, 24 h). Silver spots
are also detected in the hepatic tissue close to regions with
amoebas. These are attributed to products left behind during
amoeba motion or to proteins secreted by the trophozoites
after their arrival in the liver that may promote tissue destruction.
DISCUSSION
Liver invasion by E. histolytica with production of abscesses
is the most common extraintestinal manifestation of amoebiasis. Infection with radioactive parasites of immunocompetent
hamsters by the intraportal route allowed a description of
abscess development by radioimaging combined with histological analyses. Our study shows that E. histolytica infection of
the liver has a fast temporal program in which the parasite
INFECT. IMMUN.
crosses the endothelium of liver sinusoids, disseminates
through the organ, and adapts to the new environment before
it starts to divide and establish itself successfully to cause massive organ damage. An important finding was that parasites are
largely destroyed by the native immune system; we determined
that the period between 6 and 12 h is critical for amoeba
survival and subsequent ALA development.
In the hamster model system the parasites reach the liver in
the bloodstream through the portal vein. During early stages of
ALA development, amoebic radiolabeled material is deposited
on the surface of endothelial cells (Fig. 4), suggesting that
these cells are rapidly attacked by elements of the cytotoxic
system of parasites. This might be the initial trigger for the
inflammatory response. It was found that in humans suffering
from amoebiasis the liver endothelial cells secrete proinflammatory factors after contact with E. histolytica (14). The proinflammatory factors lead to recruitment and activation of immune cells that are responsible for the killing of parasites at
early stages postinfection. Our preliminary results indicate that
endothelial cells of the hamster sinusoids undergo apoptosis in
the presence of trophozoites (unpublished results). The resulting disturbance of the endothelial barrier likely facilitates the
passage of trophozoites between the blood circulation and the
hepatic tissue.
The variation in numbers and redistribution of trophozoites
and host immune cells in the first 24 h of ALA development
demonstrate a highly dynamic interaction between these different cells. Inflammatory cells are rapidly recruited after the
arrival of parasites to the liver, forming large foci around the
trophozoites and eliminating the majority of them. Consequently, most of the foci do not contain a live amoeba after
12 h of infestation (Fig. 3). Among the cells of the immune
system, neutrophils probably play the central role in the elimination of E. histolytica since parasite debris are normally surrounded by neutrophils in the hamster animal model (Fig. 2
and 4), and liver lesions are more prominent in neutrophildepleted mice than in normal mice (10, 13).
After the initial killing of trophozoites that leads to a significant reduction of positive foci, there is an increase of at least
sixfold in the number of foci with amoebas between 12 and
24 h postinfection. This is due to the multiplication of survivor
trophozoites (at 12 h postinfection the few positive foci detected have more than one amoeba in numerous cases [Fig. 3
and Table 1]) and to their migration in the liver tissue to
positions where new inflammatory foci are formed. Trophozoites leaving the foci or localized in their periphery can be easily
observed in histology sections of infected livers (Fig. 2). The
significant variations in the numbers of positive and negative
foci during the first 24 h of ALA development (Fig. 3) reflect
a very dynamic movement of both parasites and cells of the
immune system in the infected hamster liver.
The results presented here show that E. histolytica trophozoites that were grown under axenic conditions and injected via
the portal route are susceptible to the immunoresponse upon
their arrival into the liver. After 12 h of infection the trophozoites multiply and the immune system is not able to oppose
the fast dissemination of the parasite. It is possible that the
adaptation of trophozoites to the liver environment requires
the expression of a repertoire of genes necessary for survival
and for escape from the immune system. Evidence suggesting
FATE OF E. HISTOLYTICA IN AMOEBIC LIVER ABSCESS
VOL. 70, 2002
that complement resistance has to be acquired by E. histolytica
to survive during invasion of the human liver has been reported, for example, by Reed et al. and Walderich et al. (7, 15).
There is therefore a commitment phase necessary for ALA
development that relies on the presence of well-adapted survivor trophozoites. A most interesting question is whether,
during amoebiasis, the trophozoites that cross the intestinal
parenchyma and enter the blood circulation, which is the normal route for reaching the liver, are ready to colonize the liver
or whether they have to undergo the same adaptation discussed above. Regardless of this issue, the hamster experimental model of amoebiasis provides a very valuable system for
identifying the requirements for E. histolytica pathogenesis and
for investigating the molecular basis for how the parasite
adapts its genetic program depending on the tissue environment. Understanding this program will pave the way for the
identification of major parasite factors that account for the
aggressive behavior of E. histolytica at different stages of amoebiasis.
ACKNOWLEDGMENTS
We thank R. Perez-Tamayo and E. Orozco for kindly providing us
with the indispensable E. histolytica strain for the realization of these
experiments. We also acknowledge I. Fernandez for discussions, M.
Mavris for manuscript advice, and P. Sansonetti for continuous support and interest in the project.
This work was supported by grants from the French Ministry of
National Education through the PRFMMIP program. P.T. was supported by EMBO and FCT postdoctoral fellowships.
REFERENCES
1. Charpak, G., W. Dominik, and N. Zaganidis. 1989. Optical imaging of the
spatial distribution of beta-particles emerging from surfaces. Proc. Natl.
Acad. Sci. USA 86:17411745.
Editor: W. A. Petri, Jr.
3215
2. Cieslak, P. R., H. W. Virgin, and S. L. Stanley. 1992. A severe combined
immunodeficient (SCID) mouse model for infection with Entamoeba histolytica. J. Exp. Med. 176:16051609.
3. Crumeyrolle-Arias, M., M. Jafarian-Tehrani, A. Cardona, L. Edelman, P.
Roux, P. Laniece, Y. Charon, and F. Haour. 1996. Radioimagers as an
alternative to film autoradiography for in situ quantitative analysis of 125Iligand receptor binding and pharmacological studies. Histochem. J. 28:801
809.
4. Diamond, L. S., D. R. Harlow, and C. C. Cunnick. 1978. A new medium for
the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans.
R. Soc. Trop. Med. Hyg. 72:431432.
5. Laniece, P., Y. Charon, A. Cardona, L. Pinot, S. Maitrejean, R. Mastrippolito, B. Sandkamp, and L. Valentin. 1998. A new high resolution radioimager for the quantitative analysis of radio-labelled molecules in tissue
section. J. Neurosci. Methods 86:15.
6. Ravdin, J. I. 1995. Amebiasis. Clin. Infect. Dis. 20:14531466.
7. Reed, S. L., J. G. Curd, I. Gigli, F. D. Gillin, and A. I. Braude. 1986.
Activation of complement by pathogenic and nonpathogenic Entamoeba
histolytica. J. Immunol. 136:22652270.
8. Rougeot, C., R. Vienet, A. Cardona, L. Le Doledec, J. M. Grognet, and F.
Rougeon. 1997. Targets for SMR1-pentapeptide suggest a link between the
circulating peptide and mineral transport. Am. J. Physiol. 273:13091320.
9. Seydel, K. B., and S. L. Stanley. 1998. Entamoeba histolytica induces host cell
death in amebic liver abscess by a non-Fas-dependent, non-tumor necrosis
factor alpha-dependent pathway of apoptosis. Infect. Immun. 66:29802983.
10. Seydel, K. B., T. Zhang, and S. L. Stanley. 1997. Neutrophils play a critical
role in early resistance to amebic liver abscesses in severe immunodeficient
mice. Infect. Immun. 65:39513953.
11. Stanley, S. L. 2001. Pathophysiology of amebiasis. Trends Parasitol. 17:280
285.
12. Tsutsumi, V., R. Mena-Lopez, F. Anaya-Velazquez, and A. Martinez-Palomo.
1984. Cellular bases of experimental amebic liver abscess formation. Am. J.
Parasitol. 117:8191.
13. Velazquez, C., M. Shibayama-Salas, J. Aguirre-Garcia, V. Tsutsumi, and J.
Calderon. 1998. Role of neutrophils in innate resistance to Entamoeba histolytica liver infection in mice. Parasite Immunol. 20:255262.
14. Ventura-Juarez, J., R. Campos-Rodriguez, H. A. Rodriguez-Martinez, A.
Rodriguez-Reyes, A. Martinez-Palomo, and V. Tsutsumi. 1997. Human amebic liver abscess: expression of cellular adhesion molecules 1 and 2 and of
von Willebrand factor in endothelial cells. Parasitol. Res. 83:510515.
15. Walderich, B., A. Weber, and J. Knobloch. 1997. Sensitivity of Entamoeba
histolytica and Entamoeba dispar patient isolates to human complement.
Parasite Immunol. 19:265271.
Potrebbero piacerti anche
- Nematode Infections in Humans, France: Trichostrongylus ColubriformisDocumento2 pagineNematode Infections in Humans, France: Trichostrongylus ColubriformisBeverly FGNessuna valutazione finora
- ErosikkkDocumento20 pagineErosikkkYusrinabilla -Nessuna valutazione finora
- Salmonella Infection of Gallbladder Epithelial Cells Drives Local Inflammation and Injury in A Model of Acute Typhoid FeverDocumento38 pagineSalmonella Infection of Gallbladder Epithelial Cells Drives Local Inflammation and Injury in A Model of Acute Typhoid FeverAgussalimNessuna valutazione finora
- Feline Gastrointestinal Eosinofílica Fibroplasia EsclerosanteDocumento8 pagineFeline Gastrointestinal Eosinofílica Fibroplasia EsclerosanteCarlos Alberto Chaves VelasquezNessuna valutazione finora
- J Immunol 2007 Rydström 5789 801Documento14 pagineJ Immunol 2007 Rydström 5789 801susana1616Nessuna valutazione finora
- Jun Zou, Nathan ShankarDocumento11 pagineJun Zou, Nathan ShankarHQ HQNessuna valutazione finora
- Helmintos TisularesDocumento8 pagineHelmintos TisularesValentina Arenas RNessuna valutazione finora
- Antibiotic Resistance Profile of Staphylococci From Clinical Sources Recovered From InfantsDocumento7 pagineAntibiotic Resistance Profile of Staphylococci From Clinical Sources Recovered From InfantsCornius TerranovaNessuna valutazione finora
- Developmental and Comparative Immunology: Ying Zhang, Hui Sun, Huilin Zhao, Xingxing Chen, Jiaojiao Li, Boqing LiDocumento6 pagineDevelopmental and Comparative Immunology: Ying Zhang, Hui Sun, Huilin Zhao, Xingxing Chen, Jiaojiao Li, Boqing LiMohammad Farhan AdityaNessuna valutazione finora
- Emetine Produce Entamoeba Histolytica Death by IndDocumento6 pagineEmetine Produce Entamoeba Histolytica Death by IndJota CarlosNessuna valutazione finora
- Feline Panleukopenia Virus Infection in Various Species From HungaryDocumento9 pagineFeline Panleukopenia Virus Infection in Various Species From HungaryAlonSultanNessuna valutazione finora
- Jir 409Documento10 pagineJir 409utkarsh PromotiomNessuna valutazione finora
- A Mouse Model of Recrudescence ofDocumento4 pagineA Mouse Model of Recrudescence ofPaulo CzarnewskiNessuna valutazione finora
- Vet Pathol 2013 Delaney 172 6 DolphinDocumento6 pagineVet Pathol 2013 Delaney 172 6 DolphinKhairul Ihsan Pengusaha MudaNessuna valutazione finora
- 2014 Article 47 PDFDocumento7 pagine2014 Article 47 PDFalif bagusNessuna valutazione finora
- Fimmu 11 571816Documento20 pagineFimmu 11 571816Ricardo GomezNessuna valutazione finora
- Comparative Methods For Demonstration: Study of Tissue Culture of Toxoplasma GondiiDocumento4 pagineComparative Methods For Demonstration: Study of Tissue Culture of Toxoplasma GondiiSalim JufriNessuna valutazione finora
- Immunohistochemical Characterization of A Renal Nephroblastoma in A Trp53-Mutant and Prolyl Isomerase 1-Deficient MouseDocumento5 pagineImmunohistochemical Characterization of A Renal Nephroblastoma in A Trp53-Mutant and Prolyl Isomerase 1-Deficient Mouselily rogaNessuna valutazione finora
- Causal Agents: AmoebiasisDocumento5 pagineCausal Agents: Amoebiasisadinda sandyaNessuna valutazione finora
- Immunomodulation in The Canine Endometrium by UteropathogenicDocumento17 pagineImmunomodulation in The Canine Endometrium by UteropathogenicAndreea Blanco VeraNessuna valutazione finora
- Jced 7 E656Documento4 pagineJced 7 E656María José VázquezNessuna valutazione finora
- CHAPTER 2-ParasitDocumento109 pagineCHAPTER 2-Parasitalery ahreallyNessuna valutazione finora
- Jurnal Patogenesis AmoebiasisDocumento12 pagineJurnal Patogenesis AmoebiasisAngga NuralamNessuna valutazione finora
- Amoebiasis NewDocumento27 pagineAmoebiasis NewMutiaNessuna valutazione finora
- 1 s2.0 S0002944018305698 MainDocumento9 pagine1 s2.0 S0002944018305698 MainFrank Barreiro SanchezNessuna valutazione finora
- Diagnosis and Management of Amebiasis: State-Of-The-Art Clinical ArticleDocumento9 pagineDiagnosis and Management of Amebiasis: State-Of-The-Art Clinical ArticleSiscaSelviaNessuna valutazione finora
- Green DKK, 2015Documento14 pagineGreen DKK, 2015Nabila NatasyaNessuna valutazione finora
- Influence of Acute-Phase Parasite Load On PathologyDocumento11 pagineInfluence of Acute-Phase Parasite Load On PathologymclimacoNessuna valutazione finora
- 1 2 BRDocumento3 pagine1 2 BRrahmani bagherNessuna valutazione finora
- Cat L PDFDocumento11 pagineCat L PDFFlaviu TabaranNessuna valutazione finora
- Intestinal Protozoa Amoebae & Ciliates: ObjectivesDocumento5 pagineIntestinal Protozoa Amoebae & Ciliates: ObjectivesmicroperadeniyaNessuna valutazione finora
- Anthrax Lethal Toxin Disrupts Intestinal Barrier Function and Causes Systemic Infections With Enteric Bacteria 2012Documento10 pagineAnthrax Lethal Toxin Disrupts Intestinal Barrier Function and Causes Systemic Infections With Enteric Bacteria 2012fer123wizNessuna valutazione finora
- Recent Buzz in Malaria Research: The FEBS Journal, Editorial Office, Cambridge, UKDocumento4 pagineRecent Buzz in Malaria Research: The FEBS Journal, Editorial Office, Cambridge, UKVerlyanita SeptiariniNessuna valutazione finora
- Acute HepatotoxityDocumento11 pagineAcute HepatotoxityThially BragaNessuna valutazione finora
- Lancet - Septic Shock (Seminars)Documento16 pagineLancet - Septic Shock (Seminars)williamsbarriosNessuna valutazione finora
- Medmicro 32 2 135Documento7 pagineMedmicro 32 2 135vinty permatasariNessuna valutazione finora
- Artículo 2 - Fisiopatología de La PielonefritisDocumento17 pagineArtículo 2 - Fisiopatología de La PielonefritisCAROLINA CHUMACERO BERMEONessuna valutazione finora
- The Malaria Cell Atlas A Comprehensive RDocumento50 pagineThe Malaria Cell Atlas A Comprehensive R9868838836ankNessuna valutazione finora
- Gamberini Et AlDocumento9 pagineGamberini Et AlDeboraXiningNessuna valutazione finora
- Gut Bacteriome and Metabolome of Ascaris Lumbricoides in PatientsDocumento13 pagineGut Bacteriome and Metabolome of Ascaris Lumbricoides in PatientsprofnutrialecuriNessuna valutazione finora
- Wang 2015Documento7 pagineWang 2015Sebastian MilesNessuna valutazione finora
- 2021 Genome-Wide Association Study of Resistance To MTBDocumento18 pagine2021 Genome-Wide Association Study of Resistance To MTBAntonio Jafar GarciaNessuna valutazione finora
- Aeruginosa Strain PAO1 Is Regulated by Quorum Sensing andDocumento15 pagineAeruginosa Strain PAO1 Is Regulated by Quorum Sensing andSarah KKCNessuna valutazione finora
- Entamoba 1Documento15 pagineEntamoba 1nisaaintanNessuna valutazione finora
- Amebiasis: Review ArticleDocumento9 pagineAmebiasis: Review ArticleJouffrey Itaar MadridistaNessuna valutazione finora
- Hongos PerroDocumento4 pagineHongos PerroHelen Dellepiane GilNessuna valutazione finora
- Invasive Epithelial Mesothelioma in A Dog: F. R, B. B, K. R, E. S, D. BDocumento5 pagineInvasive Epithelial Mesothelioma in A Dog: F. R, B. B, K. R, E. S, D. BjamesyuNessuna valutazione finora
- Clinical, Morphological and Immunohistochemical Characterization of Cutaneous Lymphocytosis in 23 Cats (Pages 3-12)Documento10 pagineClinical, Morphological and Immunohistochemical Characterization of Cutaneous Lymphocytosis in 23 Cats (Pages 3-12)jenNessuna valutazione finora
- 1 s2.0 S1286457912001918 MainDocumento8 pagine1 s2.0 S1286457912001918 Mainabznaim420Nessuna valutazione finora
- Petton 2021 Frontiers Pacific MANUSCRIT2Documento26 paginePetton 2021 Frontiers Pacific MANUSCRIT2AmadorRevillaNessuna valutazione finora
- J Infect Dis.-2000-Hang-1738-48Documento11 pagineJ Infect Dis.-2000-Hang-1738-48Anonymous hXd6PJlNessuna valutazione finora
- E. Coli TITIDocumento6 pagineE. Coli TITIiggyNessuna valutazione finora
- Toxoplasma Gondii: Regulates ICAM-1 Mediated Monocyte Adhesion To TrophoblastsDocumento7 pagineToxoplasma Gondii: Regulates ICAM-1 Mediated Monocyte Adhesion To TrophoblastsCarlos Javier Castro CavadiaNessuna valutazione finora
- 423 432 PDFDocumento10 pagine423 432 PDFaldiansyahraufNessuna valutazione finora
- Original Article: A Guide To Histomorphological Evaluation of Intestinal Inflammation in Mouse ModelsDocumento21 pagineOriginal Article: A Guide To Histomorphological Evaluation of Intestinal Inflammation in Mouse ModelsOUR STORYNessuna valutazione finora
- Apoptosis in Natural Rabies Virus Infection in DogsDocumento5 pagineApoptosis in Natural Rabies Virus Infection in DogspapiipiiNessuna valutazione finora
- Candidatus Odyssella Thessalonicensis ' Gen. Nov., Sp. Nov., An Obligate Intracellular Parasite of Acanthamoeba SpeciesDocumento10 pagineCandidatus Odyssella Thessalonicensis ' Gen. Nov., Sp. Nov., An Obligate Intracellular Parasite of Acanthamoeba SpeciessomasushmaNessuna valutazione finora
- Parasitos en ReptilesDocumento13 pagineParasitos en ReptilesEduardo Pella100% (1)
- A Quantitative PCR (Taqman) Assay For Pathogenic: Leptospira SPPDocumento22 pagineA Quantitative PCR (Taqman) Assay For Pathogenic: Leptospira SPPVigneshwaran RavishankarNessuna valutazione finora
- Aikat1979 PDFDocumento5 pagineAikat1979 PDFMaithreyanNandhakumarNessuna valutazione finora
- Alternative Telomere RepaiirDocumento16 pagineAlternative Telomere RepaiirMaithreyanNandhakumarNessuna valutazione finora
- The Present and Future Role of Microfluidics PDFDocumento9 pagineThe Present and Future Role of Microfluidics PDFMaithreyanNandhakumarNessuna valutazione finora
- ThreeChannel PDFDocumento8 pagineThreeChannel PDFMaithreyanNandhakumarNessuna valutazione finora
- Extraintestinal Amebiasis': Scess Was PresentDocumento3 pagineExtraintestinal Amebiasis': Scess Was PresentMaithreyanNandhakumarNessuna valutazione finora
- Mechanotransduction PDFDocumento8 pagineMechanotransduction PDFMaithreyanNandhakumarNessuna valutazione finora
- 3microfluidic Co-Culture System For Cancer Migratory Analysis and Anti-Metastatic Drugs ScreeningDocumento11 pagine3microfluidic Co-Culture System For Cancer Migratory Analysis and Anti-Metastatic Drugs ScreeningMaithreyanNandhakumarNessuna valutazione finora
- Current Concept Same Bias IsDocumento10 pagineCurrent Concept Same Bias IsMaithreyanNandhakumarNessuna valutazione finora
- 4A Microfluidic 3D in Vitro Model For Specificity of Breast CancerDocumento18 pagine4A Microfluidic 3D in Vitro Model For Specificity of Breast CancerMaithreyanNandhakumarNessuna valutazione finora
- 5microfluidic Assay For Simultaneous Culture of Multiple Cell Types On Surfaces or Within HydrogelsDocumento29 pagine5microfluidic Assay For Simultaneous Culture of Multiple Cell Types On Surfaces or Within HydrogelsMaithreyanNandhakumarNessuna valutazione finora
- 2investigating Metastasis Using in Vitro Platforms - Madame Curie Bioscience Database - NCBI BookshelfDocumento25 pagine2investigating Metastasis Using in Vitro Platforms - Madame Curie Bioscience Database - NCBI BookshelfMaithreyanNandhakumarNessuna valutazione finora
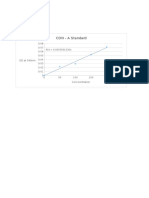
- CON - A Standard: OD at 540nmDocumento10 pagineCON - A Standard: OD at 540nmMaithreyanNandhakumarNessuna valutazione finora
- 1microfluidic ModellingDocumento22 pagine1microfluidic ModellingMaithreyanNandhakumarNessuna valutazione finora
- ReferencesDocumento4 pagineReferencesMaithreyanNandhakumarNessuna valutazione finora
- Introduction To Flow Cytometry - A Learning GuideDocumento54 pagineIntroduction To Flow Cytometry - A Learning GuideFrank FontaineNessuna valutazione finora
- Antifreeze Proteins Enhance Survival of Cells in Cryopreservation - Substituting DMSO With RmAFP#1 in Cryopreservation of Cells 1.2Documento93 pagineAntifreeze Proteins Enhance Survival of Cells in Cryopreservation - Substituting DMSO With RmAFP#1 in Cryopreservation of Cells 1.2MaithreyanNandhakumarNessuna valutazione finora
- .Documento33 pagine.3bdullah al3yuniNessuna valutazione finora
- Factors Affecting Wound HealingDocumento11 pagineFactors Affecting Wound HealingFredy Rodeardo MaringgaNessuna valutazione finora
- Pathology FinalADocumento72 paginePathology FinalAvaegmundig100% (1)
- What You Should Know About LipogemsDocumento4 pagineWhat You Should Know About LipogemsEmma KalmanNessuna valutazione finora
- Interpretation of The HemogramDocumento5 pagineInterpretation of The HemogramMargarita MujicaNessuna valutazione finora
- COVID Thrombosis: Mitochondria, The Endothelial Glycocalyx and Platelets Are KeyDocumento10 pagineCOVID Thrombosis: Mitochondria, The Endothelial Glycocalyx and Platelets Are KeyMercedes BouterNessuna valutazione finora
- DR - Bambang-Damage Control OrthopaedicsDocumento37 pagineDR - Bambang-Damage Control OrthopaedicsbadliinaaNessuna valutazione finora
- Pathophysiology of CHF Secondary To RHDDocumento89 paginePathophysiology of CHF Secondary To RHDMira MariantiNessuna valutazione finora
- Shar-Pei Dogs Hypocobalaminemia PDFDocumento10 pagineShar-Pei Dogs Hypocobalaminemia PDFFlávia UchôaNessuna valutazione finora
- Biomarcadores em IBDDocumento7 pagineBiomarcadores em IBDAndressa RigoNessuna valutazione finora
- Burns 3Documento31 pagineBurns 3Reann LeeNessuna valutazione finora
- Aktu Syllabus Bpharm 2nd Sem PDFDocumento21 pagineAktu Syllabus Bpharm 2nd Sem PDFAnkit Kumar100% (1)
- Nutrigenomics and CancerDocumento9 pagineNutrigenomics and CancerAbyatar Hugo BaskoroNessuna valutazione finora
- Pathology Notes (Class Notes)Documento106 paginePathology Notes (Class Notes)Mustafa Kamal Bangash100% (1)
- Protein Energy Wasting What Is It and What Can We Do To Prevent ItDocumento8 pagineProtein Energy Wasting What Is It and What Can We Do To Prevent ItLú VillalobosNessuna valutazione finora
- 1 Fundamentals of Nursing PracticeDocumento27 pagine1 Fundamentals of Nursing PracticeJoseph Manglallan100% (1)
- Ncma219 Lec FinalDocumento45 pagineNcma219 Lec FinalangeliqueggonzalesNessuna valutazione finora
- Novak Et Al 2011 AllergyDocumento10 pagineNovak Et Al 2011 AllergyArturo VeraNessuna valutazione finora
- Lecture 3 - Immunological System (K.Dhaliwal 2019)Documento62 pagineLecture 3 - Immunological System (K.Dhaliwal 2019)brittany AcheampongNessuna valutazione finora
- Culture of Animal Cells: Fig. 1.2. Tissue Culture ApplicationsDocumento1 paginaCulture of Animal Cells: Fig. 1.2. Tissue Culture ApplicationsCatalina ColoradoNessuna valutazione finora
- SF Journal of Biotechnology and Biomedical EngineeringDocumento5 pagineSF Journal of Biotechnology and Biomedical EngineeringFAUZANNessuna valutazione finora
- Peripatetic Cardiomyo - Genetics To ManagementDocumento11 paginePeripatetic Cardiomyo - Genetics To Managementanna67890Nessuna valutazione finora
- MelkiorDocumento5 pagineMelkiorFiLiOeiNessuna valutazione finora
- Systemic - Sclerosis Nature Review 2014Documento22 pagineSystemic - Sclerosis Nature Review 2014CT TANNessuna valutazione finora
- Robbins Chapter 2 DiagramsDocumento20 pagineRobbins Chapter 2 Diagramsjeffaguilar100% (2)
- Gastritis in DogsDocumento2 pagineGastritis in DogsPamela BriceñoNessuna valutazione finora
- Articol2 1Documento6 pagineArticol2 1Smaranda GavrilNessuna valutazione finora
- ProceedingsofROINMED2013 PDFDocumento161 pagineProceedingsofROINMED2013 PDFRazvan GheorgheNessuna valutazione finora
- SerratiopeptidaseDocumento4 pagineSerratiopeptidaseMo KhanNessuna valutazione finora
- Infrared Radiation PDFDocumento10 pagineInfrared Radiation PDFAjay Pal NattNessuna valutazione finora